Abstract
The pollen morphology and exine structure of 52 specimens of the 14 species (15 taxa) of the genus Achillea L. section Babounya (DC.) O. Hoffm. (Asteraceae) distributed in Turkey were examined with light and scanning electron (SEM) microscopes. The pollen characteristics of 13 species (except for Achillea tenuifolia Lam.) are newly reported here. The pollen grains were oblate-spheroidal, prolate-spheroidal, subprolate and generally tricolporate, but sometimes tetracolporate, and even pentacolporate. The size of grains varied, ranged from 21.20 to 45 μm on the polar axis mean, and from 18.10 to 43.14 μm on the equatorial axis mean. The structure of the exine was doubletectate (Anthemoid pattern) and mean of exine thickness varied from 3.61 to 8.16 μm. The sculpture was echinate both in light and SEM micrographs. Moreover, rugulate, microperforate and rugulate-microperforate ornamentations were observed in SEM. The results indicate that the examined species showed heterogeneity in pollen characteristics, both at an interspecies level and between specimens of a species collected from different localities. In addition, correlation between pollen size and chromosome number was discussed.
Introduction
The pollen morphology of Turkish Achillea L. (Asteraceae) is currently being studied to clarify its taxonomy and make contributions to other multidisciplinary researches on the genus. Within this scope, 14 (15 taxa) of 48 (54 taxa) Turkish Achillea species, nine of which are endemic, were investigated with light (LM) and scanning electron (SEM) microscopes in this study (Arabacı Citation2012).
The aerial parts of different species of the genus Achillea are used in Turkish folk medicine (Baytop Citation1999). Essential oil composition and antimicrobial activity of the some Achillea species growing in Turkey were described in previous studies (İşcan et al. Citation2006; Kucukbay, Kuyumcu, and Arabacı Citation2010; Kucukbay et al. Citation2011).
The pollen morphology of some Achillea species has been previously studied (Wodehouse Citation1935; Erdtman Citation1943; Yang and Ai Citation2002; Meo and Khan Citation2003; Punt and Hoen Citation2009). Six species of the Achillea section Achillea, which have ivory-white to golden-yellow ligules, were previously examined by the authors as an initial study of pollen morphology of Turkish Achillea species (Akyalçın, Arabacı, and Yıldız Citation2011). According to this study, the examined species were heterogeneous, the aperture forms generally tricolporate, the pollen wall structures Anthemoid type, and the pollen ornamentations were found to be echinate in LM, and echinate-microperforate and echinate-rugulate-microperforate in SEM analyses.
As the genus Achillea is a model for remarkable eco-geographical radiation spreading across southeastern Europe and southwestern Asia with extensions through Eurasia to North America, and demonstrated numerous cases of polyploidy (2x-4x-6x-8x), transition zones between species, hybridization and excessive polymorphism, more specimens (52) from different localities were chosen to determine the palynological features of these species (Ehrendorfer Citation1959; Vetter et al. Citation1996a, 1996b; Saukel et al. Citation2004; Guo, Ehrendorfer, and Samuel Citation2004; Guo et al. Citation2005; Ehrendorfer and Guo Citation2006; Sahin et al. Citation2006; Kıran et al. Citation2008, 2012).
The examined species are the yellow and two to eight liguled members of the traditional section of Achillea: section Santolinoidea DC., which were incorporated into sect. Babounya (DC.) O.Hoffm by Ehrendorfer and Guo (Citation2005). This section differs morphologically from other sections by one to two pinnatisect, filiform or linear leaves that have minute, transverse, imbricate or somewhat distant leaf segments (Huber-Morath Citation1975). The pollen morphology of A. santolinoides Lag. subsp. wilhelmsii (K. Koch.) Greuter (2n = 18), A. falcata L. (2n = 18), A. cucullata (Hausskn.) Bornm. (2n = 36), A. vermicularis Trin. (2n = 18), A. monocephala Boiss. & Balansa (2n = 18), A. schischkinii Sosn., A. lycaonica Boiss. & Heldr. (2n = 18), A. magnifica Hub.-Mor. (2n = 18), A. tenuifolia Lam. (2n = 36), A. phrygia Boiss. & Balansa, A. gypsicola Hub.-Mor. (2n = 54), A. boissieri (Hausskn.) Boiss. (2n = 18), A. aleppica DC., subsp. aleppica, A. aleppica subsp. zederbaueri (Hayek) Hub.-Mor. and A. pseudoaleppica Hub.-Mor. (2n = 18) are examined by using LM and SEM. In addition, taxonomic significance of these features is discussed (chromosome count references: Khaniki Citation1995; Sahin et al. Citation2006; Kıran et al. Citation2008, 2012).
Material and methods
Studies on pollen grains were made on the materials that were collected during field studies conducted in Turkey between 2002 and 2008. The specimens were identified by using basically the existing Floras and published papers (Boissier Citation1875; Post Citation1933; Huber-Morath Citation1975; Richardson Citation1976; Duman Citation2000; Ehrendorfer and Guo Citation2005, Citation2006; Arabacı and Yıldız Citation2006a, Citation2006b; Çelik and Akpulat Citation2008; Arabacı and Budak Citation2009). Voucher specimens are deposited in the Herbarium of İnönü University (INU) in Malatya, Turkey, and the examined taxa, localities and collectors are listed in Table .
Table 1. The examined taxa, localities and collectors.
