Abstract
Forest edges are key features in human-dominated landscape. Located between forest and non-forest habitats, edges induce biotic and abiotic changes, which may have profound consequences on vegetation diversity. Recent studies suggest the importance of different edge types in the modulation of edge-related responses. However, edge effect on the spatial dynamic of vegetation, from forest to non-forest habitats, remains unclear. Our aim was to compare the species richness and diversity of vegetation communities between forest and open habitats with their respective edges, in high-contrast versus low-contrast situations. The degree of contrast was defined according to the disturbance regimen of non-forest habitats. We surveyed vascular vegetation along transects in forest and open habitats and in their respective edges, in three regions of France. We showed that edge effects occur on plant diversity, whatever the region, but asymmetrically. Edge effect tends to be greater on the open side than on the forest side of the border. Species richness and diversity were generally higher in open edge than in open habitat, whereas no significant difference was observed between forest edge and forest habitat, whatever the contrast situation encountered. This study shows that the edge effects detected along a forest–edge–exterior habitat gradient may depend in large part on the disturbance regimen in open habitats as well as the vegetation pool size. We highlighted the need to carefully consider the edge types, e.g. their contrast with adjoining non-forest habitat, in further studies to identify the relevant factors and mechanisms behind edge-related response patterns of biodiversity in human-dominated landscapes.
Introduction
Human-driven changes in land-use patterns have increased the need to understand how landscape structure and configuration affect species distribution (Collinge Citation2009). Mosaic landscape results from diverse and opposite dynamics: agricultural intensification, resource extraction and timber harvesting creating open habitats (Harper et al. Citation2005), abandonment of grazing or management, tree plantations leading to the increase in forest habitats. In such landscapes, interfaces between open habitats and forests, also called forest edges, became one of the dominant features. Forest edges may induce gradual changes from the border in abiotic and biotic conditions, i.e. “edge effects”, through the modification of organisms, matter and energy flows (Cadenasso et al. Citation2003).
Hence, forest edges have a major ecological role, notably for biodiversity and associated ecological features. They can act not only as biodiversity hotspots, amalgamating species from both forest and open habitats (Matlack and Litvaitis Citation1999; Duelli, Obrist, and Fluckiger Citation2002), but also as corridors for species circulating in the landscape. Conversely, forest edges may represent ecological traps for species that select them as reproduction sites despite the high mortality risk (e.g. Ries and Fagan Citation2003). Hence, the response pattern of species to the forest edge can be positive, negative or neutral (Ries and Sisk Citation2004).
The composition and structure of forest edges seem to be of high relevance for biodiversity aspects because distribution of species and plant communities is largely shaped by the edge type, which is determined by the type of vegetation in adjacent habitat, the age of the forest, and the time since disturbance (Didham and Lawton Citation1999; Mesquita, Delamônica, and Laurance Citation1999; Harper et al. Citation2005; Chabrerie et al. Citation2013; Pellissier et al. Citation2013). Even when several factors that influence the spatial extent of the edge effect into forest patches are standardized, variability still exists (Hamberg et al. Citation2008) due to the importance of the type of edge studied. Edges with high contrast between forest and adjacent open habitat and edges with low contrast may have different structural characteristics and as a consequence, different functional characteristics (Gehlhausen, Schwartz, and Augspurger Citation2000; Laurance, Didham, and Power Citation2001; Cadenasso et al. Citation2003; Strayer et al. Citation2003; Ries et al. Citation2004). For example, López-Barrera et al. (Citation2006) showed that edge effects detected on oak seedlings along a forest–edge–open habitat gradient in the highlands of Chiapas, depend in large part on the fact that the edge had low or high contrast with adjacent habitat.
Understanding how the edge type may alter the structure and composition of the vegetation diversity is of high importance not only for understanding and identifying response patterns to edge effect (Ries et al. Citation2004; Harper et al. Citation2005) but also for predicting vegetation community composition in human-dominated landscapes. This knowledge could help to improve the management of biodiversity in forest edges where the interactions between forests and agriculture are growing, particularly through the valuation of ecosystem services, such as pollination and pest regulation. In this study, we investigated the response patterns to edge influence of vegetation communities, through their species richness and diversity, according to the contrast between forest and open habitats, defined here by the frequency of disturbance in open habitats, in three regions of France. A large part of studies on edge effects focus on the forest side (e.g. Alignier and Deconchat Citation2013) and ignored the adjacent habitat, although theoretical models predict that edge effects occur on both sides of the edge (Cadenasso et al. Citation2003; Ries and Sisk Citation2004; Ries et al. Citation2004).
