Abstract
Plants produce many terpenoids including gibberellins and many commercially important secondary metabolites. The final steps of terpenoid production involve terpene synthase (TPS) enzymes. The origin of plant TPS is not known; searches for TPS showed their presence in all plant groups except algae. Although many plants have several genes in their genome that encode TPS enzymes, the bryophyte Physcomitrella patens (Hedw.) Bruch & Schimp. possesses only one bifunctional ent-kaurene synthase (PpCPS/KS), which produces both 16α-hydroxykaurane and ent-kaurene (the precursor of gibberellins). This protein shares characteristics of two unifunctional TPS of higher plants – ent-copalyl diphosphate synthase (CPS) and ent-kaurene synthase (KS). Bifunctional TPS are also found in fungi. In this study, the bifunctional PpCPS/KS has been characterized by some bioinformatics tools. Comparative analysis of PpCPS/KS with some fungal and plant TPS as well as terpenoid-producing bacterial enzymes has been performed. The results indicate that bifunctional TPS came from fungi to bryophytes, probably by horizontal gene transfer and unifunctional TPS gradually evolved from bifunctional TPS in higher plants.
Introduction
Terpenoids are a large class of compounds that serve multiple roles in plants. They include plant hormone gibberellins as well as compounds defined as specialized metabolites that serve specific physiological and ecological roles in plants, i.e. secondary metabolites (Falara et al. Citation2011). In plants, terpenoids are synthesized by the action of terpene synthases (TPS) (Chen et al. Citation2011). Although many studies have been carried out to reveal the evolutionary history of plant TPS (Trapp and Croteau Citation2001; Hillwig et al. Citation2011; Chen et al. Citation2011), the origin of TPS genes in plants have not been studied in detail. Comparative analysis of TPS from lower plants like bryophytes and algae with TPS from higher plants can throw some light on this aspect. Vascular plants (angiosperms, gymnosperms and pteridophytes) have several genes in their genome that encode TPS (Chen et al. Citation2011). Among bryophytes, the moss Physcomitrella patens (Hedw.) Bruch & Schimp. possesses a bifunctional ent-kaurene synthase (PpCPS/KS) enzyme, which is the only TPS enzyme in this species (Hayashi et al. Citation2006; Chen et al. Citation2011). Studies of eukaryotic algal TPS have not been found in the literature. A preliminary search against NCBI protein database (http://www.ncbi.nlm.nih.gov/protein), Ensembl plants (http://plants.ensembl.org/index.html) and Phytozome (http://www.phytozome.net/) for algal TPS returned no results. A BLAST (Altschul et al. Citation1990) search for ent-copalyl diphosphate synthase and ent-kaurene synthase of Arabidopsis thaliana (L.) Heynh. (AtCPS and AtKS, respectively) against the NCBI non-redundant protein sequence database of algae returned no significant results. In addition to the BLAST searches made with AtCPS and AtKS against protein databases, BLASTn searches for conserved domains of AtCPS and AtKS against the NCBI nucleotide database limited to green algae sequences, including nucleotide collection (nr/nt), EST and transcriptome shotgun assembly, also returned only non-significant results (E-value > 0.1). Hence, among plants, bryophytes are probably the oldest to contain TPS. Characterization of the bifunctional ent-kaurene synthase (PpCPS/KS) of P. patens is therefore necessary when studying the origin of plant TPS. This enzyme produces both 16α-hydroxykaurane and ent-kaurene (Hayashi et al. Citation2006). In higher plants, ent-kaurene is one of the biosynthetic intermediates in the production of gibberellins, although there is no clear evidence of the presence of endogenous gibberellins in moss (Hayashi et al. Citation2006). In flowering plants, ent-kaurene is synthesized by a two-step cyclization from geranylgeranyl diphosphate via copalyl diphosphate by two distinct unifunctional diterpene cyclases namely ent-copalyl diphosphate synthase (CPS) and ent-kaurene synthase (KS) (Hayashi et al. Citation2006). Plant CPS contain an aspartate-rich motif DXDD (Hayashi et al. Citation2006). The second aspartate of this motif acts as the proton donor to initiate the cyclization of geranylgeranyl diphosphate (Sun and Kamiya Citation1994; Hayashi et al. Citation2006). On the other hand, KS have a DDXXD motif (Hayashi et al. Citation2006). This motif initiates the formation of an ent-kaurene skeleton by the cyclization of copalyl diphosphate (Yamaguchi et al. Citation1996, Citation1998; Hayashi et al. Citation2006). This ent-kaurene is a tetracyclic diterpene from which gibberellins are formed (Hayashi et al. Citation2006). However, the bifunctional ent-kaurene synthase from P. patens (PpCPS/KS) produces both ent-kaurene and 16α-hydroxykaurane from geranylgeranyl diphosphate via ent-copalyl diphosphate. This enzyme contains both DXDD and DDXXD motifs, as well as the sequence similarities and activities of both plant CPS and KS (Hayashi et al. Citation2006). Another example of plant bifunctional TPS is abietadiene synthase from Abies grandis (AgAS), a gymnosperm (Vogel et al. Citation1996). Here also, the DXDD motif is conserved in the CPS domain, but the KS domain contains a DDXXD motif. Bifunctional TPS are also found in fungi (Kawaide et al. Citation1997; Kawaide, Sassa, and Kamiya Citation2000; Toyomasu et al. Citation2000; Kawaide Citation2006). Fungal KS in the gibberellin-producing fungi Phaeosphaeria sp. L487 and Gibberella fujikuroi are examples of fungal bifunctional KS (Kawaide et al. Citation1997; Toyomasu et al. Citation2000; Kawaide, Sassa, and Kamiya Citation2000; Kawaide Citation2006). These fungal KS catalyse sequential conversion of copalyl diphosphate to ent-kaurene in a similar manner to PpCPS/KS (Hayashi et al. Citation2006). Moreover, they contain the DXDD motif in the CPS domain and a DEXXEA motif in the KS domain (Hayashi et al. Citation2006). These facts raise the possibility that plant TPS might have originated from fungal bifunctional TPS, probably by horizontal gene transfer. Horizontal gene transfer from fungi as well as from prokaryotes has played important roles in the evolution of land plants (Yue et al. Citation2012). Additionally, it is known that several bacteria also contain CPS and KS, which are found in an operon (Morrone et al. Citation2009). Hence, prokaryotic enzymes that synthesize terpenoids also need to be compared with the PpCPS/KS from P. patens to throw some light on the origin of plant TPS.
The bryophytes and the ancestor of vascular plants diverged more than 400 million years ago in land plant evolution (Nishiyama et al. Citation2003). The moss P. patens is a model organism for the study of the genetics and development of bryophytes (Hayashi et al. Citation2006). As this species is a representative of an early phase in the evolutionary process of land plants, its study can provide new insight into the evolution of different physiological mechanisms of land plants. Hence, characterization of PpCPS/KS of P. patens and comparative study with other plant and fungal TPS as well as bacterial CPS and KS has the potential to emphasize the origin of terpenoid biosynthesis as well as gibberellin biosynthesis in land plants.