The pollen grains for examination by LM and SEM were prepared according to the method described by Erdtman (Citation1960). Measurements and light micrographs were taken with a Leica DM 2500 microscope and DFC 280 camera. Approximately 50 pollen grains were examined for each specimen. The spine lengths were excluded in measurements. The polar axis (P), equatorial axis (E), colpus length (Clg), mesocolpium thickness (Meso), Amb, distances between colpus apices (t), exine, ectexine and endexine thicknesses, number of apertures and length of the spines were measured on the pollen grains. The shape classifications based on P/E ratios were given following the method of Erdtman (Citation1969). For SEM, pollen grains were directly mounted on stubs using double-sided adhesive tape, coated with gold and examined using a Jeol-JSM-6335F SEM. Spine lengths, spine base diameters and spine perforation diameters were measured by Image J program on SEM micrographs. The SPSS statistical program (ver. 19.0) was used to calculate the mean (M), standard deviation (SD) and variation (V) of LM measurements. The pollen terminology follows mainly Erdtman (Citation1943, 1960, 1969), Faegri and Iversen (Citation1992), Skvarla and Turner (Citation1966, 1971) and Punt et al. (Citation2007). The pollen slides were deposited in the Palynology Laboratory of Çanakkale Onsekiz Mart University, in Turkey.
Results
The main palynological features of the species examined in this study are summarized in Tables – and shown in Figures –.
Table 2. Pollen shape and morphological data (mean values, standard deviations and variations) of Achillea species in light microscopy analyses.
Figure 1. Light micrographs of pollen grains (×1000) Achillea santolinoides subsp. wilhelmsii (A, B) (from Yıldız 15238), (C, D) (from Arabacı 2231), (E–G) (from Arabacı 1452), (H, I) (from 1374), (J, K) (from Arabacı 1405), (L, M) (from Arabacı 1413); Achillea falcata (N, O) (from Arabacı 1566), (P, Q) (from Arabacı 1580), (R, S) (from Arabacı 2558); Achillea cucullata T (from Arabacı 1949). Scale bars 20 μm.
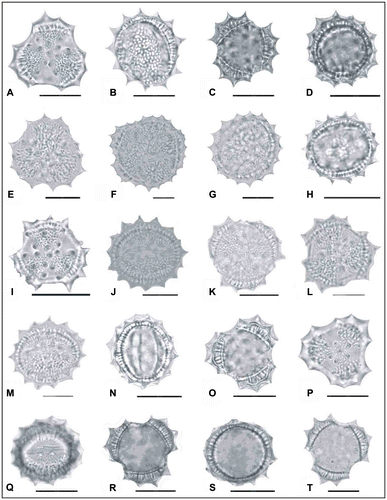
Figure 2. Light micrographs of pollen grains (×1000) Achillea cucullata (A) (from Arabacı 1949), (B, C) (from Arabacı 1763), (D, E) (from Arabacı 1884), (F, G) (from Yıldız 16832); Achillea vermicularis (H, I) (from Arabacı 1626), (J, K) (from Arabacı 1415), (L, M) (from Yıldız 15196), (N, O) (from Arabacı 2559), (P, Q) (from Yıldız 16943); Achillea monocephala (R, S) (from Arabacı 2184); Achillea schischkinii (T) (from Arabacı 1457). Scale bars 20 μm.
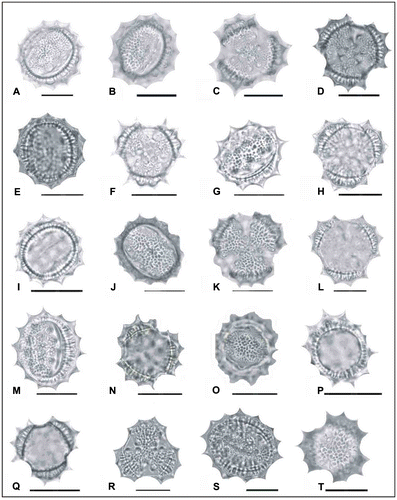
Figure 3. Light micrographs of pollen grains (×1000) Achillea schischkinii (A) (from Arabacı 1457), (B, C) (from Arabacı 1749), (D, E) (from Arabacı 1469), (F, G) (from Arabacı 2567); Achillea lycaonica (H–J) (from Arabacı 1532), (K, L) (from Arabacı 2061), (M, N) (from Arabacı 1581), (O, P) (from Arabacı 2214), (Q, R) (from Arabacı 2220); Achillea magnifica (S, T) (from Arabacı 1445). Scale bars 20 μm.
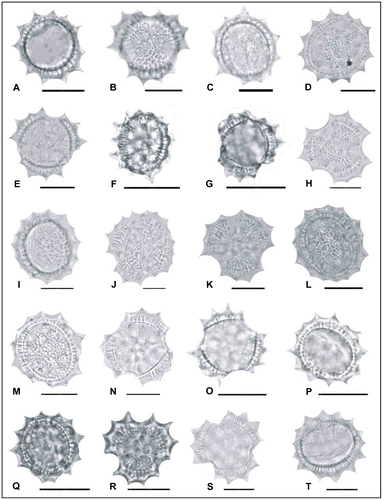
Figure 4. Light micrographs of pollen grains (×1000) Achillea magnifica (A, B) (from Arabacı 1465), (C, D) (from Arabacı 1497); Achillea tenuifolia (E, F) (from Arabacı 2582), (G, H) (from Arabacı 1619), (I, J) (from Arabacı 1618); Achillea phrygia (K, L) (from Yıldız 15258), (M, N) (from Arabacı 1530), (O, P) (from Arabacı 1480), (Q, R) (from Arabacı 2236); Achillea gypsicola (S, T) (from Arabacı 1560). Scale bars 20 μm.