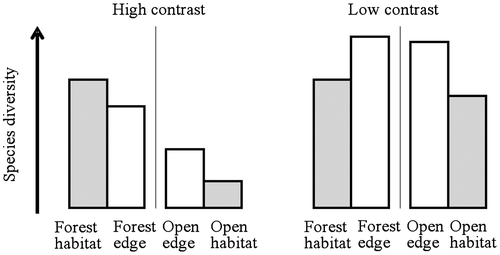
This study compares the richness and diversity of vegetation communities between a core habitat and its associated edge. Habitats considered are forests and their adjacent open habitats (fields, herbaceous communities). Edges inside the forest (i.e. forest edge) are compared to forest interior (i.e. forest habitat) and edges outside the forest (i.e. open edge) are compared to open habitat. Because the frequency and intensity of disturbance of the open habitat can be high or moderate, we distinguish respectively high- and low-contrast edges. Different patterns are expected according to the contrast between forest and adjacent open habitats, so-called edge type (Figure ). In a high-contrast situation between forest and adjacent open habitat, a gradual transition is expected. The edge could play a role as a barrier or a filter limiting the flow of organisms between habitats: for example, forest specialist species that grow preferentially in shade conditions do not come out of the forest interior and heliophilous species from open habitats do not come in (Strayer et al. Citation2003). Assuming that the forest habitat – less disturbed – is richer than the adjacent open habitat, we hypothesized that vegetation diversity decreases along the gradient forest–edge–open habitat. In low-contrast situations, plant species may disperse from one habitat to another, develop and concentrate along edges. As a result, the diversity of species may be substantially greater along edges than within any of the adjacent habitats (Matlack and Litvaitis Citation1999).
Figure 1. Expected theoretical patterns of plant species diversity in high- and low-contrast situations between forest and open habitats. In low-contrast situation, edge effect is assumed to be positive, i.e. more species are encountered in the edge than in the core habitat (in grey), whatever the side (forest or open). In high-contrast situation, it is assumed to be mixed (see the text for details).
Material and methods
Study area
The study was conducted in three regions of France (Figure ). The Aquitaine region, with almost one million hectares of maritime pines, has the largest artificial forest in Europe. The landscape is dominated by a mosaic of maritime pine plantations of different ages, clear-cuts, heathlands (dominated by Molinia caerulea (L.) Moensch, Erica cinerea L., Ulex europaeus L.), scattered deciduous forest patches and herbaceous firebreaks. As a whole, forest covers 75% of the total area and open areas (25%) are patches of different sizes disseminated in a forested landscape. The climate is thermo-Atlantic (mean annual temperature 12°C, mean annual rainfall 700 mm) and the elevation is low (c.50 m above sea level). The Midi-Pyrénées region can be classified as a temperate agro-forested landscape. Forests are fragmented with patch size between 0.5 and 35 ha and cover approximately 15% of the total area. The main tree species are Quercus robur L., Quercus pubescens Willd, Carpinus betulus L., Prunus avium (L.) L. and Sorbus torminalis (L.) Crantz. The region is hilly (250–400 m above sea level) and has a sub-Atlantic climate and slight Mediterranean influences (mean annual temperature 11°C; mean annual rainfall 750 mm). The Centre region (Loiret) is mostly dedicated to intensive crop production. Most forests (patch size between 0.5 ha and > 2200 ha) are oak–hornbeam coppice with standards used for wood and wood fuel production and cover near 35% of the region. The region is slightly hilly (110–300 m above sea level) and has a sub-Atlantic climate with some continental influence (mean annual temperature 10.5°C, mean annual precipitation 750 mm).
