Abstract
The water relations of showy laminar floral tissues (petals and tepals) were studied in 20 Mediterranean plants successively blossoming under ambient conditions. The water potential and osmotic potential of floral tissues decline according to the succession of the median day of flowering of the selected plant species. The highest (least negative) value of floral water potential (–0.32 MPa), among the examined species, was measured in petals of Anemone coronaria in March, and the lowest value (–1.25 MPa) in petals of Coridothymus capitatus in June. Low values of water potential of floral tissues coincided with constraints in declining values of osmotic potential at the onset of the dry period in the Mediterranean region; this apparently resulted in a reduction of turgor of floral tissues of Mediterranean plants subjected to water shortage. The reduced osmotic potential was correlated with enhanced soluble sugar content of floral tissues, presumably contributing to the expansion and water status of flowers under water scarcity, by decreasing water requirements.
Introduction
Flowers are among the most spectacular products of nature; their seasonal advertisement and life-span are essential for plant reproduction. Blossoming of plants is regulated by mechanisms that act to ensure that flower emergence occurs in suitable environmental conditions (Barrett Citation2010; Tooke and Battey Citation2010). Emerging flowers, which exhibit almost complete absence of sclerotic elements, have evolved mechanisms that require water supply in order to expand (Darlington and Dixon Citation1991; Lambrecht Citation2013). In the Mediterranean region, the main flowering period coincides with spring; induction of flowers before the dry summer may be interpreted as an evolved response of plants to predictably unfavourable environmental conditions when water is scarce (Bosch, Retana, and Cerda Citation1997; Tooke and Battey Citation2010; Argiropoulos and Rhizopoulou Citation2012; Scaven and Rafferty Citation2013; Farré-Armengol et al. Citation2014; Petanidou et al. Citation2014). Also, blossoming during spring appears to be an adaptation that diminishes the risk of floral senescence before fertilization. Although water availability has been identified as the main factor controlling anthesis of plant species (Skirycz and Inzé Citation2010), early summer blooming seems to be advantageous for some native Mediterranean plants, because they may benefit from the abundant pollinators and avoid competition for pollination that is prominent during spring (Voliotis Citation1984; Herrera Citation1992; Kigel et al. Citation2011). Furthermore, flowering during the drought period implies the ability of plant species to withstand water stress (Rhizopoulou et al. Citation2006; Chimona et al. Citation2012; Argiropoulos and Rhizopoulou Citation2013; Claeys and Inzé Citation2013). Summer water deficit is considered the main environmental constraint for plant growth in Mediterranean-type ecosystems; as water availability seasonally declines, it is reasonable to hypothesize that floral tissues of later-flowering species experience lower water potential and require lower osmotic potential to maintain turgor.
The objective of this study was to investigate components of the water relations of the visually striking floral tissues, i.e. petals (in dicotyledons) and tepals (in monocotyledons) of successively blossoming Mediterranean plants. Water flux from the soil to floral tissues is driven by water potential gradients; the water potential of floral tissues is related to the water status of the xylem that supplies water from the roots to the above-ground plant tissues (Forterre Citation2013). Earlier investigators studied the water relations of flowers of plant species mainly grown under controlled conditions (Trolinder, McMichael, and Upchurch Citation1993; Westgate, Passioura, and Munns Citation1996; Chapotin et al. Citation2003; field, Chatelet, and Brodribb Citation2009; van Doorn Citation2012). To the best of our knowledge, the components of water relations of petals and tepals of flowers and florets from naturally grown, native Mediterranean plants have not hitherto been investigated.
Material and methods
Twenty Mediterranean plants belonging to 16 families (Bremer et al. Citation2009; Chase and Reveal Citation2009) and growing in a nature reserve in the Botanic Garden of Diomedes (38°00’ N, 23°38’ E) were selected for the measurements of floral water status. The Botanic Garden of Diomedes is an asset of the University of Athens (Greece) that displays many elements of the Mediterranean ecosystems. The garden covers 460 acres, of which only 50 acres have been dedicated to the cultivation of ornamental plants. Landscape construction was based on the designs of Professor Herta Hammerbacher (1900–1985) of the University of Berlin (Hottentrager Citation1992), under the strict condition that the impact of landscape shaping on the natural ecosystem should be kept to a minimum, to conserve and protect native Mediterranean plants and habitats (Sarlis Citation1998; Potts et al. Citation2006; Rhizopoulou et al. Citation2012; Heywood Citation2014).