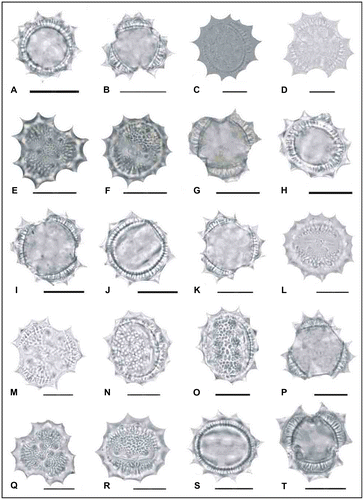
Figure 5. Light micrographs of pollen grains (×1000) Achillea gypsicola (A, B) (from Arabacı 2221), (C, D) (from Arabacı 2224), (E, F) (from Arabacı 2208); Achillea boissieri (G, H) (from Arabacı 1963); Achillea aleppica subsp. aleppica (I, J) (from Arabacı 1383), (K, L) (from Yıldız 1513), (M, N) (from Arabacı 1388), (O, P) (from Arabacı 2174); Achillea aleppica subsp. zederbaueri (Q, R) (from Arabacı 1594); Achillea pseudoaleppica (S, T) (from Arabacı 1441). Scale bars 20 μm.
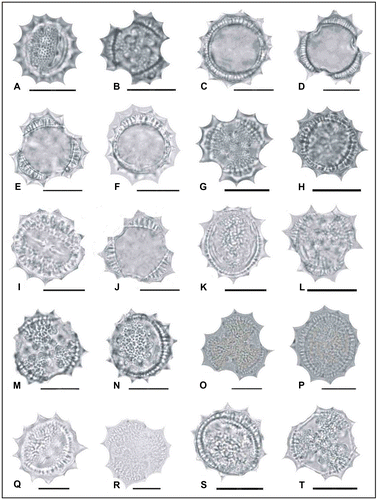
Figure 6. Light micrographs of pollen grains (×1000) Achillea pseudoaleppica (A, B) (from Arabacı 1446), (C, D) (from Arabacı 1389), (E, F) (from Arabacı 2244). Scale bars 20 μm.
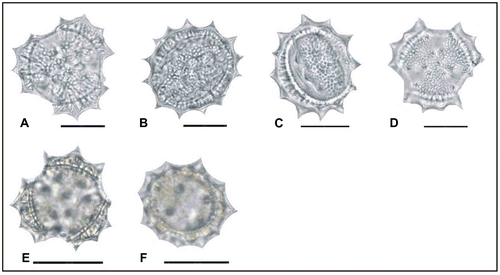
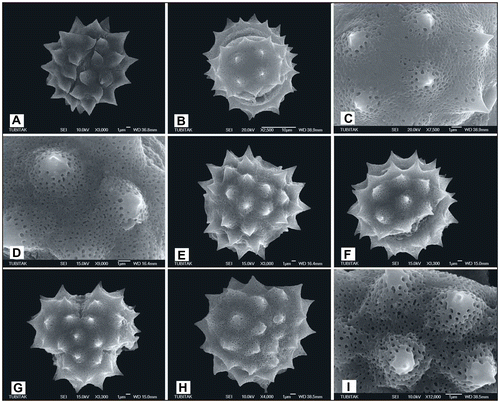
Figure 7. Scanning electron micrographs of pollen grains Achillea santolinoides subsp. wilhelmsii (A, B) (from Yıldız 15238), (C, D) (from Arabacı 2231), (E–G) (from Arabacı 1452), (H) (from Arabacı 1405), (I–K) (from Arabacı 1413); Achillea falcata (L) (from Arabacı 1566), (M) (from Arabacı 2258), (N) (from Arabacı 1580); Achillea cucullata (O) (from Arabacı 1949).
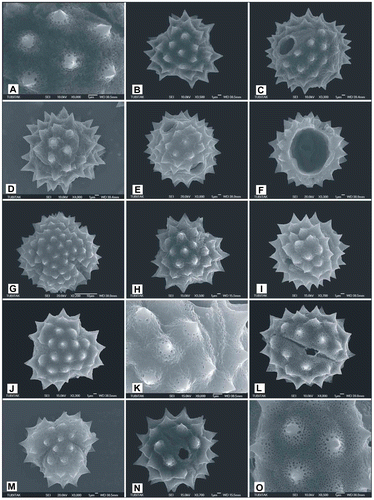
Figure 8. Scanning electron micrographs of pollen grains Achillea cucullata (A) (from Arabacı 1949), (B, C) (from Arabacı 1763), (D) (fractured grain from Arabacı 1763) (1– double tectum columellae of uniform length, 2– branching columellae, 3–basal columellae extending between double tectum and foot layer, 4– foot layer and endexine), (E–G) (from Arabacı 1884), (H, I) (from Yıldız 16832); Achillea vermicularis (J) (from Arabacı 1626), (K, L) (from Arabacı 1415), (M, N) (from Yıldız 15196), (O) (from Arabacı 2559).
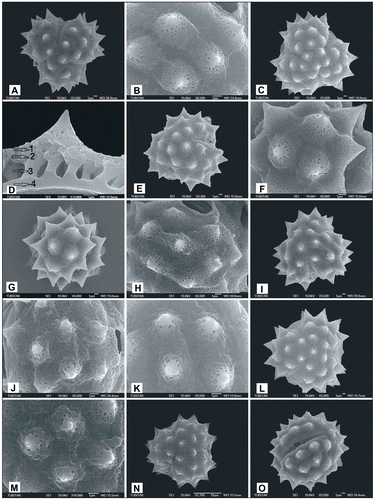
Figure 9. Scanning electron micrographs of pollen grains Achillea vermicularis (A) (from Arabacı 2559), (B, C) (from Yıldız 16943); Achillea monocephala (D) (from Arabacı 2184); Achillea schischkinii (E) (from Arabacı 1457), (F–H) (from Arabacı 1749), (I, J) (from Arabacı 2567); Achillea lycaonica (K) (from Arabacı 1532), (L, M) (from Arabacı 2061), (N, O) (from Arabacı 1581).
