Abstract
Border and isolated plant populations represent an interesting target for ecological and conservation issues. We analysed the ecological constraints and the conservation status of the eastern population of Helianthemum caput-felis Boiss. (Cistaceae), located in Sardinia. The distribution of H. caput-felis was verified via field surveys; ecological data, morphological and reproductive traits, were recorded in 40 permanent plots randomly established; the human trampling effects on plant density, plant size and plant performance were analysed. Plant density was higher in bedrock and lowland areas, in garrigue and maquis habitats; however, the differences among plants growing in different ecological conditions were not statistically significant; only human trampling intensity significantly affected plant density and lowest values were observed in areas with intense trampling pressure. All ecological variables analysed had a statistically significant effect on plant size and on the number of fruits per plant. In particular, larger plants were found in areas with the following ecological features: presence of structured soil, on the slopes, in the maquis habitat, and in areas with intensive human trampling. Conversely, plants displayed a higher fruits output per plant in deep and structured soil, in lowland areas, and in the garrigue and maquis habitats; the mean fruits output per plant increased as human trampling intensified. Human-induced threats are the main hazards threatening the remaining Sardinian population. In particular, the major threats are linked to tourism and other outdoor activities (i.e. human trampling), followed by the expansion of agricultural activities; all of these threats result in the disappearance of small localities and in reduced population size due to habitat loss and fragmentation. Our study indicates that H. caput-felis should be considered as Critically Endangered (CR) at the regional level. Urgent measures should be undertaken to protect the remaining H. caput-felis population in Sardinia and a possible integrated strategy for the conservation and management consists of a combination of in situ and ex situ measures. In particular, greater emphasis should be given to minimizing the negative impacts of unsustainable tourism and recreation use, in order to exclude human trampling and to facilitate the plant recruitment process and population renewal. In addition, an ex situ conservation strategy must be implemented and the seeds collected could be used for future translocations in suitable areas. Moreover, considering the threats observed, a long-term monitoring programme must be developed to reveal changes in the species conservation status.
Introduction
Several plant species are characterized by a disjunctive distribution in which peripheral populations can be isolated from the main home range. Plants that display a peculiar distribution and, in particular, species with border and peripheral populations, represent interesting targets in ecology, evolutionary biology and genetics (Eckert, Samis, and Lougheed Citation2008; Sexton et al. Citation2009; Pouget et al. Citation2013). In addition, they provide insight into critical phenomena, such as speciation, adaptive radiation and natural selection (Grant and Antonovics Citation1978; Holt and Keitt Citation2005).
Independent of the central/marginal model debate, border populations as well as peripheral isolated plant populations are particularly important from both ecological and genetic points of view (Lesica and Allendorf Citation1995; Conradt Citation2001; Holt and Keitt Citation2005) and require more attention from conservation biologists (Abeli et al. Citation2009). Border populations are usually considered more vulnerable and are more prone to local extinction because of their isolation and restriction to marginal habitats (e.g. Gargano et al. Citation2007; Del Vecchio et al. Citation2012; Villellas et al. Citation2013; Villellas, Morris, and García Citation2013). As suggested by international organizations (e.g. IUCN, European Council) and according to The European Strategy for Plant Conservation (Planta Europa Citation2008), border and isolated populations should be considered an important resource for biodiversity and should therefore be included in conservation programmes. Consequently, several plant species in the Mediterranean Basin that show outlying populations isolated ecologically and geographically from the rest of their distribution range have been investigated in recent years (e.g. Gargano et al. Citation2007; Del Vecchio et al. Citation2012; Fois et al. Citation2015).
Cistaceae is a medium-size family with eight genera and approximately 180 taxa distributed in temperate and subtropical regions of the northern hemisphere, and it displays the highest genus and species diversity in the Mediterranean floristic region (Guzmán and Vargas Citation2009). Within this family, Helianthemum Miller is the most diverse genus, with approximately 100 taxa that grow from sea level up to approximately 3000 m in a diverse array of substrates (limestone, dolomite, marl, gypsum, saline and sand-soils) that are concentrated in the western Mediterranean area (Próctor and Heywood Citation1968). Within this genus, Helianthemum caput-felis Boiss. deserves particular attention because it is considered the only extant representative of an ancient lineage (Arrigoni Citation1971; López-González Citation1992). Helianthemum caput-felis is a coastal plant distributed throughout the western Mediterranean Basin (southeastern Iberian Peninsula, Mallorca, Sardinia and northwest Africa) in several fragmented populations; the widest distribution and the largest populations are located in Spain (Agulló et al. Citation2011; López-González Citation1992), whereas the presence of this species in Sardinia and northwest Africa is restricted to small areas in unique or reduced places (Arrigoni Citation1971; Fenu and Bacchetta Citation2008; Quézel and Santa Citation1963).