In this study, the amino acid sequence of PpCPS/KS of P. patens has been compared with sequences of other plant, fungal and bacterial TPS to answer the question of the origin of TPS in plants. The three-dimensional structure of PpCPS/KS has been modelled by homology modelling for the first time. Additionally, the three-dimensional structure of conifer and fungal bifunctional TPS as well as Arabidopsis CPS and KS has been compared with PpCPS/KS for better understanding of the evolution of this class of enzymes.
Materials and methods
Sequence retrieval and phylogenetic analysis
Primarily, the amino acid sequences of PpCPS/KS and several other sequences from plants, fungi and bacteria were collected from the NCBI protein database (http://www.ncbi.nlm.nih.gov/protein). Two algal genomes – Chlamydomonas reinhardtii and Cyanidioschyzon merolae – were searched using the Ensembl plants database (http://plants.ensembl.org/index.html) for two Pfam domains of the TPS family, the metal binding domain (PF03936) and the TPS N-terminal domain (PF01397), which returned no results. Also, a BLAST (Altschul et al. Citation1990) search with AtCPS and AtKS against the NCBI non-redundant protein sequence database of algae returned no significant results. Hence, the final sequences comprised four plant, two fungal and five bacterial sequences. Details of the proteins are given in Table . Multiple sequence alignment was performed using ClustalW (Larkin et al. Citation2007) and alignments were manually curated using Jalview (Waterhouse et al. Citation2009). Phylogenetic analysis was performed with MEGA 5.0 (Tamura et al. Citation2011) by maximum parsimony and the bootstrap test was carried out with 2000 replicates. The resulting dendrogram was visualized with FigTree downloaded from http://beast.bio.ed.ac.uk/FigTree.
Table 1. Names of different proteins used in this study.
Physicochemical and structural analysis
Different physical and chemical parameters like molecular weight, theoretical ioselectric point (pI), instability index, aliphatic index and grand average of hydropathicity (GRAVY), were measured using ProtParam (Gasteiger et al. Citation2005) (http://web.expasy.org/protparam/). Prediction of helices, sheets and coils in the secondary structure of deduced amino acid sequences was predicted by the Self-optimized prediction method with multiple alignments (SOPMA) (Geourjon and Deléage Citation1995). The presence of different domains was analysed using the Pfam database (http://pfam.sanger.ac.uk/) (Finn et al. Citation2010) and represented with DomainDraw (Fink and Hamilton, Citation2007) server (http://domaindraw.imb.uq.edu.au.).
Homology modelling and structure validation
The three-dimensional crystal structure of AtCPS (Accession No. Q38802, PDB ID: 3PYA) was downloaded from Protein Data Bank (Berman et al. Citation2000). It was ascertained that no three-dimensional structure was available for the other proteins. Hence, the structures were built by homology modelling. Homology modelling was performed using the Phyre2 web server (http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id=index) (Kelley and Sternberg Citation2009). Energy minimizations were performed using GROMOS96 force field (van Gunsteren et al. Citation1996), implemented with Swiss-PDB Viewer (Guex and Peitsch Citation1997). The refined hypothetical protein models generated were subjected to a series of tests for testing the internal stability and reliability by submitting to Structural Analysis and Verification Server (http://nihserver.mbi.ucla.edu/SAVES/). Two programs were used inside this server, namely PROCHECK (Laskowski et al. Citation1993), and Verify3D (Eisenberg, Lüthy and Bowie Citation1997). Backbone conformation was evaluated by the inspection of the Psi/Phi Ramachandran plot obtained from PROCHECK. Verify3D determines the compatibility of an atomic model (three-dimensional) with its own amino acid sequence by assigning a structural class based on its location and environment (alpha, beta, loop, polar, nonpolar etc) and comparing the results to good structures (Bowie, Lüthy, and Eisenberg Citation1991; Lüthy, Bowie and Eisenberg Citation1992). Ramachandran plots were obtained using the RAMPAGE (Lovell et al. Citation2003) server (http://mordred.bioc.cam.ac.uk/~rapper/rampage.php). The models were further checked with the QMEAN server for model quality estimation (http://swissmodel.expasy.org/qmean/cgi/index.cgi) (Benkert, Künzli, and Schwede Citation2009). The overall model quality of the structures was also analysed on the ProSA-web server (https://prosa.services.came.sbg.ac.at/prosa.php) (Sippl Citation1993; Wiederstein and Sippl Citation2007). This program calculates Z-score, which is a measure of compatibility between sequence and structure (Wiederstein and Sippl Citation2007). The model Z-score should be comparable to the Z-scores obtained for the template (Sánchez and Šali Citation2000). ProSA-web also calculates a plot of residue score, which shows local model quality by plotting energies as a function of amino acid sequence position (Wiederstein and Sippl Citation2007). Root mean squared deviation (RMSD) between the template and the models was calculated using the UCSF Chimera package (Pettersen et al. Citation2004). The RMSD indicates the degree to which two three-dimensional structures are similar and the lower the value the greater the similarity between the structures (Syed et al. Citation2012). The visualization of different structures was performed using Chimera package (Pettersen et al. Citation2004).
Analysis of surface properties
The surface hydrophobicity of the three-dimensional structures was compared using PLATINUM server (http://model.nmr.ru/platinum/) (Efremov et al. Citation2007; Pyrkov et al. Citation2009), which has been designed to calculate match or mismatch in receptor–ligand complexes based on molecular hydrophobicity potential (Efremov et al. Citation2007; Pyrkov et al. Citation2009). The total hydrophobic and hydrophilic surfaces for a molecule can be obtained with this tool. The hydrophobic surfaces of the proteins have been viewed with color h script (obtained from http://www.pymolwiki.org/index.php/Color_h) of PyMOL (DeLano Citation2002). The color h script colours the protein surface according to the normalized consensus hydrophobicity scale according to Eisenberg et al. (Citation1984). Electrostatic potentials of the proteins were compared with the web-based version of PIPSA (Protein Interaction Property Similarity Analysis) (Blomberg et al. Citation1999; Wade, Gabdoulline, and De Rienzo Citation2001; Gabdoulline, Stein, and Wade Citation2007; Richter et al. Citation2008). The models were submitted to the PIPSA server (Richter et al. Citation2008), and Adaptive Poisson–Boltzmann Solver (APBS) software (Baker et al. Citation2001) was chosen to calculate the electrostatic potentials. The electrostatic surfaces of the proteins were calculated using APBS using PDB2PQR (Dolinsky et al. Citation2004) server (http://nbcr-222.ucsd.edu/pdb2pqr_1.8/) with default parameters. The electrostatic potential of the molecular surfaces of the proteins were visualized using PyMOL (DeLano Citation2002).
