Abstract
Potentilla fruticosa L. is a self-incompatible clonal shrub, characterized by a wide circumpolar distribution (Asia and North America). In Europe the species has many peripheral isolated populations, and within the Alps it is confined to a restricted area of the Maritime Alps (Italy and France). In alpine environments P. fruticosa is affected by a significant lack of information about current population status and little and conflicting information is reported about its potential habitat. Our study investigated P. fruticosa populations on the Italian side of the Alps to evaluate its synecology, syntaxonomy and conservation status. Results showed that six out of the seven populations inventoried in the area during the study, consisted of 20 or fewer individuals, and only one included a high number of plants. The species was observed in the study area within the Caricetum frigidae association (Caricion davallianae alliance), very close to small creeks characterized by fairly constant water levels. In the Italian Alps P. fruticosa has a very restricted geographic range, estimated at around 16 km2 (extent of occurrence). Isolation of populations affected viable seed production. A continuing decline in the quality and extent of the habitat is expected due to the continuing abandonment of pastures that began 40 years ago in the study area. According to the most recent IUCN categories and criteria the species should be listed at the regional/national level as Critically Endangered.
Introduction
Many studies have shown that spatial isolation and small population size may negatively affect many rare plant species, limiting genetic exchanges between populations, reducing plant fitness and viability, and increasing the risk of extinction (Young, Boyle, and Brown Citation1996; Frankham and Ralls Citation1998; Lu, Waller, and David Citation2005; Leimu et al. Citation2006; Aguilar et al. Citation2008; Kuss et al. Citation2008). Nonetheless, natural fragmentation is a frequent feature in alpine species, due to the effects of Quaternary history, pronounced mountainous topography and related abiotic heterogeneity (Kuss et al. Citation2008). Diverse life history traits of different plants may make them more or less vulnerable to fragmentation effects, i.e. stronger negative effects on short-lived species are expected compared with long-lived species (Young, Boyle and Brown Citation1996). Self-incompatible species are most prone to negative effects of isolation (Leimu et al. Citation2006). However, the ability of many species to reproduce clonally may limit the negative effects of fragmentation, resulting in a delay of time between generations (Honnay and Bossuyt Citation2005).
Within European taxa, Potentilla fruticosa L. represents a very interesting example of a self-incompatible clonal plant characterized by a fragmented distribution range. The species has a wide circumpolar distribution in the northern temperate zone (Asia and North America) with many peripheral isolated populations in Europe. In northern Europe it is recorded in western Ireland, northern England, the Baltic area, and the Ural Mountains (Elkington and Woodell Citation1963). In southern Europe, very small populations of P. fruticosa were observed in the French and Spanish Pyrenees, in the Maritime Alps (France and Italy) and in a single locality in Bulgaria (Elkington Citation1969).
Many studies have stressed the importance of peripheral isolated populations on species conservation, both for their ecological and genetic importance (Dvornyk Citation2001; van Rossum et al. Citation2003; Lesica and McCune Citation2004; Gapare and Aitken Citation2005; Leppig and White Citation2006). Due to the greater physiological stress associated with marginal habitat conditions, peripheral populations may be well adapted to shifting the species range in response to climate change (Hunter and Hutchinson Citation1994; Garcia-Ramos and Kirkpatrick, Citation1997; Mott Citation2010). In addition, they are potentially important for future speciation events (Lesica and Allendorf Citation1995).
In the Alps, P. fruticosa has many peripheral isolated populations with respect to the core distribution. These isolated populations are confined to a restricted area of the Maritime Alps, between the municipalities of Entraque and Valdieri (Piedmont, Italy) and the municipalities of St-Martin-Vésubie, Fontan, Belvédère and Guillaumes (France) (Pignatti Citation1982; SILENE Citation2013). Based on historical and recent observations, only four populations have been documented in the Italian Alps (Burnat, Briquet, and Cavillier Citation1892–1931; Bono Citation1965; Pascale Citation2006). According to the only comprehensive Italian Red List of threatened plants (Conti, Manzi and Pedrotti Citation1997), based on an older version of IUCN categories, P. fruticosa is included among Lower Risk (LR) species. Until now it has never been considered for an assessment according to the most recent categories and criteria (IUCN Citation2001, Citation2012a) and it is currently not included within the new Italian Red List (Rossi, Montagnani, Gargano et al. Citation2013; Rossi, Montagnani, Abeli et al., Citation2013).
The knowledge of syntaxonomy and synecology of rare plant species is essential to their conservation, e.g. phytosociological habitat descriptions provide considerable relevant information about habitat quality, syndynamic processes and related management options (Austin Citation1999; Hölzel Citation2003; Lonati and Siniscalco Citation2009a, Citation2009b, Citation2010; Lonati, Gorlier, and Lombardi Citation2011). Additionally, IUCN guidelines take into account the reduction of habitat quality during the assessment process (IUCN Citation2013).
Little and conflicting information about P. fruticosa potential habitat in the Alps has been reported. Pignatti (Citation1982) reported the species in the Italian Alps on sunny cliffs, contrasting with the observations of Pascale (Citation2006), who reported the species associated with Alnus viridis stands and along alpine streams banks in the same geographic area. Phytosociological data are also not very clear: Aeschimann et al. (Citation2004) indicated the species optimum condition in the Alps in the Caricion davallianae alliance, according to observations of Braun-Blanquet (Citation1948) in the Pyrenees. In contrast, the same species is listed by Cavallero et al. (Citation2007) within thermo-xerophilous Centaureo uniflorae–Festucetum paniculatae (Festucion variae alliance) in the Italian western Alps.
The present study investigated P. fruticosa populations in the Italian Alps to assess their conservation status according to the most recent IUCN criteria and categories (IUCN Citation2001, 2012a). The specific goals of the work are: (1) to describe actual geographic distribution and assess population size, (2) to define the phytosociological optimum of the species in the Italian Alps, (3) to test whether the vegetative performance and potential reproductive success are related to the isolation of populations, and (4) to identify the factors affecting P. fruticosa conservation.
Material and methods
Study species
Potentilla fruticosa is a deciduous branched shrub, 50–100 cm high. Both erect and prostrate branches are present in mature plants, the latter being able to easily root adventitiously. Vegetative propagation takes place by means of creeping stems just below the soil surface and may enable the plant to cover a wide area (Elkington and Woodell Citation1963).
