Abstract
Arable weeds are a key component of the biodiversity of agroecosystems, but have faced a marked decline due to agricultural intensification. Recently, the crop edge has been considered as a potential refugia for many species. Indeed, weed species richness and abundance are higher in the crop edge than in the field margin and the field core. In this study we question whether weed functional diversity also varies among field elements and whether it is higher in the crop edge. We studied the interspecific and intraspecific variation of three functional traits (specific leaf area, canopy height and above-ground biomass) related to the response of weeds to competition and to agricultural practices, for seven weed species sampled in the crop edge, the field margin and the field core area in four winter-wheat fields. We show that trait values varied significantly with the species, the field element and their interaction. Within the field, all species had high specific leaf area, low canopy height and biomass, suggesting a shade-tolerance syndrome that could be a strategy in response to both competition with the crop and the disturbances induced by agricultural practices. In the crop edge, where the functional variation was the highest, two distinct functional strategies were observed, suggesting a resource partitioning under the predominance of weed–weed competition. In conclusion, the crop edge plays a key role in sustaining weed diversity, mostly because of its intermediate environmental properties that allow the coexistence of weeds with different ecological strategies.
Introduction
Arable weeds are an important component of plant diversity in agroecosystems and play a key role in supporting biological diversity at all upper trophic levels. In particular, weeds are of primary importance as a food resource for many species of birds and insects (Robinson and Sutherland Citation2002; Marshall et al. Citation2003). Over the last decades, weed species diversity and abundance have drastically declined in several European countries due to post-war agricultural intensification and land-use change (Andreasen, Stryhn, and Streibig Citation1996; Sutcliffe and Kay Citation2000; Baessler and Klotz Citation2006; Storkey et al. Citation2012).
Weeds are not spatially limited within cultivated fields, but are present along field margins and within various semi-natural habitats embedded in the agricultural mosaics (Marshall and Arnold Citation1995). However, the management practices implemented by farmers to enhance crop production mostly impact weeds within arable fields and their surroundings. As a consequence, most studies focused on the spatial repartition of weeds in (i) the crop edge, which is the narrow linear area where the soil is tilled but uncultivated, and (ii) the field, which is generally subdivided into a field margin, i.e. the first few metres of the cultivated area, and a field core area (following the terminology of Cordeau et al. Citation2012). The biotic environment and the management intensity differ between these three elements. In the field core, the crop exerts an intense competition over many weeds because of early establishment, sowing at high density (Weiner et al. Citation2010) and important inputs of mineral fertilizers that confer to the crop individuals a long-term preferential access to light (Kleijn and van der Voort Citation1997). The intensity of weed control resulting from management practices (i.e. herbicide application, tillage and crop rotations) also varies among field elements. In general, the crop edge is characterized by a higher weed density (Marshall Citation1989), a higher weed cover (Kovács-Hostyánski et al. Citation2011) and denser seedbank (José-María and Sans Citation2011) than in the field, suggesting more favourable conditions for weed development in the crop edge. Moreover, the crop edge shows higher species richness and diversity than the field (Fried et al. Citation2009), and the field margin generally shows higher species richness than the field core (Romero, Chamorro and Sans Citation2008; Kovács-Hostyánski et al. Citation2011). Finally, decades of intense weed control in the field core have resulted in a depletion of soil seedbanks within cultivated fields (Robinson and Sutherland Citation2002), and crop edges may be considered as refugia for weed biodiversity (Fried et al. Citation2009).
Management practices affect not only the taxonomic diversity of weed communities, but also their functional diversity, i.e. the variation in the functional traits of plant species. Several functional traits determine weed response to management practices, especially the amount of fertilizer applied and the herbicide pressures (Storkey, Moss and Cussans Citation2010). The usefulness of trait-based approaches in weed science has been emphasized in many recent papers (Storkey Citation2006; Fried et al. Citation2009; Gunton, Petit and Gaba Citation2011; Fried, Kazakou and Gaba Citation2012; Navas Citation2012), but all these studies considered functional variation across large scales (national or regional). There are currently no data about the spatial variation of traits at field scale. Furthermore, previous studies have explored the weed response to cropping systems by considering mean trait values per species. However, the intraspecific variability of trait values has recently been demonstrated to be important for species coexistence and the dynamics of communities (Jung et al. Citation2010; Albert et al. Citation2011; Bolnick et al. Citation2011). Indeed, exploring the intraspecific variation allows us to detect whether individuals within a local population exhibit different ecological strategies.