Results
Physicochemical characterization of PpCPS/KS and phylogenetic study
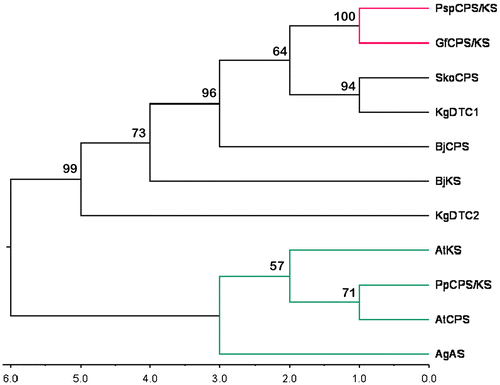
The dendrogram obtained by maximum parsimony (Figure ) clearly showed that bacterial and fungal enzymes grouped together and plant TPS formed one separate cluster. Within the first cluster, two fungal enzymes were grouped together. This combined clade was joined with a clade formed by two prokaryotic enzymes – a Streptomyces sp. KO-3988 CPS (SkoCPS) (Waksman and Henrici 1943) and a terpentedienyl-diphosphate synthase from Kitasatospora griseola (KgDTC1) (Takashi et al. 1985). This showed that these two prokaryotic proteins shared more sequence similarity with the fungal TPS. Among the plant TPS, PpCPS/KS and AtCPS grouped together. AgAS maintained a separate subclade. Hence, although AgAS is a bifunctional TPS, it is not very similar to PpCPS/KS with respect to amino acid sequences.
Figure 1. Maximum parsimony tree of the selected terpene synthase proteins from plants, fungi and bacteria. The tree was built with PpCPS/KS (ent-kaurene synthase from Physcomitrella patens); AtCPS (Arabidopsis thaliana ent-copalyl diphosphate synthase), AtKS (Arabidopsis thaliana ent-kaurene synthase); AgAS (Abies grandis abietadiene synthase); GfCPS/KS (Gibberella fujikuroi ent-kaurene synthase); PspCPS/KS (Phaeosphaeria sp. L487 ent-kaurene synthase); BjCPS (Bradyrhizobium diazoefficiens USDA 110 ent-CPP specific CPS); BjKS (Bradyrhizobium diazoefficiens USDA 110 Kaurene synthase); SkoCPS (Streptomyces sp. KO-3988 ent-copalyl diphosphate synthase); KgDTC1 (Kitasatospora griseola Terpentedienyl-diphosphate synthase) and KgDTC2 (Kitasatospora griseola Terpentetriene synthase). Bootstrap values are given at the branch points of the tree.
The molecular weight, theoretical pI, instability index, aliphatic index and GRAVY of PpCPS/KS and other investigated proteins are given in Table . It shows that the fungal KS were the heaviest of all the proteins investigated. PpCPS/KS was the heaviest among the plant TPS. AgAS, the other bifunctional TPS of plant origin, was smaller than PpCPS/KS. However, the bacterial proteins were much smaller than the plant and fungal proteins. This is in accordance with the length of the proteins (Table ). The theoretical pI of PpCPS/KS and all other plant and fungal TPS were acidic (Table ). However, bacterial proteins showed a range of theoretical pI from basic (for both the CPS (BjCPS) and the KS (BjKS) of Bradyrhizobium diazoefficiens) (Delamuta et al. 2013) to acidic (SkoCPS, KgDTC1 and KgDTC2). The instability index of PpCPS/KS was 47.72, highest among plant TPS investigated in the present study (Table ). Other plant TPS also had instability indices >40. However, the fungal KS had instability indices <40. This index is an estimate of the stability of the protein in a test tube. A protein with an instability index <40 is predicted to be stable whereas a value >40 predicts that the protein may be unstable (Guruprasad, Reddy, and Pandit Citation1990). Among bacterial proteins, SkoCPS had the lowest instability index (36.98). All other bacterial proteins had an instability index >40. The aliphatic index indicates the thermostability of a globular protein (Ikai Citation1980). The aliphatic index of PpCPS/KS was 81.79 (Table ), which is the lowest among all the plant and fungal TPS investigated in this study. The only protein with aliphatic index lower than PpCPS/KS was KgDTC1 (78.32), a bacterial protein. This showed that PpCPS/KS was the least thermostable among the plant and fungal TPS. The GRAVY of PpCPS/KS was highest (–0.248) among the plant proteinsI but was lower than that of fungal TPS. Bacterial enzymes showed a range of GRAVY values (Table ).
Table 2. ProtParam table showing different physicochemical properties of the proteins.
Structural features and domain organization
All of the investigated proteins were predominantly alpha-helical as revealed by SOPMA (Table ); >50% of all the plant and fungal proteins were constituted by alpha-helix. However, plant TPS showed a slightly higher percentage of alpha-helix (57.11–66.62%) than fungal proteins of Gibberella fujikuroi (Sawada) Wollenw. (1931) (GfCPS/KS; 53.05%) and Phaeosphaeria sp. (PspCPS/KS; 56.87%) (I. Miyake). It was also notable that random coils in fungal proteins were more than in plant TPS (Table ).
Table 3. Secondary structural features of the investigated proteins.
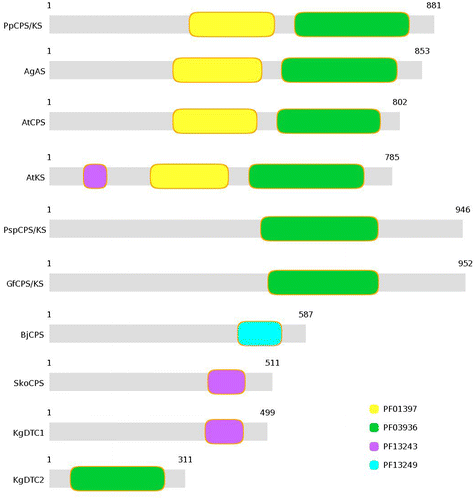
All the investigated plant TPS were characterized by two Pfam domains PF03936 (TPS family, metal-binding domain) and PF01397 (TPS, N-terminal domain) (Figure ). However, the fungal bifunctional KS lack the N-terminal domain (Figure ), as shown by Pfam results. Bacterial TPS, however, showed different domain organization. BjCPS showed the presence of only one prenyltransferase-like domain (PF13249). Another prenyltransferase-like domain (PF13243) was represented by SkoCPS and KgDTC1. This domain was also present in AtKS, although the sequence coverage was less than that of the bacterial proteins (Figure ). KgDTC2 was represented by PF03936, i.e. metal-binding domain of TPS. BjKS did not show any significant hit by Pfam.