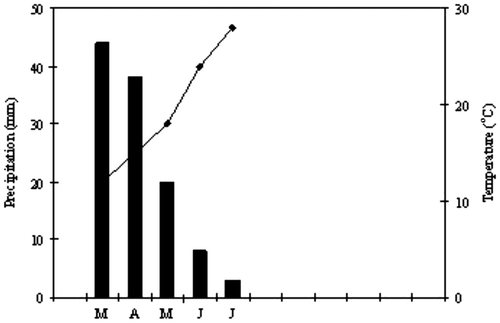
The study was conducted in the naturally occurring stands of wild shrubs and herbs in the Botanic garden of Diomedes (Rhizopoulou Citation2007; Rhizopoulou et al. Citation2012) and detailed field observations were made on a monthly basis between 2011 and 2012. The selected plants are growing under natural conditions in the Botanic Garden and are not artificially irrigated. Average monthly precipitation ranged from 44 to 3 mm and monthly temperature from 12 to 28°C between March and June, respectively, i.e. during the study period of two consecutive years (Figure ). Time and duration of flowering of all species were monitored over the two years (2011–2013); for each species, a number of individual flowers (>35) was observed from the bud to the open-corolla stage and then to wilting. Median day of flowering, which is expressed as a Julian date, was estimated on flowers per individual plant (floral stems × number of flowers per stem), i.e. 20 flowers on each of the 31 different individual plants, for each species, selected at random.
Figure 1. Climatic data of the research area during the study. The order of months is from March to July and the values represent the averages of the two years of the study; continuous line represents mean monthly temperature and bars represent monthly precipitation.
Predawn measurements of water relations were made on expanded floral tissues, i.e. during the first day of fully expanded floral corollas; 10 specimens were harvested from randomly selected plants of each species. The water potential (Ψ) was measured on discs of floral tissues (Richter Citation1997; Rhizopoulou et al. Citation2006) using C-52 sample chambers, 6 mm in diameter (Wescor Inc., Logan, UT, USA), attached to a Dew point psychrometer (HR-33T, Wescor Inc., Logan, UT, USA). The osmotic potential (Ψs) was measured on the same discs after freezing in liquid nitrogen and thawing, using the same technique (Grange Citation1983; Oosterhuis and Wullschleger Citation1989). Turgor was calculated as the algebraic difference between Ψ and Ψs. The reported values for Ψ and Ψs are means of five determinations ± standard error (SE).
Soluble sugars were extracted from dried, powdered floral material with acetone and were quantified colorimetrically in triplicate samples, according to the phenol-sulphuric acid method of Dubois et al. (Citation1956); d-glucose (Serva (Heidelberg, Germany)) solutions were used for the standard curve.
Statistics were assessed with SPSS 12 software package (SPSS, Chicago, IL, USA). Correlations between parameters were tested through linear regressions fitted to the data.