In the present study, we address the following questions: (i) does the crop edge show a higher range of response trait values compared to the field core and the field margin? (ii) if so, are there any differences in the range of ecological strategies of weeds among field elements? and (iii) does the intraspecific functional variability of weeds differ among field elements? We considered seven co-occurring common weed species and analysed their interspecific and intraspecific trait distributions across field elements in winter wheat. In the field margin and the field core, we assume a higher intensity of competition (mostly weed–crop) and of disturbances (i.e. agricultural practices) than in the crop edge. As a consequence, due to the higher environmental filtering (Cornwell and Ackerly Citation2009), the range of functional trait values is expected to be smaller in the field margin and the field core. In the crop edge, we assume that the lower disturbance and the absence of the crop competitor should promote weed–weed competition. Therefore, as predicted by the limiting similarity principle (MacArthur and Levins Citation1967), weeds should exploit different resource niches; hence the extent of trait differences among the species should be higher. We studied seven species that co-occur at different abundances among the three field elements and investigated whether these species show distinct sets of trait values and different ranges of trait variation depending on the field element where they grow.
Materials and methods
Field sites
The winter wheat fields sampled were located in Fénay, Côte d’Or (Burgundy, France 47°13′ N, 5°03′ E), within an intensive agricultural area mainly cultivated with winter crops (32% winter wheat, 20% oilseed rape, 14% mustard and 14% winter barley) in rotation with spring barley (9%). We chose four arable fields with a similar crop sequence (winter wheat – winter barley – winter oilseed rape) and a similar crop management (similar inter-row width and density, similar timing of treatment sequence). To avoid an effect of the surrounding landscape on the weed flora in field margins, the sampled areas within the four arable fields were always located along a stone path and away from forest edges and hedges.
Species selection
We studied seven common weed species known to occur in all field elements, though with different relative abundances in Fénay (Fried et al. Citation2007). All these species are dicotyledonous, have a C3 photosynthetic pathway, are therophytes, hermaphroditic and have partially overlapping flowering periods. They have different plant architectures. Veronica hederifolia L. and Veronica persica Poir. are creeping species. Fallopia convolvulus (L.) Á. Löve and Galium aparine L. are primarily climbing species while Viola arvensis Murray, Geranium dissectum L. and Papaver rhoeas L. are characterized by an erect habit.
Species traits
We use a few well-targeted functional traits that are ecologically meaningful (Bernhardt-Römermann et al. Citation2008), and chose three traits known to vary in response to disturbance and light competition: the specific leaf area (SLA), the plant canopy height and the plant above-ground biomass. SLA is a good indicator of weed–crop competition for light at the species-scale (Brainard, Bellinder, and DiTommaso Citation2005; Storkey Citation2005; Gaba et al. in press) and is also correlated with the relative growth rate of plants (Reich, Walters and Ellsworth Citation1997). A higher relative growth rate is usually linked with a higher return interval of disturbances (Gaba et al. in press). The canopy height can be considered as an indicator of light competition at the species-scale (Holt Citation1995) but also partly related to disturbance frequency (Gaba et al. in press). Finally, several studies have quantified the effect of competition among weeds and winter wheat based on the reduction of weeds above-ground biomass (Weiner et al. Citation2010). The above-ground biomass is also a surrogate of the plant performance and seed production (Lutman Citation2002; Brainard, Bellinder, and DiTommaso Citation2005).
Plant sampling and traits measurement
In each field, we delimited one area in each of the three field elements considered (the crop edge, the field margin and the field core). Up to ten individuals per species per element were collected from each of the four cultivated fields sampled. In the crop edge and the field margin, samplings were carried out along a 50 m transect. In the field core, a random sampling was performed. To avoid sampling genetically related individuals, the minimum distance between samples was always 50 cm. Measurements were conducted in early June 2012 within a single week when plants had reached the end of their vegetative growth period (numerous nodes or side shoots visible) or when they were at the beginning of their flowering period. The canopy height was measured in situ as the distance (in cm) between the top of the main photosynthetic organs and the soil. Two leaves (fully formed with no signs of damage or senescence) were then sealed in plastic bags containing moist paper towels to prevent dehydration. The above-ground biomass was collected and stored in paper bags. Immediately upon returning from the field, individual leaf area was measured using a leaf area meter (LI-3100 Area Meter, LI-COR inc, Lincoln, NE, USA). For small leaves, the surface area was determined using a software image analysis: Visilog 6.7 (Noesis, France). Then, leaves and above-ground biomass were dried at 80°C for 2 days and weighed (in g). The SLA (in m² g–1) was calculated as the ratio of the leaf surface to the leaf dry mass.