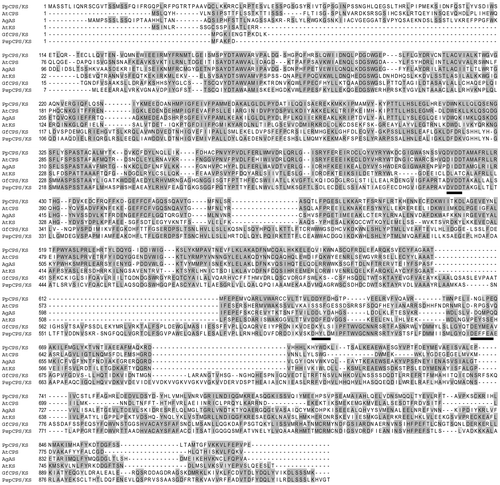
However, the sequence similarity among the plant and fungal TPS was significant as there were many conserved amino acid positions (Figure ). The DXDD motif was represented by DVDD in the bifunctional ent-kaurene synthase of fungi (GfCPS/KS and PspCPS/KS) and P. patens (PpCPS/KS). However, in AgAS and AtCPS, the valine of the second position of DXDD motif was replaced by isoleucine (Figure ). In AtKS, the first aspartate of the DXDD motif was conserved. The other three were replaced by leucine, alanine and threonine. The DDXXD motif was not present in fungal KS. Instead, they showed the presence of a DEXXEA motif and the third and fourth positions were completely different in GfCPS/KS and PspCPS/KS (Figure ). The third and fourth positions of the DDXXD were also different in plants. However, in AtKS and PpCPS/KS, they were both aromatic amino acids, whereas in AgAS there was only one aromatic amino acid (tyrosine). It is noteworthy that AtKS and PpCPS/KS produce ent-kaurene, the precursor of gibberellins, whereas AgAS produces abietadiene, a secondary metabolite.
Figure 3. Sequence alignment of ent-kaurene synthase from Physcomitrella patens (PpCPS/KS) and other diterpene synthases from plants and fungi. PpCPS/KS polypeptide was compared with Arabidopsis thaliana ent-copalyl diphosphate synthase (AtCPS), Arabidopsis thaliana ent-kaurene synthase (AtKS), Abies grandis abietadiene synthase (AgAS), Gibberella fujikuroi ent-kaurene synthase (GfCPS/KS) and Phaeosphaeria sp. L487 ent-kaurene synthase (PspCPS/KS). Identical amino acids are represented by grey shading. Underline represents conserved aspartate-rich motifs (DXDD for all enzymes containing CPS activity, DDXXD for plant enzymes with KS activity and DEXXEA for fungal enzymes).
Three-dimensional structure of PpCPS/KS
PpCPS/KS is 881 amino acids in length. Phyre2 server modelled the three-dimensional structure of this protein based on chain A of 3P5P, i.e. crystal structure of taxadiene synthase from the Pacific Yew (Taxus brevifolia) (Köksal et al. Citation2011). The modelled protein has overall structural similarities with the template (3P5P, chain A) as predicted by the RMSD value of 0.509 Å between its backbone atoms with its template. A very low percentage of residues was found to be in the unacceptable regions of the Ramachandran Plot as predicted by RAMPAGE and PROCHECK (Table ). The different software tools, like Verify3D, ProSA-web and QMEAN (Table ), also predicted a good model quality. QMEAN is a scoring function of a linear combination of structural descriptors (Benkert, Tosatto and Schomburg Citation2008). The QMEAN score ranges between 0 and 1 and a higher value reflects better quality of a three-dimensional structure (Wang and Chou Citation2011). The QMEAN score for the modelled structure of PpCPS/KS was 0.639 whereas the QMEAN score for the template was 0.773. The protein has 37 alpha helices and two beta strands. The DVDD motif is present in helix 15 and the DDYFD motif is present on helix 25 (Figure ).
Table 4. Quality assessment of modelled structure and the template by means of Ramachandran plot analysis by RAMPAGE and Procheck.
Table 5. Quality assessment of homology models and the template by various tools.
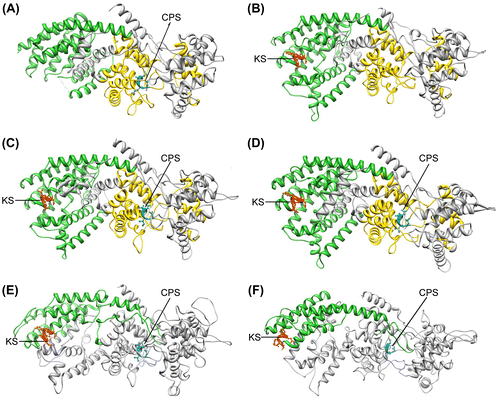
Figure 4. Three-dimensional structures of all the investigated polypeptides. (A) Arabidopsis thaliana ent-copalyl diphosphate synthase (AtCPS), (B) Arabidopsis thaliana ent-kaurene synthase (AtKS), (C) Abies grandis abietadiene synthase (AgAS), (D) Physcomitrella patens ent-kaurene synthase (PpCPS/KS), (E) Gibberella fujikuroi ent-kaurene synthase (GfCPS/KS) and (F) Phaeosphaeria sp. L487 ent-kaurene synthase (PspCPS/KS). In all cases, green region represents metal binding domain, yellow region represents N-terminal region (absent in E and F), red region is aspartate-rich motif with KS activity and blue region represents aspartate-rich motif with CPS activity.
Structures of other terpene synthases from plants and fungi
For a comparative study of PpCPS/KS with other plant and fungal bifunctional TPS, three-dimensional structures of AgAS, PspCPS/KS and GfCPS/KS were also generated. Moreover, these structures were compared with AtCPS and AtKS. The three-dimensional structure of AtCPS was found in PDB (PDB ID: 3PYA), and the three-dimensional structure of AtKS has been modelled. All the four proteins from plant and fungi were modelled on the same template as in PpCPS/KS, i.e. crystal structure of taxadiene synthase from Pacific Yew (Taxus brevifolia) (PDB ID: 3P5P, chain A) (Köksal et al. Citation2011). The different software tools, like Verify3D, ProSA-web and QMEAN, predicted good model quality (Table ) and no residues were found to be in the unacceptable regions of the Ramachandran plot as predicted by PROCKECK (Table ). As the bacterial proteins are much smaller than the plant and fungal TPS, their structures have been found to be totally different after modelling. They could not be compared with plant and fungal TPS and so have not been studied further. Among the four modelled proteins, only AtCPS showed 39 helices. This protein also has four strands. The other four proteins have 36 helices and two strands (except for AtKS, which showed no strands in its structure). Among all the proteins, AgAS showed the highest structural similarity with the template because it showed the lowest RMSD value (0.142 Å) with the template. It showed the presence of DIDD and DDLYD motifs on helix 14 and helix 25. AtKS also showed the presence of DDFFD motif on helix 25 (Figure ), but the DIDD motif was present on the helix 13 of AtCPS/KS. In the fungal proteins, the DVDD and DEXXEA motifs were present on helix 16 and helix 28. The RMSD of PpCPS/KS and the other enzymes showed that AtCPS is the closest to PpCPS/KS structurally (RMSD = 0.507 Å), whereas for AtKS RMSD = 1.975 Å. The bifunctional TPS had similar RMSD to PpCPS/KS (1.924 Å, 1.981 Å and 1.977 Å for AgAS, GfCPS/KS and PspCPS/KS, respectively).