The species is self-incompatible (Innes and Lenz Citation1991). Flowers are usually produced during the second season when the plants are 40–50 cm high (Elkington and Woodell Citation1963). Flowers are five-merous, with triangular ovate sepals, oblanceolate linear epicalyx segments, and orbicular-ovate yellow petals. The whole calyx is persistent in fruit and surrounds the achenes. Achenes are about 1 mm long, dark brown at maturity, and surrounded by a ring of hairs produced from the base.
Data collection
To assess the conservation status of P. fruticosa, we georeferenced all the existing populations and quantified the area and the total number of individuals for each population. Identification of populations was based on published sources, herbarium records, personal unpublished observations and extensive field surveys across the entire Italian range of the species during the flowering period (June and July 2010). In most cases plants were easily identified in the field as singular individuals. Due to clonal spread by the external prostrate branches, the oldest individuals appeared as polycormic plants with dense canopies and hemi-ellipsoidal crowns, probably corresponding to genets. Individual plants were patchily distributed in the field, forming dense patches of less than 20–25 m2. We used a threshold distance of 100 m between patches to differentiate individual populations (Kolb and Lindhorst Citation2006). Population perimeters were georeferenced using a GPS and the corresponding areas were quantified using Quantum GIS 1.8 (Quantum GIS Development Team, 2012). Five of the seven populations (POP1, POP3, POP4, POP5, POP6) consisted of one single patch; one population (POP2) comprised two well-separated patches; and one (POP7) included a large number of patches, often close to each other and not easily distinguishable. In all populations except POP7 the size was assessed by direct count of all the individuals (without distinction between mature and immature individuals). In POP7 direct counts of all individual plants were not feasible. Consequently, we estimated the population size by measurement of the area occupied by the patches multiplied by the average plant density (e.g. Pluess and Stöcklin Citation2004), which was determined by counting all individuals in six 28.3 m2 circular plots (3 m radius), randomly arranged inside the area. The population size was assessed during July 2010.
To describe the synecology, structure, and vegetative and reproductive fitness of P. fruticosa, in all populations but POP7, we located one or two 28.3 m2 circular permanent plots with the plot centre at the centre of each patch. In POP7, surveys were carried out in the same circular plots used to measure plant density. Thirteen plots were surveyed during 2010.
To investigate the species synecology, phytosociological surveys were carried out at each plot during June and July 2010, using the abundance–dominance values proposed by Braun-Blanquet (Citation1932). Percent cover of bare soil, rocks, herbs, lower shrub (height ≤ 1.3 m) and upper shrub (height between 1.3 and 5.0 m) layers were visually estimated. All the woody species, including P. fruticosa, were recorded within the lower or upper shrub layer. Floristic nomenclature follows Pignatti (Citation1982).
To describe the population structure we recorded or calculated the following parameters within each circular plot at the end of the growing season (September 2010):
P. fruticosa plant density, by counting all flowering and non-flowering individuals | |||||
P. fruticosa plant height (from soil level to the tip of the tallest shoot) and diameter (average of two diameters of an approximate ellipse), by measuring all the individuals, average plant height and diameter were calculated for each plot using data recorded from all individuals | |||||
Total canopy area of P. fruticosa (m2 plot−1), summing up all the canopy areas of singular individuals (shape of the canopy crown approximate to an ellipse). | |||||
Additionally, to measure the species vegetative and reproductive fitness, 10 individuals of P. fruticosa were randomly selected within each plot except in the smallest population with fewer than 10 individuals. We measured the following morphological and reproductive traits (September 2010):
Annual final shoot length (one shoot derived from the terminal bud for each selected individual);
Number of well-developed, potentially viable achenes (total count in 10 flowers randomly selected within each plot, coming from the 10 randomly selected individuals whenever possible). Well-developed fruits (filled) were easily separable from those aborted (unfilled), the latter almost exclusively comprised of the pappus.
To quantify patch isolation we calculated the following indices (Hanski, Kuussaari, and Nieminen Citation1994; Bruun Citation2000):
distance from patches I to the nearest occupied patch (Ii1), calculated using the GPS position of each plot. We also tested the mean distance to the nearest two (Ii2), three (Ii3) and four patches (Ii4), but this did not alter the results as both isolation parameters were strongly correlated (Pearson correlation: r > 0.99 and p < 0.001 with Ii2; r = 0.95 and p < 0.001 with Ii3; r = 0.88 and p < 0.001 with Ii4);
overall isolation, henceforth called isolation index (In), defined as In = –∑ exp(–dij), where dij is the distance between patches i and j in kilometres.
To assess the abiotic factors affecting vegetation at each plot, a number of variables were measured in the field or extracted from available data sets. Topographic variables (elevation, aspect, slope and distance from the nearest creek) were measured by using topographic measuring devices. Soil pH was measured potentiometrically on air-dried topsoil samples (10 cm) on the > 2 mm soil fraction of a soil/water suspension (soil/water ratio 1 : 2) using standard techniques (Soil Survey Staff Citation1999). Aspect was transformed into southness [southness = 180 – (aspect – 180)], to provide an interpretable, non-circular variable (Chang et al. Citation2004). Climate data in the study area were extrapolated for each plot, using UTM coordinates from the Climatologic Atlas of Piedmont (Biancotti et al. Citation1998).
Data analysis
Phytosociological data were transformed into numerical values according to van der Maarel (Citation1979), which were used to classify the 13 plots by cluster analysis (option for clustering: average link; resemblance coefficient: similarity ratio). Each group was assigned an association based on the presence and frequency of phytosociological characteristic species. The syntaxonomic nomenclature follows Grabherr and Mucina (Citation1993), integrated with Mucina, Grabherr, and Ellmauer (Citation1993), Oberdorfer (Citation1983) and Theurillat et al. (Citation1994). Nomenclature of associations and related syntaxa was revised according to the International Code of Phytosociological Nomenclature (Weber, Moravec, and Theurillat Citation2000).