Data analyses
All data analyses were carried out using the R software version 2.15.1 (R Development Core Team, Citation2012). Variation in functional trait values among individuals was explored with a standardized principal component analysis. For each trait, a linear mixed model was implemented, that included the field element, the species and the “field element × species” interaction as fixed effects and a random intercept field effect. To meet the assumptions of homogeneity of variance and normality of the residuals, the SLA and the height were square-root transformed and the biomass was log10-transformed. Bartlett test and Shapiro–Wilk test were performed on the standardized residuals to check the need for transformation. Mixed effects models analyses were performed using the package lme4. A likelihood-ratio test based on the χ² distribution was used to evaluate the significance of the additional effect of each variable in the model after first including all other variables (i.e. type III analysis of deviance). We applied sum to zero contrasts before fitting models. We used Tukey contrasts for multiple comparisons of means to compare significant differences between field elements.
Interspecific variation in functional traits was quantified using a measure of overlap between the two distributions of trait values obtained for each pair of species. To avoid the effect of a too-small sample size, we only considered species with a total of more than 10 individuals measured per field element. First, a measure of overlap was estimated for each trait based on non-parametric Gaussian kernel density estimation using the R functions implemented by Geange et al. (Citation2011) but with slight modifications to follow recommendations from Sheather and Jones (Citation1991) for the choice of bandwidth. Second, a multi-trait measure of overlap was estimated based on standardized trait values using the metric Fβ (Villéger, Novack-Gottshall, and Mouillot Citation2011). Fβ is a measure of functional dissimilarity between two convex hulls in a multidimensional space; it is maximal when two species have no common range of traits and minimal when they are identical. We therefore used 1 – Fβ as a measure of functional overlap.
We used a randomization method to test whether the functional overlap between species within each field element was significantly lower than under the null hypothesis of no functional divergence among species. If individuals from two species share the same distribution of trait values, their actual taxonomic identity is irrelevant; the randomization procedure is therefore a permutation of individuals, independently of their species name. We generated 10,000 null distributions and used a one-tailed direct test with significant threshold of p < 0.05 to test for a non-random structure (see Supplementary Figure 1 for details). Multiple comparisons were made; therefore a sequential Bonferroni adjustment was applied (Holm Citation1979).
Intraspecific variation in functional traits within each species was quantified using a measure of overlap between the two distributions of trait values obtained for each pair of field elements. The same overlap metrics and randomization method as described above were used to test whether the functional overlap between field elements for each species was significantly lower than under the null hypothesis of no intraspecific functional divergence between field elements. Individuals were randomized independently of the field element from which they were sampled.
Results
Over the four cultivated fields, a total of 386 plants were sampled and measured, 185 of which were located in the crop edge, 108 in the field margin and 93 in the field core area. The average number of plants sampled per species was 54 ± 24.
Trait variability among field elements and species
The first axis of the principal component analysis on individual traits’ values explained more than 60% of the variation. Individuals were classified along this axis according to their height and SLA (Figure A). The variation in biomass was correlated with the variation in height, but was relatively independent from the variation in SLA. The ordination plan showed a clear difference between the crop edge and the field. In the crop edge, a wider range of trait values was observed, especially for the biomass (Figure B). In comparison, the field core and the field margin were both characterized by a similar trend of variation of SLA values (Figure B).
Figure 1. Standardized principal component analysis of the variation of three functional traits in seven weed species, measured at the individual level within three field elements. (A) Contribution of traits to the first and second principal components. (B) Location of plants sampled from different field elements on the ordination plan; 90% confidence ellipses around field element centroids are drawn. (C) Location of plants belonging to different species on the ordination plan. EPPO codes refer to abbreviations as presented in Table .