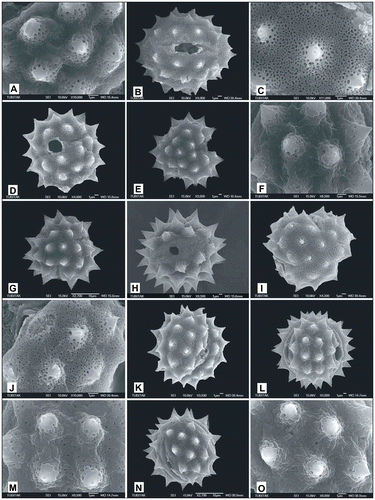
Figure 10. Scanning electron micrographs of pollen grains Achillea lycaonica (A) (from Arabacı 2214), (B, C) (from Arabacı 2220); Achillea magnifica (D–G) (from Arabacı 1445), (H, I) (from Arabacı 1465), (J, K) (from Arabacı 1497); Achillea tenuifolia (L, M) (from Arabacı 2582), (N, O) (from Arabacı 1618).
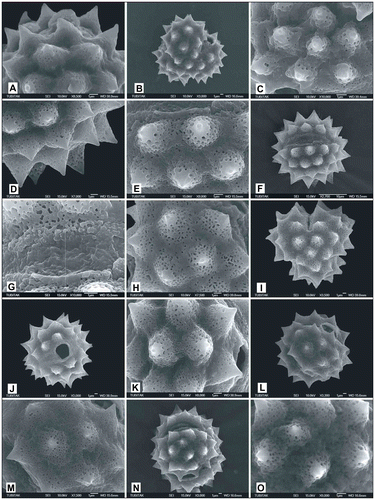
Figure 11. Scanning electron micrographs of pollen grains Achillea phrygia (A) (from Yıldız 15258), (B, C) (from Arabacı 1480), (D, E) (from Arabacı 2236); Achillea gypsicola (F) (from Arabacı 1560), (G) (from Arabacı 2208), (H, I) (from Arabacı 2224); Achillea boissieri (J) (from Arabacı 1963); Achillea aleppica subsp. aleppica (K) (from Arabacı 1383), (L) (from Arabacı 1513), (M, N) (from Arabacı 1388) (O) (from Arabacı 2174).
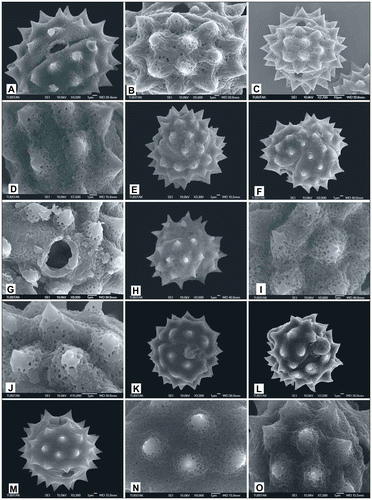
Figure 12. Scanning electron micrographs of pollen grains Achillea aleppica subsp. zederbaueri (A) (from Arabacı 1594); Achillea pseudoaleppica (B, C) (from Arabacı 1441), (D, E) (from Arabacı 1446), (F, G) (from Arabacı 1389), (H, I) (from Arabacı 2244).
Symmetry and shape
The pollen morphology of the 52 specimens from the 14 species (15 taxa) of the genus Achillea section Babounya (DC.) O. Hoffm. are radially symmetrical and isopolar. Pollen grains can be small, medium or large in size (Hesse et al. Citation2009). But the grains are slightly small/large in some of the species examined. The pollen grains are oblate-spheroidal, prolate-spheroidal and subprolate. The mean ratio of P/E is between 0.89 and 1.21. The polar axis mean ranged from 21.20 to 45 μm, and the variations were between 18 and 55 μm. The equatorial axis mean ranged from 18.10 to 43.14 μm, and the variations ranged from 16 to 50 μm. The maximum, minimum and average sizes of polar axis (P), equatorial axis (E) and ratio of polar axis to equatorial axis (P/E) are given (Table ). Amb shape is intersemi-angular. The shape is oval, compressed oval or circular in the meridional section and trilobulate (Figures –), or sometimes tetralobulate (Figures , ) in the polar optical section.
Apertures
The pollen grains are generally tricolporate in examined specimens. Some specimens of A. santolinoides subsp. wilhelmsii (1452, 1405), A. cucullata (1949) and A. tenuifolia (1949), have both tricolporate and tetracolporate grains; moreover, A. santolinoides subsp. wilhelmsii (1452) also displays pentacolporate grains (Table ) (Figures –).
Table 3. Pollen morphological data (mean values, standard deviations, and variations) of Achillea species in light microscopy analyses.
The mean length of colpi varied from 14.22 to 33.32 μm and the variations were between 12 and 40 μm, acute at apices, and margins were distinct. The sculpture was distinct at the margins between spines (Figures ,M, –K). The mean distances between the colpus apices (t) varied from 5 to 11.31 μm with the variation between 4 and 15 μm (Table ).
The apocolpium was angular. The aperture membrane was scabrous (Figures , ,N, ,H, ,G, ). The endoaperture was lalongate or alongate. In SEM analyses, the operculum was observed on the pores in the specimens that were treated with alcohol.