Comparison of three-dimensional orientation of aspartate-rich motifs
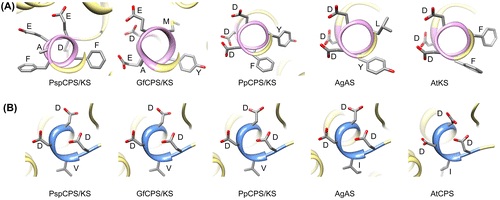
It is interesting that although the aspartate-rich motifs of KS domains of fungal and plant TPS (DEXXEA and DDXXD, respectively) did not align in the multiple sequence alignment, they showed similar three-dimensional orientation in the modelled structures (Figure ). All of them were present on an alpha-helix. The orientations of the amino acids in the DXDD motifs of the CPS domain of all TPS were also similar in three-dimensional space. Figure shows the detailed three-dimensional ordination of aspartate-rich motifs of CPS and KS domains of the investigated proteins. The orientation of the aspartate residues in all the plant aspartate-rich motifs of KS domains was similar. There was almost no difference in the structural orientation of the aspartate residues in AgAs and AtKS, whereas the orientation of the fifth aspartate of DDYFD of PpCPS/KS was slightly different compared with the other two proteins (Figure ). However, the fungal KS showed different orientations of the side chains of the amino acids in their DEXXEA motif. The aspartate-rich motif of the CPS domain, however, is structurally more conserved than the aspartate-rich motif of KS domain. The side chains of the aspartate residues of the CPS domains were similar in all five proteins. However, the side chains of the third and fourth aspartates of AtCPS were positioned differently to the others. Also, the isoleucine positioning was different from that of AgAS.
Surface properties of the proteins
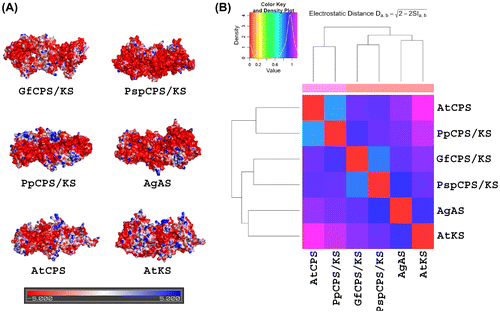
The electrostatic surfaces of the investigated proteins were mostly electronegative (Figure ). However, the fungal TPS showed very few positive potential patches over their surfaces. PpCPS/KS showed some large positive potential patches whereas other plant TPS showed scattered, small positive potential patches over their surfaces. To further quantify the similarity of surface electrostatic potentials of the TPS, the models were analysed with PIPSA. The resulting dendrogram was divided into three clusters (Figure ). The two fungal TPS clustered together. Within plants, PpCPS/KS and AtCPS formed one group while AgAS and AtKS grouped together. However, it is notable that fungal enzymes grouped with the cluster formed by AgAS and AtKS.
Figure 6. Molecular electrostatic potential surfaces and PIPSA clustering of different terpene synthases (TPS) from plants and fungi. (A) Representation of the electrostatic potential projected on the solvent accessible surface of the proteins, using the APBS electrostatic potential and the surface colours were represented at –5 (red) and +5 (blue) kTe-1. (b) The dendogram from the PIPSA analysis of the TPS from plants and fungi.
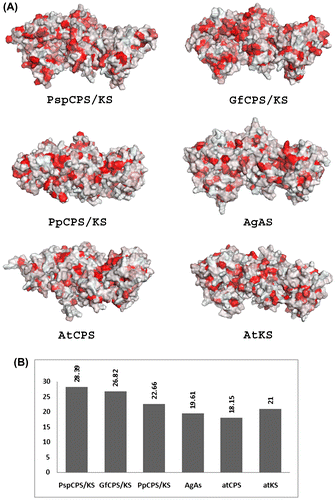
The overall surfaces of the TPS of the present study were mostly hydrophilic (Figure ). In all the proteins, there were several hydrophobic patches dispersed on the surface. However, Figure shows clearly that greater numbers of these patches were present in fungal TPS than plant TPS. The two fungal TPS were more hydrophobic (28.39% and 26.82% hydrophobicity for PspCPS/KS and GfCPS/KS, respectively) than plant TPS (which ranged from 18.15% to 22.66%) (Figure ). Among the plant TPS, PpCPS/KS showed the highest hydrophobicity (22.66%).
Figure 7. Molecular hydrophobicity potential of different terpene synthases (TPS) from plants and fungi. (A) Surface hydrophobicity of investigated TPS proteins coloured in PyMOL. Red areas represent hydrophobic surface. (B) Graphical representation of the percentage of hydrophobic surface of each protein as obtained with PLATINUM.
Discussion
In this paper, an attempt has been made to characterize the bifunctional ent-kaurene synthase from the moss P. patens as well as to investigate the origin of the TPS enzymes in plants. Representatives of this class of enzymes were also selected from gibberellin-producing fungi and bacteria because they could throw further light on the origin of this class of enzymes in plants. The sequence conservation of the aspartate-rich motif of bifunctional ent-kaurene synthases from fungi and P. patens shows that DVDD is the primitive representative of the DXDD motif of the CPS domain. The valine in the second position of this motif has been substituted by isoleucine in some TPS of higher plants like AgAS and AtCPS. However, orientation of the side chains of these amino acids in the unifunctional AtCPS was different from the bifunctional TPS of plants and fungi, which indicates that this is the result of a selective advantage for the reaction mechanism for the CPS enzymes in higher plants. Additionally, all the investigated plant TPS possess the N-terminal domain (PF01397) that contains the DXDD motif. Although the DXDD motif is present in their fungal counterparts, the N-terminal domain (PF01397) was absent. Hence, this domain originated in plants before the divergence of land plants. The AtKS also has this domain, although it does not possess the DXDD motif, which supports the findings of Hayashi et al. (Citation2006). The conserved nature of this domain indicates its role in structural stabilization of the TPS enzymes in plants. On the other hand, the metal-binding domain (PF03936) is conserved in all the investigated enzymes from plant and fungi, i.e. it is more primitive than the N-terminal domain. The primitiveness of this domain is also evident from the fact that it is also found in the bacterial enzyme KgDTC2. The KS active domains of plants and fungi are different, but they contain at least one aspartate and one aromatic amino acid, either phenylalanine or tyrosine (Figure ). The fungal enzymes possess a DEXXEA motif in the KS active sites and the plants contain a DDXXD motif. Hence plants replaced the second and fifth positioned glutamate with aspartate, both of which are structurally similar and positively charged amino acids. The probable reason behind the DXDD motif being much conserved in all the enzymes is the substrate used by this site. All of them use geranylgeranyl diphosphate and produce copalyl diphosphate (Hayashi et al. Citation2006). In contrast, the final products, i.e. diterpenes, vary among these enzymes, possibly because of the difference in KS active domain. The end product of PpCPS/KS is mainly 16α-hydroxykaurane and some ent-kaurene (Hayashi et al. Citation2006), whereas AtKS and AgAS produce ent-kaurene and abietadiene as end products, respectively (Vogel et al. Citation1996; Yamaguchi et al. Citation1998). Fungal CPS/KS also produce ent-kaurene and subsequently gibberellins (Hayashi et al. Citation2006), but the basic difference with higher plants is that gibberellin is a secondary metabolite in fungi instead of a growth regulator as in higher plants (Rademacher Citation1994; Kawaide et al. Citation1997). It is also worth mentioning that plants produce diterpenes as both primary and secondary metabolites and the variation in their structure is remarkable (Falara et al. Citation2011). In addition, the regulation of diterpene biosynthesis varies among fungi and plants (Hedden et al. Citation2002), which may be the cause of the difference in amino acid sequences at the KS active domains of these enzymes. It was definitely advantageous for plants to separate the copalyl-diphosphate-synthesizing enzyme from the end product (diterpene) -producing enzyme because the latter should be fast evolving to cope with environmental fluctuations. This is one probable explanation for the evolution of unifunctional plant CPS and KS from bifunctional diterpene synthases.