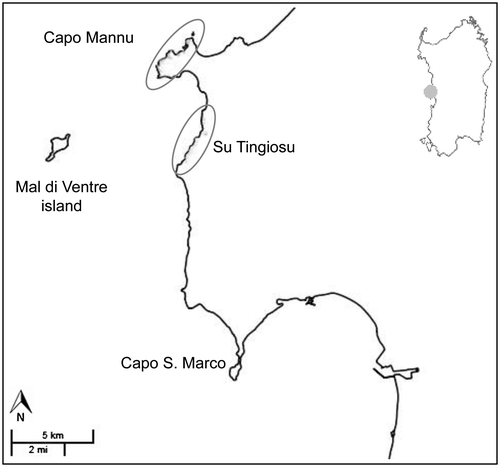
In Sardinia, H. caput-felis grows in two main localities (Capo Mannu and Su Tingiosu, central-west part of the island; Figure ) that are approximately 3 km apart; small patches are also found in the coastal cliff along the Sinis Peninsula (Arrigoni Citation1971; Fenu and Bacchetta Citation2008). This plant is found in the discontinuities of the Juniperus micro-forest and into the maquis, but it mainly grows in the coastal garrigues, where cushion chamaephytes are dominant. Helianthemum caput-felis is a member of a rupicolous coastal plant community that is rich in narrow endemics, such as Limonium lausianum Pignatti and Polygala sinisica Arrigoni, as well as other western Mediterranean plants, such as Viola arborescens L., Coris monspeliensis L. and Erica multiflora L. (Fenu et al. Citation2012).
Fig. 1. Study area in the Sinis Peninsula (CW-Sardinia); the circles indicate the main localities of Capo Mannu and Su Tingiosu where Helianthemum caput-felis grows.
Helianthemum caput-felis is an emblematic plant from a conservation point of view; it is included in the Berne Convention and Appendix II of the Habitats Directive (European Community Council Directive 92/43/EEC), and it is classified as endangered on the European red list of vascular plants (Bilz et al. Citation2011) according to the IUCN protocol (IUCN Citation2001). However, to date, no exhaustive reproductive and ecological studies have been conducted on this plant, and in particular on the Sardinian population, and no conservation actions have been implemented for this species.
Mediterranean coastal habitats have been altered by human activity for several thousand years, and coastal environments are strongly affected by tourism and related infrastructures (Davenport and Davenport Citation2006). In particular, touristic and recreational activities in coastal areas appear to be common threats to a wide variety of European threatened plants (Bilz et al. Citation2011; Ballantyne and Pickering Citation2013; Fenu et al. Citation2013; Rossi et al. Citation2015). However, few studies have focused on the effects of human trampling on Mediterranean coastal ecosystems (Comor et al. Citation2008; Kutiel, Eden, and Zhevelev Citation2000; Kerbiriou et al. Citation2008). Although for threatened plants the impact of tourism is particularly severe (Pickering and Hill Citation2007), to our knowledge, the effect of human trampling on threatened plants growing on coastal areas has yet to be accurately assessed in Mediterranean coastal habitats. In general, tolerance of species to human trampling varies, sometimes markedly. In Mediterranean coastal ecosystems some plant species (including threatened plants) are very sensitive to trampling, while others seem to be tolerant or even to benefit from trampling (Kerbiriou et al. Citation2008; Yu, Bell, and Kutiel Citation2009; Fenu et al. Citation2013). Human trampling has been demonstrated to be generally an important threat for threatened plant species in Sardinia (Quilichini and Debussche Citation2000; Fenu, Mattana, and Bacchetta Citation2011; Fenu et al. Citation2013; Rossi et al. Citation2015) and we hypothesize that also the Sardinian population of H. caput-felis is particularly affected by human disturbance, as previously reported for the Spanish populations (Dominguez Lozano et al. Citation1996).