We assessed among-group differences of topographic/environmental variables (elevation, southness, slope, soil pH) and P. fruticosa performance variables (density, average plant height and diameter, canopy area, shoot length and number of well-developed achenes), by univariate analysis of variance (ANOVA). Before the analysis, data were tested for homoscedasticity (Levene’s test) and three variables (distance from creek, average plant height and average plant diameter) were log10 transformed to meet this assumption (see Supplementary Table 1). ANOVA residuals were also tested for normality using Kolmogorov–Smirnov test (see Supplementary Table 2). Group means were compared with Bonferroni post-hoc range test (p ≤ 0.05), which takes the unbalanced replicates design into account (Soliani Citation2004; Norusis Citation2005).
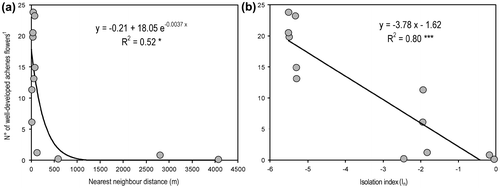
The relationship between the number of filled fruits and patch isolation (Ii1 and In) was analysed by a regression, using exponential and linear functions, respectively (p ≤ 0.05). In the linear regression, all variables were tested for normality to meet assumptions of the analysis. As a result of wild ungulate damage on flowers and fruits in one plot (POP6), the regression models were performed using 12 plots. The analysis was performed at plot scale (and not at population scale) to use a larger data set in the analysis. All statistical analyses were performed using SPSS 19 (SPSS Inc., Chicago, IL, USA).
In accordance with the IUCN categories and criteria (IUCN Citation2001, 2012a) and the most recent guidelines for their application (IUCN Citation2013), species conservation status was assessed using criterion B. We calculated the extent of occurrence (EOO) by measuring the area of the minimum convex polygon including the populations, and the area of occupancy (AOO) superimposing a 2 × 2 km grid to population locations (Gargano Citation2011). As a first step we applied the IUCN Red List categories and criteria (IUCN Citation2001, 2012a, 2013) to the Italian populations to determine the preliminary estimate of extinction risk. As a second step, according to the IUCN regional guidelines (IUCN Citation2012b), we considered the effects of French neighbouring populations on the Italian ones, and the preliminary category was up- or down-listed, when appropriate, to determine the final estimate of extinction risk in Italy.
Results
Geographic distribution and population size
We located seven populations of P. fruticosa between the municipalities of Entraque and Valdieri. Four populations were confirmed from published and herbarium data and three new populations were found (Table , Figure ).
Table 1. Code, location and size [number of total individuals (= genets)] of the studied populations.
Figure 1. Location of the studied populations and potential species range. EOO, extent of occurrence; AOO, area of occupancy.
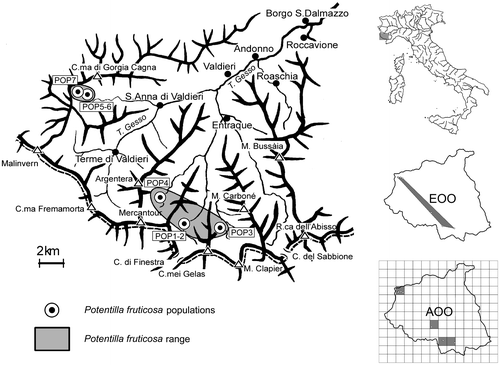
Populations ranged in size from one to about 18,000 individuals (median 13 plants). Six of the seven studied populations consisted of 20 or fewer individuals. Only one population (POP7), located in the high Vallone della Meris (Valdieri), consisted of a high number of plants (18,000 estimated individuals). The population area ranged between 0.2 and 27 m2, except for POP7, which occurred over an area of about 2.6 ha (Table ).
Based on population locations (Figure ), we identified two sub-ranges, one for each municipality. Considering all the populations together we calculated an EOO of about 18 km2 and an AOO of 16 km2.
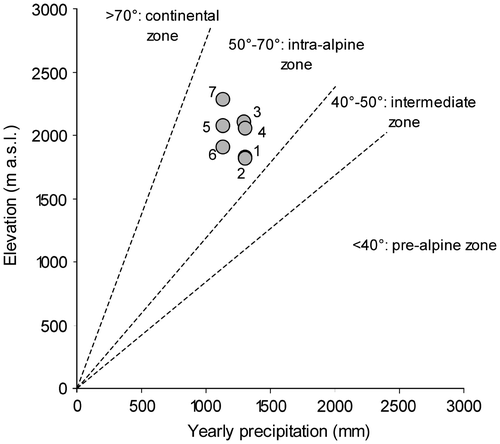
Populations ranged on average between 1800 and 2280 m above sea level (subalpine and alpine belts). They were localized in the intra-alpine zone (Gam’s continental index ranged between 54.2 and 63.5°) (Figure ).
Figure 2. Relationship between altitude (m) and yearly precipitation (mm) for the located seven populations of Potentilla fruticosa (progressive numbers according to Table 1). Lines show Gams’ continentality index thresholds (Gams’ angle) and define the ecological districts according to Ozenda (Citation1985).
Synecology
The cluster analysis, performed at the plot level, allowed the identification of three groups of plots and populations, clearly separated from each other from a phytosociological point of view (Figure , Table ):
Group 1 (two plots; two populations) included populations ascribable to thermophilous grasslands dominated by Festuca paniculata. These populations could be assigned to the association Centaureo uniflorae–Festucetum spadiceae, as confirmed by the presence of many characteristic species of the association and related syntaxa;
Group 2 (eight plots, three populations) represents over 60% of the plots and included the most important population (POP7). The vegetation could be assigned to the association Caricetum frigidae (Caricion davallianae), including oligo-mesotrophic communities in basophilous fens with low primary productivity. Carex frigida was the most abundant species in the plots. Potentilla fruticosa patches were usually close to small creeks with water available throughout the growing season (Table );
Group 3 (three plots, two populations) included populations characterized by less representative vegetation. However, due to the presence of many characteristic species from basiphilous fens (i.e. Carex frigida, Tofieldia calyculata, Pinguicula vulgaris, Parnassia palustris), we included Group 3 within the Caricetum frigidae. Potentilla fruticosa patches were usually close to small creeks, although in POP3, free water was not usually present, except after periods of very intensive rainfall or recent snowmelt. The plots included in Group 3 were, on average, localized at lower altitudes and on steeper slopes than Group 2 plots (Table ).