Results
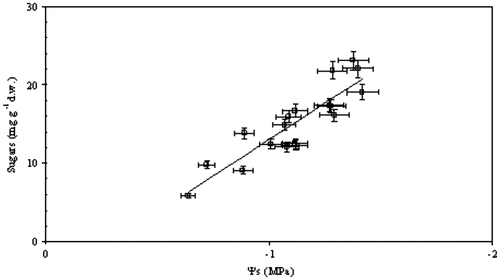
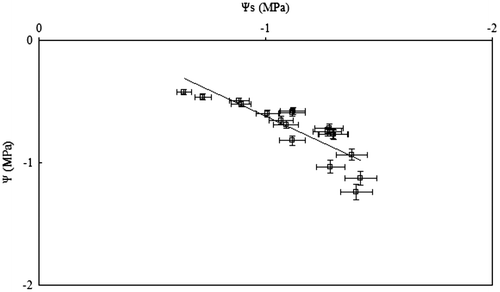
The highest (least negative) value of floral water potential (Ψ), among the 20 Mediterranean plants (Tables and ) was exhibited by Anemone coronaria (Ψ = –0.32 ± 0.05 MPa) in March and the lowest (most negative) value of floral Ψ was found in Coridothymus capitatus (Ψ = –1.25 ± 0.08 MPa) in June. In spring, Ψ of the short-lived petals of Cistus species and Eruca versicaria varied between –0.5 and –0.7 MPa, and the osmotic potential (Ψs) varied from –0.8 to –1.0 MPa; therefore, turgor of petals, representing a prerequisite for expansion of plant tissues, was sustained at approximately 0.30 MPa. Later flowering species exhibited lower perianth Ψ and Ψs in comparison with earlier blossoming species, and median Julian date of flowering was negatively correlated with Ψs (y = –0.006x – 0.14, R2 = 0.52; p = 0.05) and Ψ (y = –0.008x + 0.47, R2 = 0.34; p = 0.05) (Figure , Table ). Ψ of floral tissues reached values that were more negative than –0.7 MPa in Medicago arborea, Satureja thymbra and Ruta chalepensis. The decline in Ψ was correlated with Ψs of floral tissues of the examined species (Figure ). At the start of the dry period, more negative values of Ψ than –1.0 MPa were obtained in floral tissues, as, for example, these of Salvia fruticosa and Teucrium capitatum (Table ). However, in the petals of Capparis spinosa flowers, relatively elevated values of Ψ and Ψs were obtained during the summer drought period (Table ; Figure ). Also, the decline in Ψs of floral tissues of the considered species was substantially linked to the accumulation of soluble sugars in petals and tepals (Figure ). Soluble sugars of floral tissues of the considered species ranged from 7 to 22 mg g−1 dry weight (Table ); the lowest value was detected in the earliest flowering species, Asphodelus ramosus, and the highest value in the late-spring flowering Satureja thymbra (Table ).
Table 1. List of scientific names and families of the Mediterranean plants, presented according to the median day of flowering, expressed in Julian dates (1 for 1 January through 365 for 31 December).
Table 2. Estimates of water (Ψ) and osmotic (Ψs) potential, and soluble sugar content of floral tissues during median day of flowering.
Figure 2. Relationship between water potential (Ψ) and osmotic potential (Ψs) of floral tissues of 20 Mediterranean plants (y = 0.86x + 0.24, R2 = 0.74, p = 0.01); each spot represents mean values ± SE (n = 5).
Discussion
Ψ and Ψs of floral tissues decreased according to the succession of the median day of flowering of the Mediterranean plants. The lowered floral water status results in a gradient of water potential of plant tissues that provides the driving force for water movement along the soil–plant–atmosphere continuum during progressing soil water shortage in the Mediterranean ecosystem. Constraints in Ψs of floral tissues (Figure ) led to an array of declining turgor (being the algebraic difference between Ψ and Ψs) of expanded flowers during a period of water deficit, which can be related to the imposition of constraints to corolla size in the Mediterranean region by the water maintenance costs for flowers (Teixido and Valladares Citation2014a, Citation2014b).
Relatively low values of components of water relations obtained in floral tissues of four species belonging to the family Lamiaceae (Table ) may play a significant role in turgor maintenance and so in advertisement of flowers, represented by the spring blossoming Salvia fruticosa and Teucrium capitatum, and the late spring/early summer flowering Satureja thymbra and Coridothymus capitatus.
At the onset of the drought summer, Ψ of petals reflected the efficiency of plants at withholding water from drying soil. Although water deficiency affected floral water relations of the majority of species (Figure ), the rapidly expanded petals of the large flowers of the deeply rooted, winter deciduous perennial Capparis spinosa exhibited relatively elevated values of Ψ and Ψs during the summer drought period (Table ); the benefit of nocturnal flowering of C. spinosa reduces water loss during the dry period (Rhizopoulou Citation1990; Rhizopoulou, Heberlein, and Kassianou Citation1997; Rhizopoulou and Psaras Citation2003; Chimona et al. Citation2012; Yang et al. Citation2014).