Sampling design
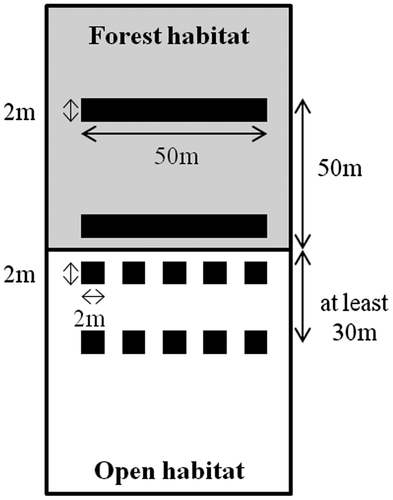
Twenty forest edges pertaining to different forest patches, were sampled in each region in May–June 2011 or 2012. For the Centre region, forest patch size varied between 0.6 ha and 818 ha (mean 71 ha). For the Midi-Pyrénées region, forest patch size varied between 0.8 and 47 ha (mean 12 ha), except for one wood of 505 ha. In each region, we considered 10 forest edges adjoining habitats with frequent soil disturbance and/or vegetation harvest (once or more per year). These high-contrast situations concerned firebreaks in Aquitaine, and oilseed rape in Midi-Pyrénées and Centre. The other 10 forest edges were located beside habitats with low disturbance (perennial communities or one vegetation disturbance per year, i.e. mowing, representing low-contrast situations) such as dune in Aquitaine, meadow in Midi-Pyrénées and orchard in Centre (Figure ). Vascular vegetation sampling was conducted in both habitats (forest and open) and their respective edges. For each forest edge, a transect was performed perpendicular to the border. The border was defined as the line formed by mature trees. Along each transect, we established 2 × 50-m plots parallel to the border, one beside the border in the edge (forest edge) and one 50 m into the forest (forest habitat) (Figure ). If edge effects can extend up to 1 km (Laurance Citation2000), they generally do not exceed 30 m in temperate forests (e.g. Piessens et al. Citation2006; Alignier and Deconchat Citation2013). We also established two sets of five 2 × 2 m plots placed parallel to the border, one beside the border (open edge) and one at least 30 m away from the border in the open habitat (Figure ). Vegetation sampling areas were defined to detect the majority of species in plant communities in each type of habitat. For that purpose, we estimated species cover with respect to the minimum sampling area method using the following standards: 100 m² for the forest side and 16 m² for the open habitats side (Guinochet Citation1973). The plots were designed to consider a similar spatial extent (50 m length) to avoid the larger environmental heterogeneity found in a larger sampling plot. At each plot, we recorded abundance–dominance of all vascular plant species pertaining to all strata (trees, shrubs and herbs) according to the Braun-Blanquet scale (Braun-Blanquet Citation1956).
Figure 3. Scheme of the sampling design. Two 100-m² plots parallel to the border were placed in the forest edge and the forest habitat, at 0 m and 50 m from the border, respectively. Two sets of five 4 m² plots were placed within the open edge and the open habitat, at 0 m and 30 m at least from the border. The border was defined as the line formed by mature trees between forest and open habitats.
Vegetation surveys in each habitat and its edge gave species richness (R) and allowed calculation of Shannon species diversity (H) according to the proportional abundance of each species (pi) as:
In open habitat and open edge, abundance values for each plant species were averaged over the five 4-m² plots.
Statistical analyses
First, we compared mean species richness and mean Shannon species diversity between habitats and their respective edges in each region using pairwise Student’s t-tests. Then, similarity in species composition was quantified for each habitat and its edge, in each region. We used the Bray Curtis similarity index, which is related to the Sorensen index and which assesses similarity between two localities based on differences in abundance of species, and not merely on presence/absence. It ranges from 0 (no similarity) to 1 (same species in both localities all, occurring at the same abundance in both sites). Because this index can only be computed with integers, abundance data were rounded up before the calculations were made; this has no effect on the resulting index values. We compared similarities in species composition according to contrast (1) for the forest side and (2) for the open side, using analysis of variance (ANOVA) tests. Statistical analyses were carried out using R 2.15.1 (R Development Core Team 2010).