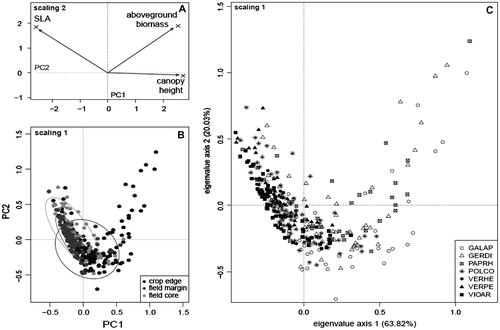
Functional traits values significantly varied with the field element, the species and the “field element × species” interaction (Table ). Important differences in trait values were observed between the weed individuals sampled in the crop edge and those sampled in the field margin. On average, individuals sampled in the crop edge were significantly taller, with higher biomass and smaller SLA values than in the field margin (Table and Figure ).
Table 1. Linear mixed model analyses on functional trait variation for seven weed species in three field elements (crop edge, field margin and field core). Tukey’s post hoc tests investigate the pairwise differences between field elements. Square-root (SLA and canopy height) or log10-transformations (above-ground biomass) were applied to meet the assumptions of homogeneity of variance (Bartlett test) and normality of the residuals (Shapiro–Wilk test). *p < 0.05, **p < 0.01, ***p < 0.001, ns, not significant.
Ecological strategies of weed species
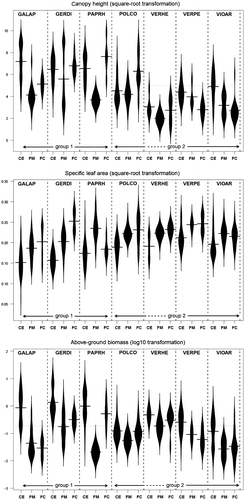
Based on functional overlap, two groups of species could be distinguished within the crop edge, respectively composed of G. aparine, G. dissectum and P. rhoeas (hereafter “group 1”) and F. convolvulus, V. persica and V. arvensis (“group 2”). Veronica hederifolia could not be included in the analyses because the sample size was too small; however, Figure suggests that it would belong to group 2. The multi-trait analysis showed a significant functional dissimilarity between these two groups, without any significant differences within a group (Table A). The single-trait analysis showed an overlap significantly lower than expected under random assumption (Table A), particularly with regard to height and biomass, which are significantly higher in group 1 (Figure ). Although SLA provided a less clear among-group distinction, species constituting group 1 showed lower values of this trait (Figure ). By contrast to the crop edge, only a small amount of trait divergence among species was observed in the field margin, except for V. hederifolia, a creeping species that differed from all other species by its height (Table B). Finally, as previously illustrated in the principal component analysis graphs (Figure ), a similar pattern was observed in the field core and the field margin with no significant functional divergence among species. But, as several species had fewer than 10 individuals in this field element, overlap estimation and randomization testing could not be implemented for the field core.
Figure 2. Distribution of trait values per species and field element. The horizontal line on each beanplot is the mean value of the distribution. The graphical representation is based on a non-parametric Gaussian kernel density estimation. The canopy height and the specific leaf area were square-root transformed while the above-ground biomass was log10-transformed. CE, crop edge; FM, field margin; FC, field core. EPPO codes refer to abbreviations as presented in Table
Table 2. Interspecific comparisons of trait distribution within each field element. Overlap in functional trait values between weed species sampled within (A) the crop edge and (B) the field margin. Non-parametric Gaussian kernel density estimation was used to measure functional overlap for each trait (top). 1 – Fβ is a measure of functional overlap in the multi-trait space (bottom). Values in bold are significantly lower than under the randomization null model after applying sequential Bonferroni–Holm adjustment (P < 0.05). Abbreviations used to represent species refer to EPPO codes as follow: Galium aparine (GALAP), Geranium dissectum (GERDI), Papaver rhoeas (PAPRH), Fallopia convolvulus (POLCO), Veronica hederifolia (VERHE), Veronica persica (VERPE) and Viola arvensis (VIOAR).