Exine
The mean exine thickness ranged from 3.61 to 8.16 μm, and the variations were between 3 and 10 μm. The ectexine thickness varied between 2 and 9 μm, and the means ranged from 2.60 to 7.12 μm. The endexine is thin; mean length varies from 0.89 to 2 μm, and the minimum and maximum thickness varies between 0.50 and 2 μm (Table ) (Figures –).
The sculpture is echinate both in LM and SEM micrographs. Moreover, rugulate, microperforate and rugulate-microperforate ornamentations were observed in SEM (Figures –). Perforations were circular, elliptical-circular or amorphous between the spines and continued around the spine base. Eleven of the 52 specimens examined were determined as amorphous in LM micrographs (Table ) (Figures –).
Spine
The measurements of spines observed in SEM analyses are detailed in Table . The mean number of spines varied from 2.2 to 9 per 100 μm2. The mean of spine length ranged from 2.5 to 6.42 μm, and spine base varied from 2.72 to 9.98 μm in LM. The spine length is between 1.38–6.45 μm, perforation diameter is between 0.09 and 0.95 μm, base diameter was between 0.29 and 10.74 μm in the equatorial optic section, and 1.50–5.11 μm, 0.10–0.77 μm, 1.51–6.87 μm in the polar section, respectively (Figures –). The spines were acute and psilate at half to a quarter of the apex. Perforations were dense, sparse or very few, homogeneous or not, circular, elliptical, or amorphous between the spines. The perforation was continuous to spine apex or finished around the spine base and a few ornamentation rows were observed on spine psilate.
Table 4. Ornamentation and spine measurements of Achillea species in SEM analyses.
Discussion
The genus Achillea has 48 species (54 taxa) growing in Turkey, 14 (15 taxa) of which are the subject of this study.
Among the examined species, A. boissieri, A. aleppica and A. pseudoaleppica are closely related species and differ from others by elliptic to oblong-cylindrical involucres, twice as long as broad. These species belong to the Irano-Turanian element. The specimens were collected between 300 and 1250 m altitude. They display morphological differences, but we have not observed distinct differences in pollen characters in LM and SEM investigations. A. magnifica, A. tenuifolia and A. lycaonica are characterized by deeply furrowed stems. The specimens were collected between 750 and 1900 m altitudes. They also did not show distinct differences in pollen characters in LM and SEM investigations.
The examined specimens of A. falcata collected from three different localities show homogeneous pollen morphology, while A. santolinoides subsp. wilhelmsii, A. cucullata, A. vermicularis, A. schischkinii, A. lycaonica, A. magnifica, A. tenuifolia, A. phrygia, A. gypsicola, A. aleppica, subsp. aleppica and A. pseudoaleppica specimens, collected from different localities, show heterogeneous pollen morphology. For the taxa A. monocephala, A. boissieri and A. aleppica subsp. zederbaueri no relevant data are available because the specimens were collected from a single locality (Table ). Similarly, heterogeneous pollen morphology was observed in six species of the Achillea sect. Achillea in previous studies (Akyalçın, Arabacı, and Yıldız Citation2011). Our results for pollen shape, exine structure and ornamentations are also in good agreement with this previous study.
The pollen aperture form of Achillea species was reported as tricolporate in several studies (Wodehouse Citation1935; Erdtman Citation1943; Skvarla and Turner Citation1966; Nilsson et al. Citation1977; Skvarla et al. Citation1977; Moore and Webb Citation1983; Moore, Webb, and Collinson Citation1991; Faegri and Iverson Citation1992; Yang and Ai Citation2002; Meo and Khan Citation2003; Jafari and Ghanbarian Citation2007; Akyalçın, Arabacı, and Yıldız Citation2011). The pollen grains are generally tricolporate in the examined specimens. However, the specimens of A. santolinoides subsp. wilhelmsii (1452, 1405), A. cucullata (1949) and A. tenuifolia (1949) have tricolporate and tetracolporate (even pentacolporate) pollen grains. The main cause of pollen heteromorphism and variation in pollen size-aperture type has been considered to be the different ploidy levels of the sporophyte (Erdtman Citation1969; Aytuğ Citation1967; İnceoğlu Citation1973, Borsch and Wilde Citation2000; Rivero-Guerra Citation2008). Indeed, A. cucullata and A. tenuifolia have 2n = 4x = 36 chromosome number, although this is not true for A. santolinoides subsp. wilhelmsii.
The exine structure is double tectate (Anthemoid pattern). The ectexine has thick or thin columellae that distinctly branched as “Y”-shaped at the apices and terminated with a layer that consists of uniform bacula (Figure ). According to Vezey et al. (Citation1994), the Anthemoid pollen type is generally characterized by a double tectum with vertical unbranching infratectal columellae joined at proximal and distal rounded expansions to form an external and internal tectal layer.
The polar and equatorial axes of the pollen grains of genus Achillea have been reported as less than 35 μm in previous studies (Wodehouse Citation1935; Erdtman Citation1943; Skvarla and Turner Citation1966; Nilsson et al. Citation1977; Skvarla et al. Citation1977; Moore and Webb Citation1983; Moore, Webb, and Collinson Citation1991; Faegri and Iverson Citation1992; Yang and Ai Citation2002; Meo and Khan Citation2003; Jafari and Ghanbarian Citation2007). According to Hesse et al. (Citation2009), pollen grains can be small, medium or large in size. In this study, the variations of the polar axis ranged between 18 and 55 μm (mean 21.20 to 45 μm), and those of the equatorial axis were between 16 and 50 μm (mean 18.10 to 43.14 μm) (Table ). Similar measurements (mean of polar axis ranging from 17.6 to 57.5 μm and mean equatorial axis ranging from 19.7 to 55.2 μm) were determined in six species of Achillea L. section Achillea (Akyalçın, Arabacı, and Yıldız Citation2011). In addition, a significant positive correlation was observed between the equatorial axis and the polar view diameter (AMB) (Table ).