Results
A total of 534 vascular plant species were recorded through the three regions. In the Aquitaine region, 154 species were recorded with 102 species (on average 18.3 ± 5.9) in high-contrast situations (n = 4 transects; firebreaks) and 60 (on average 8.7 ± 5.2) in low-contrast situations (n = 16; dunes). The assemblage was dominated in high-contrast situations by Pinus pinaster Aiton (present in 50% of surveyed plots), and in low-contrast situations by Carex arenaria L. (57.8% of surveyed plots), Cerastium diffusum Pers. (54.7% of surveyed plots) and Aira praecox L. (53.1% of surveyed plots). In the Midi-Pyrénées region, 365 species were recorded with 181 species (on average 18 ± 11.2) in high-contrast situations (n = 10 transects; oilseed rape) and 318 (on average 35.8 ± 9.7) in low-contrast situations (n = 17; meadows). The assemblage was dominated in high-contrast situations by Hedera helix L. (present in 60% of surveyed plots), Crataegus monogyna Jacq. (55% of surveyed plots) and Ligustrum vulgare L. (52% of surveyed plots) and in low-contrast situations by Dioscorea (L.) Caddick & Wilkin communis (present in 94% of surveyed plots). Rubia peregrina L. (present in 88% of surveyed plots) and Hedera helix (86% of transects). In the Centre region, 331 species were recorded with 221 species (on average 26.6 ± 13.0) in high-contrast situations (n = 10 transects; oilseed rape) and 197 (on average 25.7 ± 10.3) in low-contrast situations (n = 11; orchards). The vegetation assemblage was dominated by Galium aparine L. (present in 52% of surveyed plots) in high-contrast situations, and Hedera helix (79%), Quercus robur (68%) and Rubus fructicosus s.l. (66% of surveyed plots).
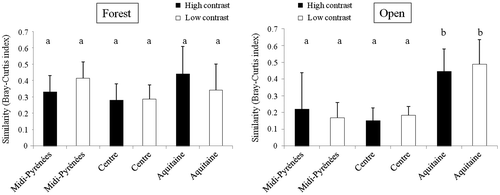
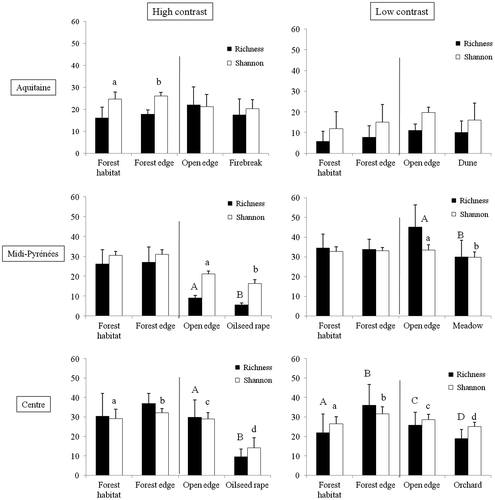
Whatever the contrast situation encountered, a clear edge effect in terms of species richness and Shannon species diversity (H) was observed between the open edge and open habitat, whereas no significant differences were observed between the forest edge and the forest habitat for both the Midi-Pyrénées and the Centre regions (Figure ; Appendix 1). Higher species richness and higher H were encountered in open edges. No significant differences were observed between habitats and their respective edges in Aquitaine (Figure ; Appendix 1).
Figure 4. Species richness (mean ± SD) and Shannon species diversity (× 10; mean ± SD) in the forest and open habitats and their respective edges in the three regions of France. Capital letters (lowercase, respectively) indicate significant differences for mean species richness (mean Shannon species diversity, respectively) at α = 0.05. Note that forest habitat was compared uniquely to forest edge and open edge uniquely to open habitat.
Similarities between vegetation communities belonging to the forest side averaged 35% and did not differ according to the contrast situation or the region (ANOVA, F = 2.95, p = 0.194; Figure ). For the open side, similarities were lower (average near 25%) than the forest side. Similarities between vegetation communities belonging to open habitat and open edge were significantly higher for the Aquitaine region (ANOVA, F = 15.16, p = 2.4 e-09; Figure ).
Discussion
Edge effects occur on plant communities in three regions of France, but in an asymmetrical way. Edge effect on plant diversity tends to be greater on the open side than on the forest side of the border. Species richness and diversity were generally higher in open edge than in open habitat whatever the contrast situation encountered. No significant difference was observed between the forest edge and the forest habitat. Expected response patterns of vegetation diversity were not all satisfied. With a view to managing vegetation diversity in the edge so as to promote ecological services in both adjacent habitats, studies dealing with edge effect according to edge types and patch contrast in human-dominated landscapes are still required.