Intraspecific trait variability
Different patterns of intraspecific variation were observed among the seven species. Intraspecific variation of F. convolvulus, V. hederifolia, V. persica and V. arvensis among field elements was mainly supported by a single functional trait, i.e. SLA, while for G. aparine, G. dissectum and P. rhoeas, intraspecific variation was important for both SLA and biomass (Figure ). At the intraspecific level, the multi-trait functional overlap measured by 1 – Fβ was always close to 0, indicating a significant difference in the multidimensional functional space between individuals sampled in adjacent field elements of the same field (Table ). Although not detected on all pairs of field elements and for all species, the importance of intraspecific variability was also broadly highlighted by the single-trait approach. The intraspecific divergence was most frequently significant for the biomass and height between the crop edge and the field margin. Within all species, plants growing in the crop edge had a higher biomass than in the field margin. Except for V. persica (a creeping species), plants growing in the crop edge were also significantly taller than their conspecifics from the field. The SLA of individuals growing in the crop edge was significantly lower than in the field margin, except for two species, G. aparine and G. dissectum. These two species are however characterized by lower SLA values in crop edge than in field margin (see Supplementary Figure 2 for details). Less consistent patterns were observed when comparing the field core with the other two elements. SLA was however generally higher for individuals sampled in the field core. Among the species studied, some are known to be more abundant in the crop edge (G. aparine, F. convolvulus and V. persica), others are known to be more abundant in the field core (V. arvensis), whereas G. dissectum and P. rhoeas are represented in all field elements (Fried et al. Citation2007). The general trends observed (Table ) do not suggest that patterns of intraspecific variability are associated with the ecological preferences of the species: intraspecific variability was not higher nor lower in the preferred habitat of each species.
Table 3. Intraspecific comparisons of trait distribution between field elements. Overlap in functional trait values between plants sampled from different field elements within each weed species. Non-parametric Gaussian kernel density estimation was used to measure functional overlap for each trait (top). 1 – Fβ is a measure of functional overlap in a multi-trait space (bottom). Values in bold are significantly lower than under the randomization null model assumption after applying sequential Bonferroni–Holm adjustment (p < 0.05). CE, crop edge; FM, field margin; FC, field core; NA, data not available due to too small sample sizes. EPPO codes refer to abbreviations as presented in Table .
Discussion
In this study we demonstrate the existence of different functional strategies of weeds among field elements based on in situ individual measurement of three key functional traits. In the field margin and the field core, we observed higher values of SLA, lower values of above-ground biomass and, although less clearly, smaller canopy height than in the crop edge. SLA is generally higher under canopy cover (Brainard, Bellinder, and DiTommaso Citation2005; Storkey Citation2005), which, in association with a smaller plant size, defines a shade-tolerance syndrome (Storkey Citation2005; Valladares and Niinemets Citation2008). This suggests that the main factor limiting the growth of individuals within the field is the lower light availability under crop canopy (Holt Citation1995). A high SLA is also known to be correlated with a high relative growth rate, an important ability in frequently disturbed environments (Gaba et al. in press). The relatively low canopy height and above-ground biomass observed within fields could also be associated with a predominance of late emerging plants that escaped important abiotic filters, such as herbicide applications or late freezing, but had a reduced growth and biomass accumulation because of an intense competition for light with the crop late in the season (Baumann, Bastiaans, and Kropff Citation2001; Chauvel, Guillemin, and Letouze Citation2005; Torra and Recasens Citation2008). Hence, within the field, disturbances and competition for light could lead to a predominance of late emerging plants with a single fast-growing shade-tolerant strategy that are able to produce seeds under canopy cover.
In contrast to the field, in the crop edge – characterized by an absence of direct crop competition and less frequent disturbances – two different functional strategies seem to coexist. The first strategy (previously defined as group 1) is characterized by a stem elongation and larger investment into supporting tissues but partially lower SLA, which can be regarded as a shade-avoidance syndrome (Franklin and Whitelam Citation2005). The second strategy (previously defined as group 2) is characterized by higher SLA values but smaller plants, which is more consistent with shade tolerance (Storkey Citation2005; Valladares and Niinemets Citation2008). The coexistence of these two light acquisition strategies could result from the predominance of weed–weed competition and a partial reduction of trait similarity among species. Studying larger sets of fields in diverse localizations will be necessary to validate these trends and a better characterization of the ecological filters at play, especially the ‘weed–crop’ and ‘weed–weed’ competitive interactions and disturbances, should be performed to fully understand how weeds respond to environmental conditions.