Table 5. Correlation between the Equatorial axis and the polar view diameter (AMB).
The specimens collected from different localities show wide variations at both at intra-species and inter-species levels in terms of pollen characteristics (Tables and ) (Figures –). Achillea vermicularis (16943) has the smallest pollen grains with mean 21.20 ± 1.06 μm and variation between 19 and 24 μm, while A. magnifica (1497), an endemic Turkish species, has the largest pollen grains with mean 45 ± 2.58 μm and variation between 40 and 51 μm for the polar axis. In equatorial axis, A. schischkinii (2567) has the smallest pollen grains with mean 18.10 ± 1.08 μm and variation between 16 and 20 μm, and A. magnifica (1497) has the largest pollen grains with mean 43.14 ± 3.00 μm and variation from 38 to 50 μm. Species within the genus Achillea have generally been reported as diploid (2n = 2x = 18), but polyploid taxa, often 4x, sometimes 6x, and even 8x have been reported (Ehrendorfer and Guo Citation2006; Sahin et al. Citation2006; Kıran et al. Citation2008, 2012). As for pollen heteromorphism and variation in pollen size–aperture type, the pollen grain size is often correlated with the ploidy level of the gamete (Ehrendorfer Citation1949; Brochman Citation1992). It was reported that the number of chromosomes increased with the size of pollen grains in the genus Thymus L. and Mentha L. (Martonfi Citation1997; Celenk et al. Citation2008). This correlation was not seen in the examined species. The largest pollen grains, on average, were observed in A. magnifica (1497), and the smallest pollen grains, on average, were in A. schischkinii (2567) and A. vermicularis (16943) (Table ). The chromosome numbers of these species were recorded as 2n = 2x = 18 in previous studies (Sahin et al. Citation2006; Kıran et al. Citation2012). In addition, the hexaploid species A. gypsicola (2n = 6x = 54) has polar axis mean varying from 24.72 to 32.56 μm, and an equatorial axis mean ranging from 22.42 to 33.84 μm.
Achillea pseudoaleppica (2n = 18) shows a wide variation in pollen size. The specimen labelled 2244 has 20–27 μm polar axis variation and 23–28 μm equatorial axis variation, while the specimen labelled 1446 has a polar axis variation between 37 and 45 μm and an equatorial axis variation ranging from 32 to 43 μm (Table ). These volume changes in the pollen grains could be produced either as a response to occupation of new habitats, or by variation in the humidity of the existing environment (Thanikaimoni Citation1986; Pardo Citation1990).
Meo and Khan (Citation2003) reported the polar axis mean as 23.7 μm with variation between 20 and 26.5 μm, and the equatorial axis mean as 22.3 μm, with variation 20–24 μm in A. santolina L., which is now considered a synonym of A. tenuifolia. In our study, similar measurements were observed in A. tenuifolia specimens with polar axis mean between 26.42 and 29.54 μm (variation 24–35 μm) and equatorial axis mean between 29.36 and 32.26 μm (variation 27–36 μm) (Table ).
This study established the pollen morphology and exine structure of 52 specimens of the 14 species (15 taxa) of the genus Achillea section Babounya (Asteraceae) distributed in Turkey. The pollen characteristics of 13 species (except for A. tenuifolia) are newly reported here. Eleven of the 15 taxa collected from different localities show heterogeneous pollen morphology, possibly because of different environmental conditions. These are valuable characteristics for further studies on the palynology and may help in the systematic analysis of the genus Achillea from Turkey.
Acknowledgements
The authors want to thank TUBİTAK (Project No: 104T291) for the financial support and TUBİTAK-MAM for SEM microphotographs. Also, we would like to thank Dr Tuncay Dirmenci and Dr. Ümit Budak for their help during field studies, and Orhan İpek and Cem Berk for their help with the SEM microphotographs.
References
- Akyalçın, H., T. Arabacı, and B. Yıldız. 2011. “Pollen Morphology of Six Achillea L. Sect. Achillea (Asteraceae) Species in Turkey.” Turkish Journal of Botany 35: 183–201.
- Arabacı, T. 2012. “Achillea L.” In Türkiye Bitkileri Listesi (Damarlı Bitkiler) [A Checklist of the Flora of Turkey (Vascular Plants)], edited by A. Güner, S. Aslan, T. Ekim, M. Vural and M.T. Babaç, 108–12. İstanbul: Nezahat Gökyiğit Botanik Bahçesi ve Flora Araştırmaları Derneği Yayını.
- Arabacı, T., and Ü. Budak. 2009. “Achillea hamzaoglui (Asteraceae), a new species from Turkey.” Annales Botanici Fennici 46: 459–463.
- Arabacı, T., and B. Yıldız. 2006a. “Achillea salicifolia Besser subsp. salicifolia (Asteraceae) in Turkey, with taxonomic remarks.” Turkish Journal of Botany 30: 171–174.
- Arabacı, T., and B. Yıldız. 2006b. “Rediscovery of Achillea boissieri Hausskn. ex Boiss. later 140 years.” Feddes Repertorium 117: 459–465.