Higher species richness was found in vegetation communities for the Centre and Midi-Pyrénées regions than for the Aquitaine region. Indeed, the vegetation pool size in the Aquitaine region, dominated by pine plantations, was only about half as important as in the other regions, dominated by broadleaved forests. Natural forests are usually more suitable as habitat for a wider range of native forest species than plantation forests (Bremer and Farley Citation2010). But there is growing evidence that plantation forests can provide valuable habitats, even for some threatened and endangered species, and may contribute to the conservation of biodiversity by various mechanisms (Carnus et al. Citation2006; Brockerhoff et al. Citation2008). Another explanation could be related to local environmental conditions, especially to available light. The development of the forest cover from a quasi-pure pine plantation to a multi-storeyed mixed forest contributes to attenuation in radiation beneath the canopy (Porté, Huard, and Dreyfus Citation2004). Pine plantations displayed understorey habitats with more light available than in broadleaved forest, so the contrast between forest habitat and forest edge was reduced. In addition, the open edge may be less influenced by adjacent forest in pine forest, leading to homogenization between habitats. This may particularly endanger the rare, often habitat-specific, species that represent a large part of species richness (Gaston Citation1994).
Addressing the edge-related response of vegetation communities, we observed some variability by comparison with expected patterns (Figure ). In high-contrast situations, species richness tended to decrease according to the gradient forest–edge–open habitat, in spite of there being no significant difference between forest edge and forest habitat. This pattern, in accordance with our expectations, was confirmed for the Midi-Pyrénées and Centre regions, but not for the Aquitaine region. Although edge effects in temperate forests generally do not exceed 30 m (e.g. Piessens et al. Citation2006; Alignier and Deconchat Citation2013), the lack of significant difference between forest edge and forest habitat indicates either that edge effect was longer than 50 m or that the size of our forest patches was too small to guarantee the presence of a core habitat zone. In that sense, forest patches can be considered to be entirely under the influence of the edge (Laurance and Yensen Citation1991) or of multiple edge effects (Fletcher Citation2005). Nevertheless, this last assumption is more debatable in the large forests of the Aquitaine and Centre regions. In low-contrast situations, the patterns were less clear. Only species richness in the Centre region was substantially greater in edges than in adjacent habitats, as expected. This result tends to demonstrate that although a low-contrast situation allows more dispersal than a high-contrast situation, species do not necessarily concentrate in edges and may ignore them. However, to justify labelling a species as insensitive, one should be able to demonstrate a consistent lack of response at several edge types (Ries and Sisk Citation2010). Focusing on Shannon diversity, only patterns observed in high-contrast situations for the Midi-Pyrénées region and in low-contrast situations for the Centre region conformed to our expectations. We have to note that no edge effect was observed in any contrast situation for the Aquitaine region. A likely explanation for the absence of vegetation response to edge effect is that we assumed that the forest habitat was richer than the open adjacent habitat, but it appears evident that it was not the case for the Aquitaine region. The dune and some firebreak habitats were richer than the forest habitat. As noted previously, stands are composed of intensive and homogeneous plantations of maritime pines in the Aquitaine region that may reduce the habitat heterogeneity and so impoverish vegetation communities (Bremer and Farley Citation2010). Conversely, open habitats like permanent grasslands could be as rich or even richer than forest habitats (Łuczaj and Sadowska Citation1997). Hence, our dichotomy of edge type, based on disturbance frequency, needs to be nuanced, as the contrast perceived by the human observer does not necessarily represent relevant heterogeneity to vegetation communities. A way to fix this could be to improve the description of disturbance regimens in each habitat and to relate them to the evolutionary history of the different plant communities.
Similarity in vegetation communities between edge and habitat averaged 35% in the forest side over all regions. Plant communities were as rich in forest edges as in forest habitats, but plant assemblages were quite different. This change in species composition may reflect the functional response of species. Indeed, forest habitat specialists are generally shade-tolerant and are known to avoid edges (Ranney, Bruner, and Levenson Citation1981) whereas edges advantage light-tolerant species (Brothers and Spingarn Citation1992). Similarity in vegetation communities between edge and habitat is lower in the open side. This result would indicate that exchanges – through dispersal – between vegetation communities in the open side were more limited than in the forest side. Similarity in vegetation communities between habitats and their respective edges did not differ according to the contrast, but according to the region. The Aquitaine region showed a significantly higher similarity between open edge and open habitat than in other regions. However, similarity increased where species richness decreased. So, inter-regional variation in similarity pattern was probably linked to the vegetation pool size.