Domain organization in the present study indicates that fungal TPS have been originated from bacterial proteins probably by fusion of two types of bacterial TPS. One type contained the metal-binding domain, i.e. PF03936 (like KgDTC2 or BjKS), and another type contained the prenyltransferase-like domain (like BjCPS, SkoCPS or KgDTC1). The transfer of the fused protein from bacteria to fungi may have occurred by horizontal gene transfer. Horizontal gene transfer from bacteria to fungi is well known (Fitzpatrick, Logue, and Butler Citation2008; Schmitt and Lumbsch Citation2009). This hypothesis, that fungal TPS originated from bacterial TPS, is also supported by the length of the proteins. BjKS (which showed insignificant hit for PF03936) and KgDTC2 were shorter (311 and 300 amino acids, respectively) than BjCPS, SkoCPS and KgDTC1 (587, 511 and 499 amino acids, respectively). Hypothetical fusion proteins of these two classes would result in proteins with around 800–900 amino acids. The fungal proteins studied in this work were 946 and 952 amino acids long. This provides strong evidence that fungal TPS have originated from the fusion of two different types of bacterial TPS. Among the plant TPS, PpCPS/KS (from Physcomitrella) was the longest. The length of this protein, as well as the fact that it also showed prenyltransferase-like domain as insignificant Pfam results, also indicates that horizontal gene transfer might have occurred from bacteria to bryophytes. However, the dendrogram (Figure ) based on multiple sequence alignment showed that fungal TPS (but not plant TPS) were more similar to bacterial TPS. Additionally, TPS of both Physcomitrella and fungi showed remarkable similarity in aspartate-rich motifs, especially DXDD. GfCPS/KS, PspCPS/KS and PpCPS/KS possess the same sequence (DVDD) of the DXDD motif. The three-dimensional arrangements of the DVDD motif of these three proteins are also strikingly similar (Figure ). All of this suggests that the bifunctional TPS of bryophytes have been acquired from fungi. Recently, Yue et al. (Citation2012) discussed the impact of horizontal gene transfer on land plant colonization. According to them, P. patens have acquired 57 families of genes either from bacteria, viruses or fungi. The acquired genes are mainly related to plant defence and stress tolerance. It is also notable that one of the products of the bifunctional TPS of P. patens is 16α-hydroxykaurane, which is also the starting point for the production of gibberellic acid in Gibberella fujikuroi (Sawada) Wollenw. (Dayan and Romagni Citation2001). This 16α-hydroxykaurane is also widespread in fungi as it is found in several lichenizing and non-lichenizing fungi (Dayan and Romagni Citation2001). Yue et al. (Citation2012) showed that one entry point for foreign DNA into the moss genome is the young gametophyte. Zhang and Guo (Citation2007) reported several arbuscular mycorrhizal fungi associated with mosses, suggesting moss–fungus associations. All of these indicate that the bifunctional TPS of P. patens came from some fungal genome.
The three-dimensional structures of the TPS proteins also highlight the events related to the origin and subsequent evolution of plant TPS. As evolution progresses, the surface properties of the proteins have changed. Although the overall structures of the proteins are similar (Figure ), surface hydrophobicity of the proteins showed that fungal TPS are slightly more hydrophobic than plant TPS (Figure ) and PpCPS/KS showed intermediate values. This was also supported by the GRAVY values of the proteins (Table ). PpCPS/KS had the highest GRAVY values among the plants; a low value of GRAVY indicates the possibility of better interaction with water (Sahay and Shakya Citation2010; Roy et al. Citation2011). Hence, after the acquisition of bifunctional TPS by bryophytes, the proteins became more hydrophilic with evolution. Additionally, surface electrostatic comparisons showed that the proteins became more positively charged with evolution (Figure ).
In conclusion, it can be said that early land plants and fungi possessed bifunctional TPS as they produce diterpenes mainly as secondary metabolites. This study indicates that fungal TPS originated from bacterial TPS and plant TPS originated from fungal TPS by horizontal gene transfer. With the increasing complexity in plants, TPS genes also diverged in structure and function and primarily became unifunctional. With the increasing complexity in morphology and physiology within plants as well as increasing interaction with organisms in the external environment, terpenoids became crucial both as growth regulators and secondary metabolites. This resulted in the diversity of TPS genes found in higher plants. However, to better understand the origin and evolution of these enzymes, more TPS from other lower plants, fungi and bacteria should be investigated.
Acknowledgements
The facility situated in the Department of Botany, Dinabandhu Mahavidyalaya is gratefully acknowledged.
References
- Altschul, S.F., W. Gish, W. Miller, E.W. Myers, and D.J. Lipman. 1990. “Basic local alignment search tool.” Journal of Molecular Biology 215(3): 403–410.
- Baker, N.A., D. Sept, S. Joseph, M.J. Holst, and J.A. McCammon. 2001. “Electrostatics of nanosystems: application to microtubules and the ribosome.” Proceedings of the National Academy of Sciences of the United State of America 98 (18): 10037–10041.
- Benkert, P., M. Künzli, and T. Schwede. 2009. “QMEAN Server for Protein Model Quality Estimation.” Nucleic Acids Research. 37: W510–W514.
- Benkert, P., S.C.E. Tosatto, and D. Schomburg. 2008. “QMEAN: a comprehensive scoring function for model quality assessment.” Proteins 71 (1): 261–277.