The decline in Ψs of floral tissues, substantially linked to the accumulation of soluble sugars (Figure ), may contribute to turgor maintenance in petals and tepals (Tarpley and Sassenrath Citation2006; Rosas et al. Citation2013; Beauzamy, Nakayama, and Boudaoud Citation2014). Indeed, petal and tepal expansion appears to be due to a pulsed increase in the concentration of soluble sugars (Evans and Reid Citation1986; Bieleski, Elgar, and Heyes Citation2000; van Doorn and van Meeteren Citation2003; van Doorn Citation2004; van Doorn and Kamdee Citation2014), which may be derived by both chlorophyllous parts of floral tissues and surrounding leaves, subsequently reducing the cost of the reproductive process and demand (Aschan and Phanz Citation2003, Citation2006; Rhizopoulou et al. Citation2006; Chimona et al. Citation2012). Also, there appears to be an influence on water uptake via osmotic adjustment; therefore, reduced values of floral Ψs may increase the capacity of Mediterranean plants to extract water from drying soil.
Authors’ contribution
The two authors contributed equally to this work.
Acknowledgements
The research was supported by the National and Kapodistrian University of Athens (ELKE 11238).
References
- Argiropoulos, A., and S. Rhizopoulou. 2012. “Topography and nanosculpture of petals’ surfaces of short-lived flowers of the wild species Cistus creticus, Cistus salviifolius, Eruca sativa and Sinapis arvensis.” Botαnical Studies 53(4): 479–488.
- Argiropoulos, A., and S. Rhizopoulou. 2013. “Morphological features of petals of Nerium oleander L.” Plant Biosystems 147(3): 638–644.10.1080/11263504.2013.763863
- Aschan, G., and H. Pfanz. 2003. “Non-foliar photosynthesis: a strategy of additional carbon acquisition.” Flora-Morphology, Distribution, Functional Ecology of Plants 198(2): 81–79.10.1078/0367-2530-00080
- Aschan, G., and H. Pfanz. 2006. “Why snowdrop (Galanthus nivalis L.) tepals have green marks?.” Flora-Morphology, Distribution, Functional Ecology of Plants 201(8): 623–632.10.1016/j.flora.2006.02.003
- Barrett, S. C. 2010. “Darwin’s legacy: the forms, function and sexual diversity of flowers.” Philosophical Transactions of the Royal Society B: Biological Sciences 365(1539): 351–368.10.1098/rstb.2009.0212
- Beauzamy, L., N. Nakayama, and A. Boudaoud. 2014. “Flowers under pressure: ins and outs of turgor regulation in development.” Annals of Botany 114: 1517–1533.10.1093/aob/mcu187
- Bieleski, R., J. Elgar, and J. Heyes. 2000. “Mechanical aspects of rapid flower opening in Asiatic lily.” Annals of Botany 86(6): 1175–1183.10.1006/anbo.2000.1291
- Bosch, J., J. Retana, and X. Cerda. 1997. “Flowering phenology, floral traits and pollinator composition in an herbaceous Mediterranean plant community.” Oecologia 109(4): 583–591.10.1007/s004420050120
- Bremer, B., K. Bremer, M. W. Chase, M. F. Fay, J. L. Reveal, D. E. Soltis, P. S. Soltis, et al. 2009. “An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III.” Botanical Journal of the Linnean Society 161(2): 105–121.
- Chapotin, S. M., N. M. Holbrook, S. R. Morse, and M. V. Gutierrez. 2003. “Water relations of tropical dry forest flowers: pathways for water entry and the role of extracellular polysaccharides.” Plant Cell and Environment 26(4): 623–630.10.1046/j.1365-3040.2003.00998.x
- Chase, M. W., and J. L. Reveal. 2009. “A phylogenetic classification of the land plants to accompany APG III.” Botanical Journal of the Linnean Society 161(2): 122–127.10.1111/(ISSN)1095-8339
- Chimona, C., A. Stamellou, A. Argiropoulos, and S. Rhizopoulou. 2012. “Study of variegated and white flower petals of Capparis spinosa expanded at dusk in arid landscapes.” Journal of Arid Land 4(2): 171–179.10.3724/SP.J.1227.2012.00171
- Claeys, H., and D. Inzé. 2013. “The agony of choice: how plants balance growth and survival under water-limiting conditions.” Plant Physiology 162(4): 1768–1779.10.1104/pp.113.220921
- Darlington, A. B., and M. A. Dixon. 1991. “The hydraulic architecture of roses (Rosa hybrida).” Canadian Journal of Botany 69(4): 702–710.10.1139/b91-095
- Dubois, M., K. A. Gilles, J. K. Hamilton, P. A. Rebers, and F. Smith. 1956. “Colorimetric method for determination of sugars and related substances.” Analytical Chemistry 28(3): 350–356.10.1021/ac60111a017
- Evans, R. Y., and M. S. Reid. 1986. “Control of petal expansion during diurnal opening of roses.” Acta Horticulturae 181: 55–63.