- Aytuğ, B. 1967. Polen Morfolojisi ve Türkiye’nin Önemli Gymnospermleri Üzerinde Palinolojik Araştırmalar [Pollen Morphology and Palynological Studies on Major Gymnospermae from Turkey]. İstanbul: İstanbul Üniversitesi Orman Fakültesi Yayınları, Kutulmuş Matbaası.
- Baytop, T. 1999. Türkiye’de Bitkiler ile Tedvi Geçmişten Bugüne [Therapy with Medicinal Plants in Turkey-Past and Present]. 2nd ed. İstanbul: Nobel Tıp Basımevi.
- Boissier, E.P. 1875. Flora Orientalis. vol. 3. Geneva: H. Georg.
- Borsch, T., and V. Wilde. 2000. “Pollen variability within species, populations and individuals, with particular reference to Nelumbo.” In Pollen and spores: Morphology and Biology, edited by M.M. Harley, C.M. Morton and S. Balckmore, 285–289. Kew: Royal Botanic Garden.
- Brochman, C. 1992. “Pollen and seed morphology of Nordic Draba (Brassicaceae): Phylogenetic and ecological implications.” Nordic Journal of Botany 1: 657–673.
- Celenk, S., G. Tarımcılar, A. Bıcakcı, G. Kaynak, and H. Malyer. 2008. “A palynological study of the genus Mentha L. (Lamiaceae).” Botanical Journal of the Linnean Society 157: 141–154.
- Çelik, N., and H.A. Akpulat. 2008. “Achillea sivasica (Asteraceae: sect. Babounya (DC.) O. Hoffm.), a new species from Inner Anatolia, Turkey.” Kew Bulletin 63: 485–489.
- Duman, H.2000. Achillea L. In Flora of Turkey and the East Aegean Islands (supplement), eds A. Güner, N. Özhatay, T. Ekim, and K.H.C. Başer, vol. 11, 158–159. Edinburgh: Edinburgh University Press.
- Ehrendorfer, F. 1949. “Zur phylogenic der gattung Galium. I. Polyploidic und geographisch–ökologische Einheiten in der gruppe des Galium pumilum Murray (Sekt. Leptogalium Lange sensu Rouy) im österreichischen Alpennarum.” Oesterreichische Botanische Zeitschrift 96: 109–138.
- Ehrendorfer, F. 1959. “Differentiation–hybridization cycles and polyploidy in Achillea.” Cold Spring Harbor Symposia on Quantitative Biology 24: 141–152.
- Ehrendorfer, F., and Y.P. Guo. 2005. “Changes in the circumscription of the genus Achillea (Compositae-Anehemideae) and its subdivision.” Willdenowia 35: 1–6.
- Ehrendorfer, F., and Y.P. Guo. 2006. “Multidisciplinary studies on Achillea sensu lato (Compositae-Anthemideae): new data on systematics and phylogeography.” Willdenowia 36: 1–19.
- Erdtman, G.1943. An Introduction to Pollen Analysis. Waltham, Mass., Chronica Botanica.
- Erdtman, G. 1960. “The Acetolysis Method. A Revised Description.” Svensk Botanisk Tidskrift 54: 561–564.
- Erdtman, G. 1969. Handbook of Palynology. New York: Haffner Press.
- Faegri, K., and J. Iverson. 1992. Textbook of Pollen Analysis. New York: Haffner Press.
- Guo, Y.P., F. Ehrendorfer, and R. Samuel. 2004. “Phylogeny and systematics of Achillea (Asteraceae-Anthemideae) inferred from nrITS and plastid trnL-F DNA sequences.” Taxon 53: 657–672.
- Guo, Y.P., J. Saukel, R. Mittermayr, and F. Ehrendorfer. 2005. “AFLP analyses demonstrate genetic divergence, hybridization, and multiple polyploidization in the evolution of Achillea (Asteraceae-Anthemideae).” New Phytologist 166: 273–290.
- Hesse, M., H. Halbritter, H. Zetter, M. Weber, R. Buchner, A. Frosch-Radivo, and S. Ulrich. 2009. Pollen Terminology: An illustrated handbook. Wien, New York: Springer.
- Huber-Morath, A.1975. Achillea L. In Flora of Turkey and the East Aegean Islands, ed. P.H. Davis, vol. 5, 224–52. Edinburgh: Edinburgh University Press.
- İnceoğlu, Ö. 1973. “Asyneuma canescens (W.K.) Griseb ve Schenk’in polen morpholojisi ve heteromorf polenler [Heteromorphic pollens and Pollen morphology of Asyneuma canescens (W.K.) Griseb ve Schenk].” Türk Biyoloji Dergisi 23: 89–94.
- İşcan, G., N. Kırımer, M. Kürkçüoglu, T. Arabacı, E. Küpeli, and K.H.C. Başer. 2006. “Biological Activity and Composition of the Essential Oils of Achillea schischkinii Sosn. and Achillea aleppica DC. subsp. aleppica.” Journal of Agricultural and Food Chemistry 54: 170–173.
- Jafari, E., and G.H. Ghanbarian. 2007. “Pollen Morphological Studies on Selected Taxa of Asteraceae.” Journal of Plant Sciences 2: 195–201.
- Khaniki, G.B. 1995. “Chromosome numbers and morphometry in Achillea (Anthemideae, Compositae).” Nucleus 38: 104–111.
- Kıran, Y., T. Arabacı, A. Şahin, and I. Turkoglu. 2008. “Karyological notes on another eight species of Achillea (Asteraceae) from Turkey.” Biologia 63: 343–348.