- Berman, H.M., J. Westbrook, Z. Feng, G. Gilliland, T.N. Bhat, H. Weissig, I.N. Shindyalov, and P.E. Bourne. 2000. “The protein data bank.” Nucleic Acids Research 28 (1): 235–242.
- Blomberg, N., R.R. Gabdoulline, M. Nilges, and R.C. Wade. 1999. “Classification of protein sequences by homology modeling and quantitative analysis of electrostatic similarity.” Proteins 37 (3): 379–387.
- Bowie, J.U., R. Lüthy, and D. Eisenberg. 1991. “A method to identify protein sequences that fold into a known three-dimensional structure.” Science 253 (5016): 164–170.
- Chen, F., D. Tholl, J. Bohlmann, and E. Pichersky. 2011. “The family of terpene synthases in plants: a mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom.” The Plant Journal 66 (1): 212–229.
- Dayan, F.E., and J.G. Romagni. 2001. “Lichens as a potential source of pesticides.” Pesticide Outlook 12 (6): 229–232.
- DeLano, W.L. 2002. The PyMOL Molecular Graphics System. San Carlos, CA, USA: DeLano Scientific.
- Dolinsky, T.J., J.E. Nielsen, J.A. McCammon, and N.A. Baker. 2004. “PDB2PQR: an automated pipeline for the setup, execution, and analysis of Poisson-Boltzmann electrostatics calculations.” Nucleic Acids Research 32: W665–W667.
- Efremov, R.G., A.O. Chugunov, T.V. Pyrkov, J.P. Priestle, A.S. Arseniev, and E. Jacoby. 2007. “Molecular lipophilicity in protein modeling and drug design.” Current Medicinal Chemistry 14 (4): 393–415.
- Eisenberg, D., R. Lüthy, and J.U. Bowie. 1997. “VERIFY3D: assessment of protein models with three-dimensional profiles.” Methods in Enzymology 277: 396–404.
- Eisenberg, D., E. Schwarz, M. Komaromy, and R. Wall. 1984. “Analysis of membrane and surface protein sequences with the hydrophobic moment plot.” Journal of Molecular Biology 179 (1): 125–142.
- Falara, V., T.A. Akhtar, T.T.H. Nguyen, E.A. Spyropoulou, P.M. Bleeker, I. Schauvinhold, Y. Matsuba, et al. 2011. “The Tomato terpene synthase gene family.” Plant Physiology 157 (2): 770–789.
- Fink, J.L., and N. Hamilton. 2007. “DomainDraw: a macromolecular feature drawing program.” In silico Biology 7 (2): 145–150.
- Finn, R.D., J. Mistry, J. Tate, P. Coggill, A. Heger, J.E. Pollington, O.L. Gavin, et al. 2010. “The Pfam protein families database.” Nucleic Acids Research 38: D211–D222.
- Fitzpatrick, D.A., M.E. Logue, and G. Butler. 2008. “Evidence of recent interkingdom horizontal gene transfer between bacteria and Candida parapsilosis.” BMC Evolutionary Biology 8: 181.
- Gabdoulline, R.R., M. Stein, and R.C. Wade. 2007. “qPIPSA: relating enzymatic kinetic parameters and interaction fields.” BMC Bioinformatics 8: 373.
- Gasteiger, E., C. Hoogland, A. Gattiker, S. Duvaud, M.R. Wilkins, R.D. Appel, and A. Bairoch. 2005. “Protein identification and analysis tools on the ExPASy server.” In The Proteomics Protocols Handbook, edited by J.M. Walker, 571–607. New York: Humana Press.
- Geourjon, C., and G. Deléage. 1995. “SOPMA: significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments.” Computer Applications in the Biosciences 11 (6): 681–684.
- Guex, N., and M.C. Peitsch. 1997. “SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling.” Electrophoresis 18 (15): 2714–2723.
- Guruprasad, K., B.V.B. Reddy, and M.W. Pandit. 1990. “Correlation between stability of a protein and its dipeptide composition: a novel approach for predicting in vivo stability of a protein from its primary sequence.” Protein Engineering 4 (2): 155–161.
- Hayashi, K., H. Kawaide, M. Notomi, Y. Sakigi, A. Matsuo, and H. Nozaki. 2006. “Identification and functional analysis of bifunctional ent-kaurene synthase from the moss Physcomitrella patens.” FEBS Letters 580 (26): 6175–6181.
- Hedden, P., A.L. Phillips, M.C. Rojas, E. Carrera, and B. Tudzynski. 2002. “Gibberellin biosynthesis in plants: a case of convergent evolution?” Journal of Plant Growth Regulation 20 (4): 319–331.
- Hillwig, M.L., M. Xu, T. Toyomasu, M.S. Tiernan, G. Wei, G. Cui, L. Huang, and R.J. Peters. 2011. “Domain loss has independently occurred multiple times in plant terpene synthase evolution.” The Plant Journal 68 (6): 1051–1060.
- Ikai, A.J. 1980. “Thermostability and aliphatic index of globular proteins.” Journal of Biochemistry 88 (6): 1895–1898.
- Kawaide, H. 2006. “Biochemical and molecular analyses of gibberellin biosynthesis in fungi.” Bioscience, Biotechnology, and Biochemistry 70 (3): 583–590.
- Kawaide, H., R. Imai, T. Sassa, and Y. Kamiya. 1997. “ent-Kaurene synthase from the fungus Phaeosphaeria sp. L487cDNA isolation, characterization, and bacterial expression of abifunctional diterpene cyclase in fungal gibberellin biosynthesis.” Journal of Biological Chemistry 272 (35): 21706–21712.
- Kawaide, H., T. Sassa, and Y. Kamiya. 2000. “Functional analysis of the two interacting cyclase domains in ent-kaurene synthase from the fungus Phaeosphaeria sp. L487 and a comparison with cyclases from higher plants.” Journal of Biological Chemistry 275 (4): 2276–2280.
- Kelley, L.A., and M.J.E. Sternberg. 2009. “Protein structure prediction on the web: a case study using the Phyre server.” Nature Protocols 4 (3): 363–371.
- Köksal, M., Y. Jin, R.M. Coates, R. Croteau, and D.W. Christianson. 2011. “Taxadiene synthase structure and evolution of modular architecture in terpene biosynthesis.” Nature 469 (7328): 116–120.
- Larkin, M.A., G. Blackshields, N.P. Brown, R. Chenna, P.A. McGettigan, H. McWilliam, F. Valentin, et al. 2007. “ClustalW and ClustalX version 2.” Bioinformatics 23 (21): 2947–2948.
- Laskowski, R.A., M.W. MacArthur, D.S. Moss, and J.M. Thornton. 1993. “PROCHECK: a program to check the stereochemistry of protein structures.” Journal of Applied Crystallography 26 (2): 283–291.