Figure 3. Dendrogram of phytosociological surveys (Ward’s method, similarity ratio). Plot codes (PL01÷13), population codes (POP1÷7) and their repartition in clusters (GR01÷3) are shown below the dendrogram.
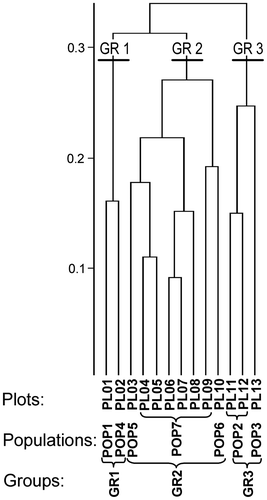
Table 2. Phytosociological surveys.
Table 3. Differences in environmental, structural and growth/reproductive performance variables within groups, tested by univariate analysis of variance.
The topsoil reaction was significantly different among the three groups. Nevertheless, the limited range of variation (6.0–6.4 on average) is not particularly relevant from an ecological point of view.
Several ingressive species (belonging to the classes Seslerietea albicantis, Loiseleurio–Vaccinietea, Mulgedio–Aconitetea, Querco–Fagetea, Calluno–Ulicetea and Molinio–Arrhenatheretea) were observed in all the groups (Table ), supplying additional information about the synecology (see Discussion).
Vegetative performance and potential reproductive success
Vegetation groups differed significantly in plant size and other traits (Table ). Within the Caricetum frigidae association (Groups 2 and 3), plots located at lower altitude (Group 3) supported, on average, the significantly largest plant dimensions (diameter and height) and the longest shoot length. The plant dimensions of Group 1 (Centaureo uniflorae–Festucetum spadiceae) were similar to those in Group 2; the length of vegetative shoots was not significantly different from the other two groups.
Plant density did not differ significantly between groups, although the highest values were observed for Group 2. A significantly highest number of well-developed achenes was observed in Group 2 (Table ).
The reproductive success, quantified by the number of well-developed achenes per flower, showed a significant inverse relation with plot isolation (Figure ). The number of well-developed achenes was significantly fitted by an inverse exponential regression with the minimum distance from the nearest neighbour plots and by an inverse linear regression with the overall isolation index (In).
Discussion
Potentilla fruticosa in the Italian Alps has a fairly broad ecological tolerance. We observed the species both in moist and dry conditions (Caricion davallianae and Festucion variae alliances, respectively). However, five of the seven known populations were observed in moist conditions in the Caricetum frigidae association in particular (Groups 2 and 3). Consequently, in the study area the Caricion davallianae should probably be designated as the optimum condition for the species, which is consistent with reports by other authors in southern Europe (Aeschimann et al. Citation2004, for the Alps; Braun-Blanquet Citation1948, for the Pyrenees).
Group 2, observed close to small creeks with water available throughout the growing season, supported the most viable population (POP7), characterized by the highest number of well-developed achenes and the highest demographic turnover, as shown by younger and so smaller plants. In Group 3, P. fruticosa probably grew in less optimal conditions because of irregular water availability, lower altitude and steeper slopes than Group 2. Steep slopes tend to be well drained (and hence drier) and therefore do not generally favour P. fruticosa. Nevertheless, the fact that they support shallow soils (which may result from erosion) limits the re-colonization by competing trees at low altitudes, which may benefit P. fruticosa.
The basophilous characteristics of the Caricetum frigidae were confirmed by the high frequency of several ingressive species belonging to the Seslerietea albicantis, indicating the presence of calcareous rocks in the study area or calcium in the soil solution, although topsoil reaction was slightly acidic (according to Soil Survey Staff Citation1999). The equilibrium in occurrence and abundance between characteristic species of the Calluno–Ulicetea (mainly Nardetalia) and Molinio–Arrhenatheretea classes highlighted a transition between oligotrophic and eutrophic conditions that characterizes the communities with low primary productivity included in the Caricion davallianae (Hájek and Hájková Citation2011; Biondi et al Citation2010).
Potentilla fruticosa occasionally occurred on dry sites (Group 1, Centaureo uniflorae–Festucetum spadiceae association), probably due to the presence of nearby populations established in optimal habitat. The presence of several ingressive species belonging to Festuco–Brometea class (e.g., Stipa pennata, Armeria plantaginea) also indicates very dry conditions. The ecological tolerance of P. fruticosa is confirmed by Elkington and Woodell (Citation1963), who observed good growth rates under garden conditions and low rainfall, and in well-drained soil without extra watering. Similarly, in dry conditions (Group 1), we observed vegetative growth (i.e. length of vegetative annual shoots) to be not significantly different from the populations ascribable to the Caricetum frigidae (Group 2 and 3).
Many studies have reported negative effects of small population size on species survival (Ouborg Citation1993; Fischer and Stöcklin Citation1997). Matthies et al. (Citation2004) indicated that the probability of survival for many perennial species increased significantly with population size and that very small populations with fewer than 26 plants were doomed to extinction. In the study area, six of the seven populations of P. fruticosa included a very small number of individuals (equal to or fewer than 20), and only POP7 could be considered a large viable population (about 18,000 individuals). The high proportion of very small populations probably jeopardizes conservation of P. fruticosa. Additionally, in long-lived species like P. fruticosa, the negative consequences of reduced population size and increased isolation may not become noticeable for a long time, because established plants often have low mortality (Oostermeijer, Van’t Veer, and Den Nijs Citation1994; Colling, Matthies, and Reckinger Citation2002; Matthies et al. Citation2004).
Several studies have shown that isolation and habitat fragmentation can decrease seed production as a result of pollination limitation in both self-incompatible and self-compatible species (Aizen and Feinsinger Citation1994; Cunningham Citation2000; Aguilar and Galetto Citation2004; Kolb Citation2005). In P. fruticosa, a strong decrease in potentially viable seed production was observed in isolated patches, probably as the result of the combined effects of small numbers of individuals (Kolb and Lindhorst Citation2006) and the self-incompatibility that characterized hermaphroditic accessions (Innes and Lenz Citation1991). The regression analyses showed that starting at a distance of 300–500 m from the nearest patch, potentially viable seed set was reduced to zero, but a more detailed experimental approach is probably needed to identify pollination-limiting distances. However, clonality may promote vegetative reproduction, thereby decreasing population extinction risk and promoting long-term persistence even in isolated populations (Stöcklin and Fischer Citation1999).