This study has found a great variability in the response patterns of vegetation communities to edge effect. These patterns did not all correspond to the expected response patterns, depending on the region. The range of edge-related response patterns observed suggests that the contrast between forest and open habitats is not uniform and that vegetation diversity depends mainly on the region – through pool size – and the disturbance regimen. Taken together, our results highlight the importance of (1) improving the description of disturbance regimen of both forest and non-forest habitats, and (2) additional tests of the effects of different edge types (Ries et al. Citation2004) or patch contrast (Harper et al. Citation2005) on vegetation communities. Considering species assemblages and their functional traits (e.g. specialists versus generalists) may provide more accurate response patterns to edge influence than by considering the whole species through diversity indices. In addition, variability observed between regions suggests that these factors should be addressed more explicitly in further studies. To develop a comprehensive theory of edge effects and effective management of vegetation diversity in edges, future studies should identify the relevant factors and mechanisms behind edge-related response patterns of biodiversity in human-dominated landscapes.
Notes on contributors
Audrey Alignier’s research is targeted at the understanding of the spatial distribution patterns of vegetation communities. Didier Alard is a plant ecologist interested in conservation ecology and biodiversity monitoring. Richard Chevalier’s research is targeted on the identification of the main biotic and abiotic drivers shaping forest plant communities. Emmanuel Corcket is a plant ecologist working on relationships between biotic processes and biodiversity.
Didier Alard, Emmanuel Corcket and Richard Chevalier conceived and performed the vegetation surveys. Audrey Alignier conducted data analysis. Audrey Alignier, Didier Alard and Emmanuel Corcket wrote the manuscript.
Acknowledgements
This work was part of the BGF-ECOFOR Bilisse project and was supported by grants from MEDDTL and the Aquitaine and Midi-Pyrénées regions.
References
- Alignier, A., and M. Deconchat. 2013. “Patterns of forest vegetation responses to edge effect as revealed by a continuous approach.” Annals of Forest Science 70: 601–609.
- Braun-Blanquet, J. 1956. “Plant Sociology: The study of plant communities.” In The study of Plant Communities, edited by H.J. Oosting. San Francisco: W.H. Freemannand.
- Bremer, L.L., and K.A. Farley. 2010. “Does plantation forestry restore biodiversity or create green deserts? A synthesis of the effects of land-use transitions on plant species richness.” Biodiversity and Conservation 19: 3893–915.
- Brockerhoff, E.G., H. Jactel, J.A. Parrotta, C.P. Quine, and J. Sayer. 2008. “Plantation forests and biodiversity: oxymoron or opportunity?” Biodiversity and Conservation 17: 925–951.
- Brothers, T.S., and A. Spingarn. 1992. “Forest fragmentation and alien plant invasion of Central Indiana old-growth forests.” Conservation Biology 6: 91–100.
- Cadenasso, M.L., S.T.A. Pickett, K.C. Weathers, S.S. Bell, T.L. Benning, M.M. Carreiro, and T.E. Dawson. 2003. “A framework for a theory of ecological boundaries.” BioScience 53: 750–758.
- Carnus, J.-M., J. Parrotta, E.G. Brockerhoff, M. Arbez, H. Jactel, A. Kremer, D. Lamb, K. O’Hara, and B. Walters. 2006. “Planted forests and biodiversity.” Journal of Forestry 104: 65–77.
- Chabrerie, O., A. Jamoneau, E. Gallet-Moron, and G. Decocq. 2013. “Maturation of forest edges is constrained by neighboring agricultural land management.” Journal of Vegetation Science 24: 58–69.
- Collinge, S.K. 2009. Ecology of fragmented landscapes. Baltimore MD: The Johns Hopkins University Press.
- Didham, R.K., and J.H. Lawton. 1999. “Edge structure determines the magnitude of changes in microclimate and vegetation structure in tropical forest fragments.” Biotropica 31: 17–30.
- Duelli, P., M.K. Obrist, and P.F. Fluckiger. 2002. “Forest edges are biodiversity hotspots–also for Neuroptera.” Acta Zoologica Academiae Scientarum Hungaricae 48: 75–87.
- Fletcher, R.J. 2005. “Multiple edge effects and their implications in fragmented landscapes.” Journal of Animal Ecology 74: 342–352.
- Gaston, K.J. 1994. Rarity. London: Chapman and Hall.
- Gehlhausen, S.M., M.W. Schwartz, and C.K. Augspurger. 2000. “Vegetation and microclimatic edge effects in two mixed-mesophytic forest fragments.” Plant Ecology 147: 21–35.