One salient result of our study is the substantial amount of intraspecific variation of weeds among field elements. Significant changes in trait values in response to varying environmental conditions were observed for all species. Furthermore, the large intraspecific variation observed in some species fully accounts for the presence of additional functional syndromes in the crop edge as compared to the field. Intraspecific trait variation may have two sources, i.e. genetic variability or phenotypic plasticity. Our study scheme does not allow distinguishing among the possible sources of intraspecific variation; however, we think that phenotypic plasticity is more likely. First, a high phenotypic plasticity in response to competition and light environment was previously described in weeds able to grow in a wide array of different habitats (e.g. Griffith and Sultan Citation2012). Second, as the spatial scales considered here are very small (i.e. about 1 m between the crop edge and the field margin, up to 20 m compared with the field core), genetic differentiation among field elements is unlikely. Moreover, most weeds are typically considered barochorous (Benvenuti Citation2007), constraining dispersal to a few metres away from the mother plant. However, seeds can travel much farther via secondary dispersal by humans (i.e. anthropochory), especially by soil-working machinery, reducing the likelihood of genetic differentiation. At the field scale, both the biotic environment and the management intensity differ between the field elements, which may select for genotypes adapted to the field elements’ environmental conditions. This would result in fine-scale genetic differentiation, as observed previously in some weeds (Hettwer and Gerowitt Citation2004). However, the three adjacent field elements considered in this study are all tilled, which probably results in a genetic homogenization of the seed bank over time due to secondary seed dispersal. Hence, the intraspecific variability observed in this study would be mostly due to phenotypic plasticity but further studies would be necessary to characterize the extent and role of plasticity and dispersion in the weed species studied here.
From a practical point of view, the occurrence of large intraspecific variation implies that the use of fixed mean field approach based on average trait values for analysing the functional diversity of weed communities is likely to be misleading. Whereas such an approach could be relevant when comparing the trait distribution over large environmental gradients (e.g. across a country), it should be avoided when analysing species response to environmental factors that vary at a finer scale, especially for plastic traits such as SLA and canopy height. In situ measurement of individual traits seems necessary to better understand how weed trait distribution is structured across various habitats within agroecosystems.
Using a functional approach, our study confirms that crop edges are favourable habitats for the development of many weeds. Several development strategies unexpressed in the cultivated field itself are observed within crop edges. In addition, the higher biomass production, regardless of the species, suggest a higher potential for the replenishment of soil seed banks within crop edges as compared to fields (José-María and Sans Citation2011). This is in agreement with previous results from Kohler et al. (Citation2011) showing that, for threatened arable weeds, seed production is maximized in the presence of ploughing but without crop competition, two conditions that characterize the crop edge area.
Supporting Information
Download MS Word (169.6 KB)Acknowledgements
This work was funded by a grant from INRA, Research Division “Plant Health and the Environment”. The authors thank Audrey Alignier, Rémy Destrebecq, Camille Drouhin, Gilles Louviot and Quentin Martinez for their assistance in the field.
References
- Albert, C. H., F. Grassein, F. M. Schurr, G. Vieilledent, and C. Violle. 2011. “When and How Should Intraspecific Variability Be Considered in Trait-Based Plant Ecology?” Perspectives in Plant Ecology, Evolution and Systematics 13 (3): 217–225.
- Andreasen, C., H. Stryhn, and J. C. Streibig. 1996. “Decline of the Flora in Danish Arable Fields.” Journal of Applied Ecology 33: 619–626.
- Baessler, C., and S. Klotz. 2006. “Effects of Changes in Agricultural Land Use on Landscape Structure and Arable Weed Vegetation over the Last 50 Years.” Agriculture Ecosystems & Environment 115 (1–4): 43–50.
- Baumann, D. T., L. Bastiaans, and M. J. Kropff. 2001. “Effects of Intercropping on Growth and Reproductive Capacity of Late-Emerging Senecio Vulgaris L., with Special Reference to Competition for Light.” Annals of Botany 87: 209–217.
- Benvenuti, S. 2007. “Weed Seed Movement and Dispersal Strategies in the Agricultural Environment.” Weed Biology and Management 7 (3): 141–157.
- Bernhardt-Römermann, M., C. Römermann, R. Nuske, A. Parth, S. Klotz, W. Schmidt, and J. Stadler. 2008. “On the Identification of the Most Suitable Traits for Plant Functional Trait Analyses.” Oikos 117: 1533–1541.
- Bolnick, D. I., P. Amarasekare, M. S. Araùjo, R. Bürger, J. M. Levine, M. Novack, V. H. W. Rudolf, S. J. Schreiber, M. C. Urban, and D. A. Vasseur. 2011. “Why Intraspecific Trait Variation Matters in Community Ecology.” Trends in Ecology and Evolution 26 (4): 183–192.