The main aims of this study were to verify the distribution range and population size of H. caput-felis in Sardinia (eastern periphery of its distribution range), to analyse plant size and reproductive traits, to analyse the effect of human disturbance, to identify its principal threats and to assess its conservation status at the regional level following the IUCN protocol.
Methods
Study species
Helianthemum caput-felis is a perennial small shrub that grows to a height of 35(50) cm. Its flowers, which are arranged in inflorescences at the tip of new branches, are yellow and hermaphroditic, open at dawn and close at dusk, and have a short lifespan (3–4 days; Rodríguez-Pérez Citation2005). Based on studies carried out in Spain, the flowering period is from late February to late May, and the fruiting season runs from late April to July–August (Rodríguez-Pérez Citation2005). Tébar, Gil, and Llorens (Citation1997) and Rodríguez-Pérez (Citation2005) reported the allogamous character of this species, being a generalist entomogamous plant. Fruits are capsules that detach at maturity, and seed germination occurs in autumn, at the onset of the rainy season (Rodríguez-Pérez Citation2005).
From an ecological point of view, H. caput-felis is a thermophilous plant that typically grows in coastal environments under the direct influence of the sea, mostly on calcareous rocky cliffs (0–200 m above sea level) with garrigues or scrublands (Arrigoni Citation1971; Agulló et al. Citation2011; Fenu et al. Citation2012); some populations also grow on different habitats, such as sand dunes (Mallorca and Melilla), rocky slopes bordering inland ravines (Melilla; Agulló et al. Citation2011) or, rarely, in open wooded areas (Raynaud Citation1999).
Data sampling
The distribution of H. caput-felis was verified by field surveys conducted in the localities for which herbarium specimens and/or published data (Arrigoni Citation1971; Fenu and Bacchetta Citation2008) were available. The geographical limits of the main localities of Capo Mannu and Su Tingiosu (CM and ST, hereafter) were mapped using a global positioning system, and the areas where the plants were found were estimated using ArcView v. 3.2 (ESRI, Redlands, CA, USA). Localities containing only scattered plants were excluded a priori in this study (Figure ).
Forty permanent plots of 2 × 1 m (20 per locality) were randomly established (in the area where the plant was found) to estimate the plant densities and extrapolate the population size. Over 1 year, plants were monitored bimonthly, approximately on the 10th and 20th days of each month. Within the plots, all plants (including seedlings) were counted, marked and measured from March to August, in order to analyse the whole reproductive season of the plant species. During each monitoring, survival, morphological (height, maximum and minimum diameter) and reproductive traits (number of flowers and fruits per plant) were recorded for every plant. Each plant was assigned to one of the following habitats: garrigue, maquis and micro-forest. Similarly, each plant was assigned to a geomorphology and substrate (lowland versus slope areas and soil versus bedrock, respectively).
Human trampling intensity was visually estimated for each plot and four levels of intensity were considered: absent, low (≤ 30% of the plot surface), moderate (31–60%), and intense (≥ 61%); 10 plots for each category were considered in this study.
Phenological and reproductive traits were monitored in 378 marked plants. Phenological phases were considered present when > 5% of the plants showed the same pattern and each plant was classified as being reproductive based on the presence of flowers/fruits (censuses were carried out in May). The average number of fruits per plant was determined at the peak of fruiting season (May) from a ratio of the total number of fruits/total number of plants monitored. Seed-set (considered as number of seeds per fruit) was calculated in May by collecting randomly 400 mature fruits from 40 randomly selected plants. Seeds were extracted and counted, and the average number of seeds per fruit was multiplied by the average number of fruits per reproductive plant to predict the mean reproductive capacity of each plant.