Under favourable environmental conditions, clonal reproduction in P. fruticosa enabled small populations and individual genets to persist for a very long time. Spread of Alnus viridis, particularly in low-altitude populations (subalpine belt), may be a possible threat for P. fruticosa survival, because of its intolerance to shade (Elkington and Woodell Citation1963). A number of ingressive species belonging to the Mulgedio–Aconitetea class indicated close dynamic relations to A. viridis stands. In addition, the presence of many characteristic species of the Querco–Fagetea (mainly belonging to Fagetalia sylvaticae) evidenced potential tree invasion processes, particularly at the lower altitudes. Conversely, at high altitudes the local dominance of the dwarf shrub Juniperus nana limited the vegetative spread of P. fruticosa from its external prostate branches. Juniperus nana was locally abundant within studied populations and was frequently observed together with other woody ingressive species belonging to Loiseleurio–Vaccinietea class.
Since the presence of P. fruticosa is related to the openness of vegetation, the positive effects of grazing on shrub control (Ascoli et al. Citation2013; Probo et al. Citation2013) may be very important for P. fruticosa conservation. In Britain and Ireland, P. fruticosa is generally avoided by grazing stock, particularly where more palatable species are available (Elkington and Woodell Citation1963). During our study, we observed only minor damage to fruits in a plot grazed by wild ungulates. Only POP7 was located in an area grazed by sheep, and the high number of P. fruticosa plants seems to indicate a positive effect of extensive grazing. All other populations were located in abandoned grasslands, where trees and shrubs have extensively recolonized open herbaceous habitats. A continuing decline in the quality and extent of habitat is therefore expected and may be a potential threat for P. fruticosa conservation. General trends showed that on the Italian side of the Maritime Alps, socio-economic changes have affected traditional livestock farming systems over the last 40 years, with the number of livestock farms and the pasture area decreasing by 70% between 1970 and 2010 (Valle Citation2013). This pastoral abandonment has probably compounded the fragmentation of Caricetum frigidae, which was already naturally very fragmented in the study area, due to its dependence on proximity to small streams.
IUCN assessment and implication for species conservation
The geographical range of P. fruticosa in the Italian Alps is very restricted, estimated at around 16 km2 (EOO). According to the IUCN categories and criteria (IUCN Citation2001, 2012a) and the most recent guidelines for their application (IUCN Citation2013), taking the criterion B1ab(iii) into account (EOO < 100 km2, taxon severely fragmented and continuing to decline in habitat quality due to grazing abandonment), we preliminarily categorized P. fruticosa into the Critically Endangered (CR) IUCN category. At regional/national levels, due to the isolation from the neighbouring French populations caused by the orography of the Alps, immigration of propagules from neighbouring regions is not expected to be significant. Under this criterion, the category was not up- or down-listed at regional/national levels [CR B1ab(iii)] (IUCN Citation2012b). A future revision of the old comprehensive Italian Red List of threatened plants (Conti, Manzi, and Pedrotti Citation1997) that categorized the species in the LR – Lower Risk category, should consider moving P. fruticosa to a higher threat category (CR – Critically Endangered).
Actions that could be implemented for the conservation of P. fruticosa in the Italian Alps include:
shrub clear cutting close to actual known populations, especially at low altitudes,
defining a favourable stocking rate and monitoring grazing effects. Extensive grazing is expected to have a positive effect on nutrient balance if herbage removal exceeds dung deposition, with a slight decrease in nutrient availability favourable to the oligotrophic species belonging to the Caricion davallianae alliance (Hájek and Hájková Citation2011). On the contrary, the presence of night pens could increase nitrophilous species and contribute to habitat loss. Additionally, trampling and seed transport may have contrasting effects depending on the grazing intensity (Ascoli et al. Citation2013; Probo et al. Citation2013; Tocco et al. Citation2013),
ex situ conservation, which might be facilitated by the species adaptability to garden conditions at low altitude (Elkington and Woodell Citation1963; Pascale and Lonati, pers. obs.), and
restocking of small isolated populations, where seed production is inconsistent (IUCN Citation2012c, Pérez, Anadón, and Díaz Citation2012).
Author contribution
All authors contributed equally to this work.
Notes on contributors
Michele Lonati is Professor of Botany at the University of Torino. His main expertise lies in the areas of applied botany, phytosociology, vegetation characterization and vegetation dynamics.
Marziano Pascale is a botanist. He is the Editor of the Note floristiche Piemontesi edited by the Museum of Natural Science of Carmagnola.
Beatrice Operti is a graduate in Forestry Science at the University of Torino, presently working in her organic farm.
Giampiero Lombardi is Professor of Alpine Rangeland Management and Ecology at the University of Torino. His main expertise lies in the areas of alpine grazing system ecology, grazing-land management and vegetation dynamics in relation to management changes.
Supplementary material
Download PDF (563.7 KB)SUPPLEMENTAL_MATERIAL_TABLE1-2.doc
Download MS Word (66.5 KB)Acknowledgements
We thank Paolo Molinaro for field work and Patrizia Rossi and the Parco delle Alpi Marittime for providing logistic and technical support to this study. The authors would also like to thank Lisa Bush for the English revision and two Journal referees for their comments on the manuscript.
References
- Aeschimann, D., K. Lauber, M.D. Moser, and J.P. Theurillat. 2004. Flora alpina. Bologna: Zanichelli.
- Aguilar, R., and L. Galetto. 2004. “Effects of forest fragmentation on male and female reproductive success in Cestrum parqui (Solanaceae).” Oecologia 138: 513–520.
- Aguilar, R., M. Quesada, L. Ashworth, Y. Herrerias-Diego, and J. Lobo. 2008. “Genetic consequences of habitat fragmentation in plant populations: susceptible signals in plant trait and methodological approaches.” Molecular Ecology 17: 5177–5188.