- Lovell, S.C., I.W. Davis, W.B. Arendall III, P.I.W. de Bakker, J.M. Word, M.G. Prisant, J.S. Richardson, and D.C. Richardson. 2003. “Structure validation by Cα geometry: ϕ, ψ and Cβ deviation.” Proteins 50 (3): 437–450.
- Lüthy, R., J.U. Bowie, and D. Eisenberg. 1992. “Assessment of protein models with three-dimensional profiles.” Nature 356 (6364): 83–85.
- Morrone, D., J. Chambers, L. Lowry, G. Kim, A. Anterola, K. Bender, and R.J. Peters. 2009. “Gibberellin biosynthesis in bacteria: separate ent-copalyl diphosphate and ent-kaurene synthases in Bradyrhizobium japonicum.” FEBS Letters 583 (2): 475–480.
- Nishiyama, T., T. Fujita, T. Shin-I, M. Seki, H. Nishide, I. Uchiyama, A. Kamiya, P. Carninci, Y. Hayashizaki, K. Shinozaki, et al. 2003. “Comparative genomics of Physcomitrella patens gametophytic transcriptome and Arabidopsis thaliana: implication for land plant evolution.” Proceedings of the National Academy of Sciences of the United States of America 100 (13): 8007–8012.
- Pettersen, E.F., T.D. Goddard, C.C. Huang, G.S. Couch, D.M. Greenblatt, E.C. Meng, and T.E. Ferrin. 2004. “UCSF Chimera-a visualization system for exploratory research and analysis.” Journal of Computational Chemistry 25 (13): 1605–1612.
- Pyrkov, T.V., A.O. Chugunov, N.A. Krylov, D.E. Nolde, and R.G. Efremov. 2009. “PLATINUM: a web tool for analysis of hydrophobic/hydrophilic organization of biomolecular complexes.” Bioinformatics 25 (9): 1201–1202.
- Rademacher, W. 1994. “Gibberellin formation in microorganisms.” Plant Growth Regulation 15 (3): 303–314.
- Richter, S., A. Wenzel, M. Stein, R.R. Gabdoulline, and R.C. Wade. 2008. “webPIPSA: a web server for the comparison of protein interaction properties.” Nucleic Acids Research 36: W276–W280.
- Roy, S., N. Maheshwari, R. Chauhan, N.K. Sen, and A. Sharma. 2011. “Structure prediction and functional characterization of secondary metabolite proteins of Ocimum.” Bioinformation 6 (8): 315–319.
- Sahay, A., and M. Shakya. 2010. “In silico analysis and homology modelling of antioxidant proteins of spinach.” Journal of Proteomics & Bioinformatics 3 (5): 148–154.
- Sánchez, R., and A. Šali 2000. “Comparative Protein Structure Modeling: Introduction and Practical Examples with Modeller.” In Protein Structure Prediction: Methods and Protocols. Methods in Molecular Biology, Vol. 143, edited by D.M. Webster, 97–129. Totowa, NJ, USA: Humana Press.
- Schmitt, I., and H.T. Lumbsch. 2009. “Ancient horizontal gene transfer from bacteria enhances biosynthetic capabilities of fungi.” PLOS One 4 (2): e4437.
- Sippl, M.J. 1993. “Recognition of errors in three-dimensional structures of proteins.” Proteins 17 (4): 355–362.
- Sun, T.P., and Y. Kamiya. 1994. “The Arabidopsis GA1 locus encodes the cyclase ent-kaurene synthetase A of gibberellin biosynthesis.” Plant Cell 6 (10): 1509–1518.
- Syed, R., R. Rani, T.A. Sabeena, G. Shafi Masoodi, and K. Alharbi. 2012. “Functional analysis and structure determination of alkaline protease from Aspergillus flavus.” Bioinformation 8 (4): 175–180.
- Tamura, K., D. Peterson, N. Peterson, G. Stecher, M. Nei, and S. Kumar. 2011. “MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods.” Molecular Biology and Evolution 28 (10): 2731–2739.
- Toyomasu, T., H. Kawaide, A. Ishizaki, S. Shinoda, M. Otsuka, W. Mitsuhashi, and T. Sassa. 2000. “Cloning of a full-length cDNA encoding ent-kaurene synthase from Gibberella fujikuroi: functional analysis of a bifunctional diterpene cyclase.” Bioscience, Biotechnology, and Biochemistry 64 (3): 660–664.
- Trapp, S.C., and R.B. Croteau. 2001. “Genomic organization of plant terpene synthases and molecular evolutionary implications.” Genetics 158 (2): 811–832.
- van Gunsteren, W.F., S.R. Billeter, A. Eising, P.H. Hünenberger, P. Krüger, A.E. Mark, W.R.P. Scott, and I.G. Tironi. 1996. Biomolecular Simulations: The GROMOS96 Manual and User Guide. Zurich: VdF Hochschulverlag ETHZ.
- Vogel, B.S., M.R. Wildung, G. Vogel, and R. Croteau. 1996. “Abietadiene synthase from Grand Fir (Abies grandis). cDNA isolation, characterization, and bacterial expression of a bifunctional diterpene cyclase involved in resin acid biosynthesis.” Journal of Biological Chemistry 271 (38): 23262–23268.
- Wade, R., R.R. Gabdoulline, and F. De Rienzo. 2001. “Protein interaction property similarity analysis.” International Journal of Quantum Chemistry 83 (3–4): 122–127.
- Wang, J.-F., and K.-C. Chou. 2011. “Insights from modeling the 3D structure of New Delhi metallo-β-lactamase and its binding interactions with antibiotic drugs.” PLoS One 6 (4): e18414.
- Waterhouse, A.M., J.B. Procter, D.M.A. Martin, M. Clamp, and G.J. Barton. 2009. “Jalview Version 2 – a multiple sequence alignment editor and analysis workbench.” Bioinformatics 25 (9): 1189–1191.
- Wiederstein, M., and M.J. Sippl. 2007. “ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins.” Nucleic Acids Research 35: W407–W410.
- Yamaguchi, S., T. Saito, H. Abe, H. Yamane, N. Murofushi, and Y. Kamiya. 1996. “Molecular cloning and characterization of a cDNA encoding the gibberellin biosynthetic enzyme ent-kaurene synthase B from pumpkin (Cucurbita maxima L.).” The Plant Journal 10 (2): 203–213.
- Yamaguchi, S., T. Sun, H. Kawaide, and Y. Kamiya. 1998. “The GA2 locus of Arabidopsis thaliana encodes ent-Kaurene synthase of gibberellin biosynthesis.” Plant Physiology 116 (4): 1271–1278.
- Yue, J., X. Hu, H. Sun, Y. Yang, and J. Huang. 2012. “Widespread impact of horizontal gene transfer on plant colonization of land.” Nature Communications 3: 1152.
- Zhang, Y., and L.-D. Guo. 2007. “Arbuscular mycorrhizal structure and fungi associated with mosses.” Mycorrhiza 17 (4): 319–325.