- Guinochet, M. 1973. Phytosociologie. Paris: Masson et Cie.
- Hamberg, L., S. Lehvävirta, M.L. Minna, H. Rita, and D.J. Kotze. 2008. “The effects of habitat edges and trampling on understorey vegetation in urban forests in Helsinki, Finland.” Applied Vegetation Science 11: 83–98.
- Harper, K.A., S.E. Macdonald, P.J. Burton, J.Q. Chen, K.D. Brosofske, S.C. Saunders, E.S. Euskirchen, D. Roberts, M.S. Jaiteh, and P.A. Esseen. 2005. “Edge influence on forest structure and composition in fragmented landscapes.” Conservation Biology 19: 768–782.
- Laurance, W.F. 2000. “Do edge effects occur over large spatial scales?” Trends in Ecology and Evolution 15: 134–135.
- Laurance, W.F., R.K. Didham, and M.E. Power. 2001. “Ecological boundaries: a search for synthesis.” Trends in Ecology and Evolution 16: 70–71.
- Laurance, W.F., and E. Yensen. 1991. “Predicting the impacts of edge effects in fragmented habitats.” Biological Conservation 55: 77–92.
- López-Barrera, F., R.H. Manson, M. González-Espinosa, and A.C. Newton. 2006. “Effects of the type of montane forest edge on oak seedling establishment along forest–edge–exterior gradients.” Forest Ecology and Management 225: 234–244.
- Łuczaj, Ł., and B. Sadowska. 1997. “Edge effect in different groups of organisms: vascular plant, bryophyte and fungi species richness across a forest-grassland border.” Folia Geobotanica and Phytotaxonomica 32: 343–353.
- Matlack, G.R., and J.A. Litvaitis. 1999. Forest edges: Maintaining biodiversity in forest ecosystems. Cambridge: Cambridge University Press.
- Mesquita, R.C., P. Delamônica, and W.F. Laurance. 1999. “Effect of surrounding vegetation on edge-related tree mortality in Amazonian forest fragments.” Biological Conservation 91: 129–134.
- Pellissier, V., L. Bergès, T. Nedeltcheva, M.-C. Schmitt, C. Avon, C. Cluzeau, and J.-L. Dupouey. 2013. “Understory plant species show long-range spatial patterns in forest patches according to distance-to-edge.” Journal of Vegetation Science 24: 9–24.
- Piessens, K., O. Honnay, R. Devlaeminck, and M. Hermy. 2006. “Biotic and abiotic edge effects in highly fragmented heathlands adjacent to cropland and forest.” Agriculture, Ecosystems and Environment 114 (2): 335–342.
- Porté, A., F. Huard, and P. Dreyfus. 2004. “Microclimate beneath pine plantation, semi-mature pine plantation and mixed broadleaved-pine forest.” Agricultural and Forest Meteorology 126: 175–182.
- Ranney, J.W., M.C. Bruner, and J.B. Levenson. 1981. “The importance of edge in the structure and dynamics of forest islands.” In Forest island dynamics in man-dominated landscapes, edited by R.L. Burgess and D.M. Sharpe, 67–95. Berlin: Springer Verlag.
- Ries, L., and W.F. Fagan. 2003. “Habitat edges as a potential ecological trap for an insect predator.” Ecological Entomology 28: 567–572.
- Ries, L., and T.D. Sisk. 2004. “A predictive model of edge effects.” Ecology 85: 2917–2926.
- Ries, L., R.J. Fletcher Jr, J. Battin, and T.D. Sisk. 2004. “Ecological responses to habitat edges: mechanisms, models, and variability explained.” Annual Review of Ecology, Evolution, and Systematics 35: 491–522.
- Ries, L., and T.D. Sisk. 2010. “What is an edge species? The implications of sensitivity to habitat edges.” Oikos 119: 1636–1642.
- Strayer, D.L., M.E. Power, W.F. Fagan, S.T. Pickett, and J. Belnap. 2003. “A classification of ecological boundaries.” BioScience 53: 723–729.
Appendix 1. Values of p for pairwise Student’s t-tests
Pairwise Student’s t-tests were used to compare (1) the species richness and (2) the Shannon species diversity between the forest and open habitats and their respective edges, in the three regions of France. In bold, significant tests at α = 0.05.