- Brainard, D. C., R. R. Bellinder, and A. DiTommaso. 2005. “Effects of Canopy Shade on the Morphology, Phenology, and Seed Characteristics of Powell Amaranth (Amaranthus Powellii).” Weed Science 53 (2): 175–186.
- Chauvel, B., J. P. Guillemin, and A. Letouze. 2005. “Effect of Intra-Specific Competition on Development and Growth of Alopecurus Myosuroides Hudson.” European Journal of Agronomy 22 (3): 301–308.
- Cordeau, S., S. Petit, X. Reboud, and B. Chauvel. 2012. “Sown Grass Strips Harbour High Weed Diversity but Decrease Weed Richness in Adjacent Crops.” Weed Research 52 (1): 88–97.
- Cornwell, W. K., and D. D. Ackerly. 2009. “Community Assembly and Shifts in Plant Trait Distributions across an Environmental Gradient in Coastal California.” Ecological Monographs 79 (1): 109–126.
- Franklin, K. A., and G. C. Whitelam. 2005. “Phytochromes and Shade-Avoidance Responses in Plants.” Annals of Botany 96 (2): 169–175.
- Fried, G., C. Girod, M. Jacquot, and F. Dessaint. 2007. “Répartition de la Flore Adventice à l’Echelle d’un Paysage Agricole: Analyse de la Diversité des Pleins Champs et des Bordures.” Paper presented at AFPP – Vingtième Conférence du COLUMA, Dijon, December 11–12.
- Fried, G., E. Kazakou, and S. Gaba. 2012. “Trajectories of Weed Communities Explained by Traits Associated with species’ Response to Management Practices.” Agriculture, Ecosystems & Environment 158: 147–155.
- Fried, G., S. Petit, F. Dessaint, and X. Reboud. 2009. “Arable Weed Decline in Northern France: Crop Edges as Refugia for Weed Conservation?” Biological Conservation 142 (1): 238–243.
- Gaba, S., G. Fried, E. Kazakou, B. Chauvel, and M. L. Navas. forthcoming. Agroecological Weed Control Using a Functional Approach. a Review of Cropping Systems Diversity. Agronomy for Sustainable Development.
- Geange, S. W., S. Pledger, K. Burns, and J. S. Shima. 2011. “A Unified Analysis of Niche Overlap Incorporating Data of Different Types.” Methods in Ecology and Evolution 2 (2): 175–184.
- Griffith, T., and S. E. Sultan. 2012. “Field-Based Insights to the Evolution of Specialization: Plasticity and Fitness across Habitats in a Specialist/Generalist Species Pair.” Ecology and Evolution 2 (4): 778–791.
- Gunton, R. M., S. Petit, and S. Gaba. 2011. “Functional Traits Relating Arable Weed Communities to Crop Characteristics.” Journal of Vegetation Science 22 (3): 541–550.
- Hettwer, U., and B. Gerowitt. 2004. “An Investigation of Genetic Variation in Cirsium Arvense Field Patches.” Weed Research 44 (4): 289–297.
- Holm, S. 1979. “A Simple Sequentially Rejective Multiple Test Procedure.” Scandinavian Journal of Statistics 6: 65–70.
- Holt, J. S. 1995. “Plant Responses to Light: A Potential Tool for Weed Management.” Weed Science 43: 474–482.
- José-María, L., and F. X. Sans. 2011. “Weed Seedbanks in Arable Fields: Effects of Management Practices and Surrounding Landscape.” Weed Research 51 (6): 631–640.
- Jung, V., C. Violle, C. Mondy, L. Hoffmann, and S. Muller. 2010. “Intraspecific Variability and Trait-Based Community Assembly.” Journal of Ecology 98 (5): 1134–1140.
- Kleijn, D., and A. C. van der Voort. 1997. “Conservation Headlands for Rare Arable Weeds: The Effects of Fertilizer Application and Light Penetration on Plant Growth.” Biological Conservation 81: 57–67.
- Kohler, F., C. Vandenberghe, R. Imstepf, and F. Gillet. 2011. “Restoration of Threatened Arable Weed Communities in Abandoned Mountainous Crop Fields.” Restoration Ecology 19: 62–69.