- Kıran, Y., I. Turkoglu, F. Kirilmaz, T. Arabaci, A. Sahin, and E. Bagci. 2012. “Karyological investigation of six Achillea L. (Asteraceae) species growing in Turkey.” Caryologia 65: 101–105.
- Kucukbay, F.Z., E. Kuyumcu, and T. Arabacı. 2010. “The essential oil of Achillea boissieri.” Chemistry of Natural Compounds 46: 824–825.
- Kucukbay, F.Z., E. Kuyumcu, S. Gunal, and T. Arabacı. 2011. “Composition and antimicrobial activity of the essentıal oil of Achillea formosa subsp. amanica.” Chemistry of Natural Compounds 47: 300–302.
- Martonfi, P. 1997. “Pollen morphology of Thymus sect. Serpyllum (Labiatae: Mentheae) in the Carpathians and Pannonia.” Grana 36: 261–270.
- Meo, A.A., and M.A. Khan. 2003. “Pollen Morphology of Achillea (Compositae-Anthemoideae) Species from Pakistan.” Pakistan Journal of Weed Science Research 9: 253–258.
- Moore, P.D., and J.A. Webb. 1983. An Illustrated Guide to Pollen Analysis. London: Hodder and Stoughton.
- Moore, P.D., J.A. Webb, and M.E. Collinson. 1991. Pollen Analysis. London, Oxford: Blackwell Scientific Publications.
- Nilsson, S., J. Praglowski, L. Nilsson, and N.O. Kultur. 1977. Atlas of airborne pollen grains and spores in Northern Europe. Sweden: Ljungforetagen Orebro.
- Pardo, C.1990. “Tipos harmome’gatas en Gleditschia triacanthos (Papilionaceae, Caeselpinoideae).” In Pollen, Esporas Y Sus Aplicaciones, eds Blanca G., C. Díaz De La Guardia, M.C. Fernández, M. Garrido, Mi. Rodríguez-García, and Y.A.T. Romero, vol. 1, 211–16. Granada: Universidad de Granada.
- Post, G.E., ed. 1933. Flora of Syria, Palestina and Sina, vol. 2. Beirut: American Press.
- Punt, W., and P.P. Hoen. 2009. “The Northwest European Pollen Flora, 70: Asteraceae-Asteroideae.” Review of Palaeobotany and Palynology 157: 22–183.
- Punt, W., P.P. Hoen, S. Blackmore, S. Nilsson, and A. Le Thomas. 2007. “Glossary of pollen and spore terminology.” Review of Palaeobotany and Palynology 143: 1–81.
- Richardson, I.B.K.1976. Achillea L. In Flora Europaea, eds T.G. Tutin, V.H. Heywood, N.A. Burges, D.M. Moore, D.H.Valentine, S.M. Walters, and D.A. Webb, vol. 4, 159–65. Cambridge: Cambridge University Press.
- Rivero-Guerra, A.O. 2008. “Cytogenetics, geographical distribution, and pollen fertility of diploid and tetraploid cytotypes of Santolina pectinata Lag. (Asteraceae: Anthemideae).” Botanical Journal of the Linnean Society 156: 657–667.
- Sahin, A., Y. Kıran, T. Arabaci, and I. Turkoglu. 2006. “Karyological notes on eight species of Achillea L. (Asteraceae, Santolinoideae) from Turkey.” Botanical Journal of the Linnean Society 151: 573–580.
- Saukel, J., M. Anchev, Y-P. Guo, A. Vitkova, A. Nedelcheva, V. Goranova, A., Konakchievet al. 2004. “Comments on the biosystematics of Achillea (Asteraceae-Anthemideae) in Bulgaria.” Phytologia Balcanica 9: 361–400.
- Skvarla, J.J., and B.L. Turner. 1966. “Systematic implications from electron microscopic studies of Compositae pollen – A review.” Annals of the Missouri Botanical Garden 53: 220–256.
- Skvarla, J.J., and B.L. Turner. 1971. “Fine structure of the pollen of Anthemis nobilis L. (Anthemideae-Compositae).” Proceedings of the Oklahoma Academy of Science 51: 61–62.
- Skvarla, J.J., B.L. Turner, V.C. Patel, and A.S. Tomb. 1977. “Pollen morphology in the Compositae and in morphologically related families.” In The biology and chemistry of the Compositae, edited by V.H. Heywood, J.B. Harborne and B.L. Turner, 41–248. London: Academic Press.
- Thanikaimoni, G. 1986. “Pollen apertures: Form and function.” In Pollen and spores: Form and function, edited by S. Blackmore and I.K. Ferguson, 119–136. London: Academic Press.
- Vetter, S., M. Lambrou, C.H. Franz, and F. Ehrendorfer. 1996a. “Cytogenetics of experimental hybrids within the Achillea millefolium complex (yarrow).” Caryologia 49: 1–12.
- Vetter, S., M. Lambrou, C.H. Franz, F. Ehrendorfer, and J. Saukel. 1996b. “Chromosome numbers of experimental tetraploid hybrids and self-pollinated progenies within the Achillea millefolium complex (Compositae).” Caryologia 49: 227–231.
- Vezey, E.L., L.E. Watson, J.J. Skvarla, and J.R. Estes. 1994. “Plesiomorphic and Apomorphic Pollen Structure Characteristics of Anthemideae (Asteoideae: Asteraceae).” American Journal of Botany 81: 648–657.
- Wodehouse, P.P.1935. Pollen Grains. New York: Mc Graw–Hill.
- Yang, Y.F., and T.M. Ai. 2002. “Studies on the pollen morphology of ten species of Achillea.” Zhongguo Zhong Yao Za Zhi 27: 338–341.