- Farré-Armengol, G., I. Filella, J. Llusià, U. Niinemets, and J. Peñuelas. 2014. “Changes in floral bouquets from compound-specific responses to increasing temperatures.” Global Change Biology. doi:10.1111/gcb.12628.
- Feild, T. S., D. S. Chatelet, and T. J. Brodribb. 2009. “Giant flowers of southern magnolia are hydrated by the xylem.” Plant Physiology 150(3): 1587–1597.10.1104/pp.109.136127
- Forterre, Y. 2013. “Slow, fast and furious: understanding the physics of plant movements.” Journal of Experimental Botany 64(15): 4745–4760.10.1093/jxb/ert230
- Grange, R. I. 1983. “Solute production during the measurement of solute potential on disrupted tissue.” Journal of Experimental Botany 34(6): 757–764.10.1093/jxb/34.6.757
- Herrera, M. C. 1992. “Individual flowering time and maternal fecundity in a summerflowering Mediterranean shrub: making the right prediction for the wrong season.” Acta Oecologica 13(1): 13–24.
- Heywood, V. H. 2014. “An overview of in situ conservation of plant species in the Mediterranean.” Flora Mediterranea 24: 5–24.10.7320/flmedit01.001
- Hottentrager, G. 1992. “New flowers, new gardens: residential gardens designed by Karl Foerster, Hermann Mattern and Herta Hammerbacher (1928–c.1943).” The Journal of Garden History 12(3): 207–227.10.1080/01445170.1992.10410559
- Kigel, J., I. Konsens, N. Rosen, G. Rotem, A. Kon, and O. Fragman-Sapir. 2011. “Relationships between flowering time and rainfall gradients across Mediterranean-desert transects.” Israel Journal of Ecology & Evolution 57(1–2): 91–109.
- Lambrecht, S. C. 2013. “Floral water costs and size variation in the highly selfing Leptosiphon bicolor (Polemoniaceae).” International Journal of Plant Science 174(1): 74–84.10.1086/668230
- Oosterhuis, D. M., and S. D. Wullschleger. 1989. “Psychrometric water potential analysis in leaf discs.” In: Gases in Plant and Microbial Cells, Modern Methods of Plant Analysis, edited by H.-F. Linskens, and J. F. Jackson, 113–133. Heidelberg: Springer Berlin.
- Petanidou, T., A. S. Kallimanis, S. P. Sgardelis, A. D. Mazaris, J. D. Pantis, and N. M. Waser. 2014. “Variable flowering phenology and pollinator use in a community suggest future phenological mismatch.” Acta Oecologica 59: 104–111.10.1016/j.actao.2014.06.001
- Potts, S. G., T. Petanidou, S. Roberts, C. O’Toole, A. Hulbert, and P. Willmer. 2006. “Plant–pollinator biodiversity and pollination services in a complex Mediterranean landscape.” Biological Conservation 129(4): 519–529.10.1016/j.biocon.2005.11.019
- Rhizopoulou, S. 1990. “Physiological responses of Capparis spinosa L. to drought.” Journal of Plant Physiology 136(3): 341–348.10.1016/S0176-1617(11)80060-X
- Rhizopoulou, S. 2007. The Julia and Alexander Diomedes Botanic Garden. Athens: Diavlos.