- Kovács-Hostyánski, A., P. Batáry, A. Báldi, and A. Harnos. 2011. “Interaction of Local and Landscape Features in the Conservation of Hungarian Arable Weed Diversity.” Applied Vegetation Science 14 (1): 40–48.
- Lutman, P. J. W. 2002. “Estimation of Seed Production by Stellaria Media, Sinapis Arvensis and Tripleurospermum Inodorum in Arable Crops.” Weed Research 42 (5): 359–369.
- MacArthur, R. H., and R. Levins. 1967. “The Limiting Similarity Convergence and Divergence of Coexisting Species.” American Naturalist 101: 377–385.
- Marshall, E. J. P. 1989. “Distribution Patterns of Plants Associated with Arable Field Edges.” Journal of Applied Ecology 26: 247–257.
- Marshall, E. J. P., and G. M. Arnold. 1995. “Factors Affecting Field Weed and Field Margin Flora on a Farm in Essex, UK.” Landscape and Urban Planning 31: 205–216.
- Marshall, E. J. P., V. K. Brown, N. D. Boatman, P. J. W. Lutman, G. R. Squire, and L. K. Ward. 2003. “The Role of Weeds in Supporting Biological Diversity within Crop Fields.” Weed Research 43 (2): 77–89.
- Navas, M.-L. 2012. “Trait-Based Approaches to Unravelling the Assembly of Weed Communities and Their Impact on Agro-Ecosystem Functioning.” Weed Research 52 (6): 479–488.
- Development Core Team, R. 2012. R: a Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing.
- Reich, P. B., M. B. Walters, and D. S. Ellsworth. 1997. “From Tropics to Tundra: Global Convergence in Plant Functioning.” Proceedings of the National Academy of Sciences of the United States of America 94: 13730–13734.
- Robinson, R. A., and W. J. Sutherland. 2002. “Post-War Changes in Arable Farming and Biodiversity in Great Britain.” Journal of Applied Ecology 39 (1): 157–176.
- Romero, A., L. Chamorro, and F. X. Sans. 2008. “Weed Diversity in Crop Edges and Inner Fields of Organic and Conventional Dryland Winter Cereal Crops in NE Spain.” Agriculture, Ecosystems and Environment 124 (1–2): 97–104.
- Sheather, S. J., and M. C. Jones. 1991. “A Reliable Data-Based Bandwidth Selection Method for Kernel Density Estimation.” Journal of the Royal Statistical Society: Series B 53: 683–690.
- Storkey, J. 2005. “Modelling Assimilation Rates of 14 Temperate Arable Weed Species as a Function of the Environment and Leaf Traits.” Weed Research 45 (5): 361–370.
- Storkey, J. 2006. “A Functional Group Approach to the Management of UK Arable Weeds to Support Biological Diversity.” Weed Research 46 (6): 513–522.
- Storkey, J., S. Meyer, K. S. Still, and C. Leuschner. 2012. “The Impact of Agricultural Intensification and Land-Use Change on the European Arable Flora.” Proceedings of the Royal Society B 279: 1421–1429.
- Storkey, J., S. R. Moss, and J. W. Cussans. 2010. “Using Assembly Theory to Explain Changes in a Weed Flora in Response to Agricultural Intensification.” Weed Science 58 (1): 39–46.
- Sutcliffe, O. L., and Q. O. N. Kay. 2000. “Changes in the Arable Flora of Central Southern England since the 1960s.” Biological Conservation 93 (1): 1–8.
- Torra, J., and J. Recasens. 2008. “Demography of Corn Poppy (Papaver Rhoeas) in Relation to Emergence Time and Crop Competition.” Weed Science 56 (6): 826–833.
- Valladares, F., and U. Niinemets. 2008. “Shade Tolerance, a Key Plant Feature of Complex Nature and Consequences.” Annual Review of Ecology, Evolution, and Systematics 39: 237–257.
- Villéger, S., P. M. Novack-Gottshall, and D. Mouillot. 2011. “The Multidimensionality of the Niche Reveals Functional Diversity Changes in Benthic Marine Biotas across Geological Time.” Ecology Letters 14 (6): 561–568.
- Weiner, J., S. B. Andersen, W. K.-M. Wille, H. W. Griepentrog, and J. N. Olsen. 2010. “Evolutionary Agroecology: the Potential for Cooperative, High Density, Weed-Suppressing Cereals.” Evolutionary Applications 3 (5–6): 473–479.