- Aizen, M.A., and P. Feinsinger. 1994. “Forest fragmentation, pollination, and plant reproduction in a chaco dry forest, Argentina.” Ecology 75: 330–351.
- Ascoli, D., M. Lonati, R. Marzano, G. Bovio, A. Cavallero, and G. Lombardi. 2013. “Prescribed burning and browsing to control tree encroachment in southern European heathlands.” Forest Ecology and Management 289: 69–77.
- Austin, M.P. 1999. “The potential contribution of vegetation ecology to biodiversity research.” Ecography 22 (5): 465–484.
- Biancotti, A., G. Bellardone, S. Bovo, B. Cagnazzi, L. Giacomelli, and C. Marchisio. 1998. Distribuzione regionale di piogge e temperature [Regional distribution of rainfall and temperature]. Torino: Cima Icam.
- Biondi, E., C. Blasi, S. Burrascano, S. Casavecchia, R. Copiz, E. Del Vico, D. Galdenzi, et al. 2010. Manuale italiano di interpretazione degli habitat Direttiva 92/43/CEE [Italian handbook for the interpretation of the habitats 92/43/EEC]. Roma: Progetto Artiser.
- Bono, G. 1965. “La Valle Gesso e la sua vegetazione (Alpi Marittime). La Flora [The Valle Gesso and its vegetation. The Flora].” Webbia 20 (1): 1–216.
- Braun-Blanquet, J. 1932. Plant sociology. New York and London: McGraw-Hill Book Company.
- Braun-Blanquet, J. 1948. La végétation alpine des Pyrénées orientales. Manresa: Imp. Ramon Torra S.C.
- Bruun, H.H. 2000. “Deficit in community species richness as explained by area and isolation of sites.” Diversity and Distribution 6: 129–135.
- Burnat, E., J. Briquet, and F. Cavillier. 1892–1931. Flore des Alpes Maritimes [Flora of Maritimes Alps]. Genéve: H. Georg.
- Cavallero, A., P. Aceto, A. Gorlier, G. Lombardi, M. Lonati, B. Martinasso, and C. Tagliatori. 2007. I tipi pastorali delle Alpi piemontesi [Pasture vegetation types of Piemontese Alps]. Bologna: Alberto Perdisa Editore.
- Chang, C., P. Lee, M. Bai, and T. Lin. 2004. “Predicting the geographical distribution of plant communities in complex terrain: a case study in Fushian Experiment Forest, northeast Taiwan.” Ecology 27: 577–588.
- Colling, G., D. Matthies, and C. Reckinger. 2002. “Population structure and establishment of the threatened long-lived perennial Scorzonera humilis in relation to environment.” Journal of Applied Ecology 39: 310–320.
- Conti, F., A. Manzi, and F. Pedrotti. 1997. Liste rosse regionali delle piante d’Italia [Regional Red List of Italian plants]. Camerino: Università di Camerino.
- Cunningham, S.A. 2000. “Depressed pollination in habitat fragments causes low fruit set.” Proceedings of the Royal Society B 267: 1149–1152.
- Dvornyk, V. 2001. “Genetic variability and differentiation of geographically marginal Scots pine populations from Ukraine.” Silvae Genetica 20: 64–65.
- Elkington, T.T., and S.R.J. Woodell. 1963. “Biological Flora of the British Isles: Potentilla fruticosa L.” Journal of Ecology 53: 769–781.
- Elkington, T.T. 1969. “Cytotaxonomic variation in Potentilla fruticosa L.” New Phytologist 68: 151–160.
- Fischer, M., and J. Stöcklin. 1997. “Local extinctions in remnants of extensively used calcareous grassland 1950–1985.” Conservation Biology 11: 727–737.
- Frankham, R., and K. Ralls. 1998. “Conservation biology – inbreeding leads to extinction.” Nature 392: 441–442.
- Gapare, W.J., and S.N. Aitken. 2005. “Strong spatial genetic structure in peripheral but not core populations of sitka spruce [Picea sitchensis (Bong.) Carr.].” Molecular Ecology 14: 2659–2667.
- Garcia-Ramos, G., and M. Kirkpatrick. 1997. “Genetic models of adaptation and gene flow in peripheral populations.” Evolution 51: 21–28.
- Gargano, D. 2011. “Verso la redazione di nuove Liste Rosse della flora d’Italia: una griglia standard per la misura dell’Area of Occupancy (AOO) [Towards the preparation of a new Red List of Italian flora: a standard grid to measure the Area of Occupancy (AOO)].” Informatore Botanico Italiano 43: 455–458.
- Grabherr, G., and L. Mucina. 1993. Die Pflanzengesellschaften Österreichs 2, Natürliche waldfreie Vegetation [The plant communities of Austria 2, Natural vegetation]. Jena: G. Fischer.
- Hájek, M., and P. Hájková. 2011. Caricion davallianae Klika 1934. In Vegetace České republiky. 3. Vodní a mokřadní vegetace [Vegetation of the Czech Republic. 3. Aquatic and wetland vegetation], edited by M. Chytrý, 619–623. Praha: Academia.
- Hanski, I., M. Kuussaari, and M. Nieminen. 1994. “Metapopulation structure and migration in the butterfly Melitaea cinxia.” Ecology 75 (3): 747–762.
- Hölzel, N. 2003. “Re-assessing the ecology of rare flood-meadow violets (Viola elatior, V. pumila and V. persicifolia) with large phytosociological data set.” Folia Geobotanica 38: 281–298.
- Honnay, O., and B. Bossuyt. 2005. “Prolonged clonal growth: escape route or route to extinction?” Oikos 108: 427–432.
- Hunter Jr, M.L., and A. Hutchinson. 1994. “The virtues and shortcomings of parochialism: conserving species that are locally rare, but globally common.” Conservation Biology 8: 1163–1165.
- Innes, R.L., and L.M. Lenz. 1991. “A genetic analysis of self-incompatibility and double flowers in Potentilla fruticosa L.” Euphytica 51: 241–248.
- IUCN. 2001. IUCN Red List categories and criteria: version 3.1. Gland and Cambridge: IUCN Species Survival Commission.
- IUCN. 2012a. IUCN Red List categories and criteria: version 3.1. Second edition. Gland and Cambridge: IUCN Species Survival Commission.