- Rhizopoulou, S., and G. Psaras. 2003. “Development and structure of drought-tolerant leaves of the Mediterranean shrub Capparis spinosa L.” Annals of Botany 92(3): 377–383.10.1093/aob/mcg149
- Rhizopoulou, S., K. Heberlein, and A. Kassianou. 1997. “Field water relations of Capparis spinosa L.” Journal of Arid Environment 36(2): 237–248.10.1006/jare.1996.0207
- Rhizopoulou, S., E. Ioannidi, N. Alexandredes, and A. Argiropoulos. 2006. “A study on functional and structural traits of the nocturnal flowers of Capparis spinosa L.” Journal of Arid Environment 66(4): 635–647.10.1016/j.jaridenv.2005.12.009
- Rhizopoulou, S., A. Lykos, P. Delipetrou, and I. Vallianatou. 2012. “Living collection of Flora Graeca Sibthorpiana: from the folios of the monumental edition to the beds of a botanic garden in Greece.” Sibbaldia 10: 171–196.
- Richter, H. 1997. “Water relations of plants in the field: some comments on the measurement of selected parameters.” Journal of Experimental Botany 48(1): 1–7.10.1093/jxb/48.1.1
- Rosas, T., L. Galiano, R. Ogaya, J. Penuelas, and J. Martinez-Vilalta. 2013. “Dynamics of non-structural carbohydrates in three Mediterranean woody species following long-term experimental drought.” Frontiers in Plant Science 4: 400.
- Sarlis, G. P. 1998. “Contribution to the study of the flora of Attica (Greece).” Lagascalia 17: 229–256.
- Scaven, V. L., and N. E. Rafferty. 2013. “Physiological effects of climate warming on flowering plants and insect pollinators and potential consequences for their interactions.” Current Zoology 59(3): 418–426.
- Skirycz, A., and D. Inzé. 2010. “More from less: plant growth under limited water.” Current Opinion in Biotechnology 21(2): 197–203.10.1016/j.copbio.2010.03.002
- Tarpley, L., and G. F. Sassenrath. 2006. “Carbohydrate profiles during cotton floral bud (square) development.” Journal of Agronomy and Crop Science 192(5): 363–372.10.1111/jac.2006.192.issue-5
- Teixido, A. L., and F. Valladares. 2014a. “Disproportionate carbon and water maintenance costs of large corollas in hot Mediterranean ecosystems.” Perspectives in Plant Ecology, Evolution and Systematics 16(2): 83–92.10.1016/j.ppees.2014.02.002
- Teixido, A. L., and F. Valladares. 2014. “Large flowers tend to be short-lived in Mediterranean ecosystems: insights from three Cistus species.” Plant Biosystems. doi:10.1080/11263504.2014.948095.
- Tooke, F., and N. H. Battey. 2010. “Temperate flowering phenology.” Journal of Experimental Botany 61(11): 2853–2862.10.1093/jxb/erq165
- Trolinder, N. L., B. L. McMichael, and D. R. Upchurch. 1993. “Water relations of cotton flower petals and fruit.” Plant Cell and Environment 16(6): 755–760.10.1111/pce.1993.16.issue-6
- van Doorn, W. G. 2004. “Is petal senescence due to sugar starvation?.” Plant Physiology 134(1): 35–42.10.1104/pp.103.033084
- van Doorn, W. G. 2012. “Water relations of cut flowers: an update.” Horticultural Reviews 40: 55–96.10.1002/9781118351871.ch2
- van Doorn, W. G., and C. Kamdee. 2014. “Flower opening and closure: an update.” Journal of Experimental Botany 65: 5749–5757.10.1093/jxb/eru327
- van Doorn, W. G., and U. van Meeteren. 2003. “Flower opening and closure: a review.” Journal of Experimental Botany 54(389): 1801–1812.10.1093/jxb/erg213
- Voliotis, D. 1984. “A phenological study of flowering period and flower colours of aromatic plants in Greece.” Vegetatio 56(3): 129–137.
- Westgate, M. E., J. B. Passioura, and R. Munns. 1996. “Water status and ABA content of floral organs in drought-stressed wheat.” Functional Plant Biology 23(6): 763–772.
- Yang, M., L. Yin, C. Yan, M. L. Zhang, F. Kong, and S. J. Li. 2014. “The characteristic variation of the flowers of Capparis spinosa L. during the extended flowering process and the influence of the rate of seed-setting.” Pakistan Journal of Botany 46(1): 95–100.