- IUCN. 2012b. Guidelines for application of IUCN Red List criteria at regional and national levels: version 4.0. Gland and Cambridge: IUCN Species Survival Commission.
- IUCN. 2012c. IUCN Guidelines for reintroductions and other conservation translocations. Cambridge: IUCN Species Survival Commission.
- IUCN. 2013. Guidelines for using the IUCN Red List categories and criteria: version 10.1. IUCN Accessed 10 September 2013. http://www.iucnredlist.org/documents/RedListGuidelines.pdf
- Kolb, A. 2005. “Reduced reproductive success and offspring survival in fragmented populations of the forest herb Phyteuma spicatum.” Journal of Ecology 93: 1226–1237.
- Kolb, A., and S. Lindhorst. 2006. “Forest fragmentation and plant reproductive success: a case study in four perennial herbs.” Plant Ecology 185: 209–220.
- Kuss, P., A.R. Pluess, H.H. Ægisdóttir, and J. Stöcklin. 2008. “Spatial isolation and genetic differentiation in naturally fragmented plant populations of the Swiss Alps.” Plant Ecology 1 (3): 149–159.
- Leimu, R., P. Mutikainen, J. Koricheva, and M. Fisher. 2006. “How general are positive relationships between plant population size, fitness and genetic variation?” Journal of Ecology 94: 942–952.
- Leppig, G., and J.W. White. 2006. “Conservation of peripheral plant populations in California.” Madroño 53 (3): 264–274.
- Lesica, P., and F.W. Allendorf. 1995. “When are peripheral populations valuable for conservation?” Conservation Biology 9: 753–760.
- Lesica, P., and B. McCune. 2004. “Decline of arctic-alpine plants at the southern margin of their range following a decade of climatic warming.” Journal of Vegetation Science 15: 679–690.
- Lonati, M., A. Gorlier, and G. Lombardi. 2011. “Syntaxonomy and synecology of Hedysarum brigantiacum communities in the western Italian Alps.” Acta Botanica Gallica 158 (4): 473–486.
- Lonati, M., and C. Siniscalco. 2009a. “Populations status, syntaxonomy and synecology of Scopolia carniolica Jacq. in the Western Alps (Piedmont, Italy).” Acta Botanica Gallica 156 (2): 245–258.
- Lonati, M., and C. Siniscalco. 2009b. “Syntaxonomy, synecology and conservation of Pseudostellaria europaea Schaeftlein communities in NW Italy in comparison with Estern Alps populations.” Plant Biosystem 143 (1): 120–136.
- Lonati, M., and C. Siniscalco. 2010. “Syntaxonomy and synecology of Erica cinerea L. communities in the Alps (north-western Italy).” Acta Botanica Gallica 157 (3): 493–504.
- Lu, Y., D.M. Waller, and P. David. 2005. “Genetic variability is correlated with population size and reproduction in American wild-rice (Zizania palustris var. palustris, Poaceae) populations.” American Journal of Botany 92 (6): 990–997.
- Matthies, D., I. Braüer, W. Maibom, and T. Tscharntke. 2004. “Population size and the risk of local extinction: empirical evidence from rare plants.” Oikos 105: 481–488.
- Mott, C.L. 2010. “Environmental constraints to the geographic expansion of plant and animal species.” Nature Education Knowledge 3 (10): 72.
- Mucina, L., G. Grabherr, and T. Ellmauer. 1993. Die Pflanzengesellschaften Österreichs 1, Anthropogene Vegetation [The plant communities of Austria 1, Anthropogenic vegetation]. Jena: G. Fischer.
- Norusis, M.J. 2005. SPSS 13.0 Statistical Procedures Companion. Chicago, IL: SPSS Inc.
- Oberdorfer, E. 1983. Pflanzensoziologische Excursionflora [Phytosociological Flora]. Stuttgart: E. Ulmer.
- Oostermeijer, J.G.B., R. Van’t Veer, and J.C.M. Den Nijs. 1994. “Population structure of the rare, long-lived perennial Gentiana pneumonanthe in relation to vegetation and management in the Netherlands.” Journal of Applied Ecology 31: 428–438.
- Ouborg, N.J. 1993. “Isolation, population size and extinction: the classical and metapopulation approaches applied to vascular plants along the Dutch Rhine system.” Oikos 66: 298–308.
- Ozenda, P. 1985. La végétation de la chaîne alpine dans l’espace montagnard européen [The vegetation of the Alps within the European mountains]. Paris: Masson.
- Pascale, M. 2006. “Nuove stazioni di alcune specie di Fanerogame rare nelle Alpi cuneesi (Piemonte, Italia Nord-occidentale) [New finds of some rare Angiosperms in the Alps of Cuneo, Piedmont, north-western Italy].” Bollettino Museo Regionale Scienze Naturali Torino 23: 730–731.
- Pérez, I., J. Anadón, and M. Díaz. 2012. “What is wrong with current translocations? A review and a decision-making proposal.” Frontiers in Ecology and the Environment 10: 494–501.
- Pignatti, S. 1982. Flora d’Italia. Bologna: Edagricole.
- Pluess, A.R., and J. Stöcklin. 2004. “Population genetic diversity of the clonal plant Geum reptans (Rosaceae) in the Swiss Alps.” American Journal of Botany 91(12): 2013–2021.
- Probo, M., A. Massolo, M. Lonati, D.W. Bailey, A. Gorlier, L. Maurino, and G. Lombardi. 2013. “Use of mineral mix supplements to modify the grazing patterns by cattle for the restoration of sub-alpine and alpine shrub-encroached grasslands.” Rangeland Journal 35 (1): 85–93.
- Quantum Gis Development Team. 2012. Quantum GIS Geographic Information System. Open Source Geospatial Foundation Project. Accessed 15 June 2012. http://qgis.osgeo.org
- Rossi, G., C. Montagnani, D. Gargano, T. Abeli, S. Ravera, A. Cogoni, G. Fenu, et al. 2013. Lista rossa della Flora italiana. 1. Policy species e altre specie minacciate [Red List of Italian Flora. Status and other threatened species]. Comitato Italiano IUCN e Ministero dell’Ambiente e della Tutela del Territorio e del Mare. Roma: Stamperia Romana.
- Rossi, G., C. Montagnani, T. Abeli, D. Gargano, L. Peruzzi, G. Fenu, S. Magrini, et al. 2013. “Are Red Lists useful for plant conservation? The New Red List of the Italian Flora in the perspective of National Conservation policies.” Plant Biosystems. doi:10.1080/11263504.2013.868375.
- SILENE. 2013. Système d’Information et de Localisation des Espèces Natives et Envahissantes – Cartographie [Information and Localisation System of Native and Exotic Invasive Species - Cartography]. Accessed 10 September 2013. http://flore.silene.eu/index.php?cont=accueil
- Soil Survey Staff. 1999. Soil taxonomy: a basic system of soil classification for making and interpreting soil surveys. Washington: US Government Printing Office.
- Soliani, L. 2004. Manuale di statistica per la ricerca e la professione [Handbook of Statistics for research and profession]. Parma: Uni Nova.
- Stöcklin, J., and M. Fischer. 1999. “Plants with longer-lived seeds have lower local extinction rates in grassland remnants 1950-1985.” Oeocologia 120: 539–543.
- Theurillat, J.P., D. Aeschimann, P. Küpfer, and R. Spichiger. 1994. “The higher vegetation units of the Alps.” Colloques Phytosociologiques 23: 190–239.
- Tocco, C., M. Probo, M. Lonati, G. Lombardi, M. Negro, B. Nervo, A. Rolando, and C. Palestrini. 2013. “Pastoral practices to reverse shrub encroachment of sub-alpine grasslands: dung beetles (Coleoptera, Scarabaeoidea) respond more quickly than vegetation.” PLoS ONE 8 (12): e83344. doi:10.1371/journal.pone.0083344.
- Valle, M. 2013. Spazio transfrontaliero Marittime Mercantour. La diversità naturale e culturale al centro dello sviluppo sostenibile e integrato del territorio [Transboarder coastal Mercantour space. Natural and cultural diversity in the centre of sustainable and integrated development]. Beinasco: AGIT MARIOGROS.
- van der Maarel, E. 1979. “Trasformation of cover-abundance values in phytosociology and its effects on community similarity.” Vegetatio 39: 97–144.
- van Rossum, F., X. Vekemans, E. Gratia, and P. Meerts. 2003. “A comparative study of allozyme variation in peripheral and central populations of Silene nutans L. (Caryophyllaceae) from western Europe: implications for conservation.” Plant Systematics and Evolution 242: 49–61.
- Weber, H.E., J. Moravec, and J.P. Theurillat. 2000. “International code of phytosociological nomenclature.” Journal of Vegetation Science 11: 739–768.
- Young, A., T. Boyle, and T. Brown. 1996. “The population genetic consequences of habitat fragmentation for plants.” Trends in Ecology & Evolution 11: 413–418.
Appendix I.
Date and accidental species of relevés (Table )
PL01: 29/06/2010; Asphodelus albus (1), Aster alpinus (1), Astragalus monspessulanum (1), Alchemilla alpina s.l. (+), Arabis brassica (+), Arabis hirsuta (+), Centaurea triumfettii (+), Cerastium arvense (+), Cruciata glabra (+), Dianthus sylvestris (+), Erysimum jugicola (+), Hieracium sylvaticum (+), Laburnum alpinum (+), Leucanthemum coronopifolium (+), Lychnis flos-jovis (+), Myosotis alpestris (+), Sempervivum montanum (+), Silene rupestris (+). PL02: 19/07/2010; Alchemilla alpina s.l. (+), Astragalus monspessulanus (+), Gnaphalium norvegicum (+), Myosotis alpestris (+), Platanthera chlorantha (+), Ranunculus montanus (+), Sedum annuum (+), Silene rupestris (+). PL03: 26/07/2010; Achillea erba-rotta (+), Aster alpinus (+), Rubus fruticosus (+), Saxifraga aspera (+), Saxifraga stellaris (+), Sedum annuum (+), Silene rupestris (+), Viola rupestris (+). PL04: 26/07/2010; Agrostis alpina (+), Saxifraga stellaris (+). PL05: 26/07/2010; Polystichum lonchitis (+), Potentilla crantzii (+), Soldanella alpina (+). PL06: 26/07/2010; Cirsium spinosissimum (+), Potentilla crantzii (+), Sedum anacampseros (+), Soldanella alpina (+). PL07: 26/07/2010; Silene rupestris (1), Agrostis alpina (+), Aster alpinus (+), Luzula sylvatica (+), Melica nutans (+), Potentilla crantzii (+), Sempervivum arachnoideum (+). PL08: 11/08/2010; Alchemilla alpina s.l. (+), Athyrium filix-fcemina (+), Cirsium spinosissimum (+), Polystichum lonchitis (+). PL11: 29/06/2010; Carex flacca (2), Cruciata glabra (+), Leucanthemum coronopifolium (+), Linum catharticum (+), Silene rupestris (+), Solidago virgaurea (+), Sorbus aucuparia (+). PL09: 11/08/2010; Athyrium filix-fœmina (+), Polystichum lonchitis (+), Potentilla crantzii (+), Ranunculus montanus (+), Sempervivum arachnoideum (+), Solidago virgaurea (+). PL10: 10/08/2010; Potentilla crantzii (1), Ranunculus montanus (1), Agrostis tenuis (+), Alchemilla alpina s.l. (+). PL12: 29/06/2010; Carex flacca (1), Lamium garganicum (1), Asphodelus albus (2), Alchemilla alpina s.l. (+), Arabis brassica (+), Cerastium arvense (+), Cruciata glabra (+), Fragaria vesca (+), Geum rivale (+), Hieracium sylvaticum (+), Leucanthemum coronopifolium (+), Linum catharticum (+), Lychnis flos-jovis (+), Mentha longifolia (+), Pulmonaria picta (+), Saxifraga stellaris (+), Senecio fuchsii (+). PL13: 13/07/2010; Clematis alpina (1), Carex flacca (+), Lonicera sp. (+), Saxifraga aspera (+), Sedum anacampseros (+), Solidago virgaurea (+), Tulipa australis (+).