IUCN assessment at regional level
Globally, the IUCN Red List procedure is the most widely used protocol for species risk assessment because it facilitates objective, repeatable and flexible assessments (e.g. Rodrigues et al. Citation2006; Grammont De and Cuarón Citation2006; Rossi et al. Citation2015). For IUCN assessment, we evaluated all of the localities where H. caput-felis grows (Fenu and Bacchetta Citation2008). IUCN categories and criteria version 3.1 (IUCN Citation2001) were applied according to the procedure for regional assessment (IUCN Citation2003). According to the New Italian Red List project (Rossi et al. Citation2013), a grid of 2 × 2 km generated by the ESRI® ArcGis™ 9.2 package and superimposed onto a map of Italy was used to assess the area of occupancy (AOO, defined as the area within the extent of occurrence, EOO, that is occupied by a taxon, where EOO is defined as the area contained within the shortest continuous imaginary boundary that can be drawn to encompass all the known sites of occurrence of a taxon, excluding cases of vagrancy, sensu IUCN Citation2001, Citation2014a). All of the parameters required by the IUCN protocol (i.e. EOO, locations, decline rate) and conservation status were assessed following the latest guidelines of the IUCN (Citation2014a).
The major threats to H. caput-felis were determined through field observations and categorized following the version 3.2 of IUCN threats classification scheme (IUCN Citation2014b).
Statistical analysis
Considering that the peak of the reproductive season occurred in May, only the data of this month have been used in the analyses. Morphometric traits collected in the field were used to calculate the volume of each plant (plant size, hereafter). We calculated plant size (V, expressed in dm3) using individual height (h, expressed in dm) and the maximum and minimum diameter (dM and dm, respectively), according to the following formula:
The Pearson correlation value was calculated to correlate plant size with reproductive parameter. To evaluate the effect of locality, ecological parameters (geomorphology, substrate and habitat type) and disturbance on plant density, plant size and fruit production, three independent generalized linear models were fitted using a normal function for continuous variables, Poisson error distribution and log as a link function for count data. GLM analyses were performed by a stepwise procedure using JMP 7.0 (SAS, SAS Institute, Cary, NC, USA).
The non-parametric Mann–Whitney U-test was performed to verify differences between localities in the mean percentage of seeds per fruit; these analyses were performed using Statistica 8.0 software (Statsoft, Tulsa, OK, USA).
Results
Surface-area and plant density
The overall surface-area occupied by the H. caput-felis population is 30.61 ha (17.87 ha for CM and 12.74 ha for ST); these locations represent almost the entire Sardinian population (99.99%), although a few isolated plants grow in some southern coastal sites along the Sinis Peninsula (data not shown).
The estimated mean density was 4.73 ± 2.31 plants/m2, and it varied from 4.63 ± 2.25 (CM) to 4.83 ± 2.42 (ST) plants/m2; the minimum and maximum density values ranged from 2 to 9.5 plants/m2 for CM and from 1 to 8.5 plants/m2 for ST (Table ).
Table 1. Helianthemum caput-felis localities and their population traits: area, mean density, estimated population range, mean plant size, percentage of reproductive plants, mean number of flowers and fruits per plant, percentage of empty fruits and mean number of seeds per fruit.
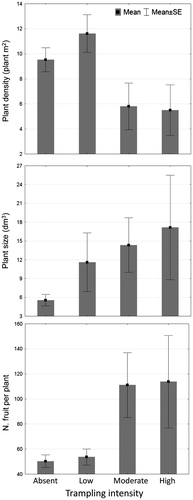
Plant density was higher in bedrock and lowland areas, and the highest density value was observed in garrigue, followed by maquis (Table ); however, the differences among plants growing in different ecological conditions are not statistically significant (p > 0.05; Table ). Our results revealed that only human trampling intensity significantly affected plant density (< 0.05; Table ): in fact, the lowest plant density was observed in the plot with intense trampling pressure (6.67 ± 4.27 plants/m2) compared with undisturbed areas (trampling low and absent, 10.38 ± 4.61 and 10.48 ± 4.96 plants/m2, respectively; Figure ).
Table 2. Helianthemum caput-felis traits at population and plant levels in relation to substrate, geomorphology and habitat in Sardinia.
Table 3. Generalized linear models results: effects of locality and ecological parameters on plant density.
Plant size
The mean plant size was 8.61 ± 35.48 dm3, ranging from a minimum of 0.20 × 10−3 dm3 to a maximum of 628.32 dm3. Plants growing in CM (mean value 11.21 ± 48.68 dm3) were larger than those growing in ST (mean value 6.11 ± 13.72 dm3); indeed, a higher percentage of large plants (plant volume > 25.00 dm3) was observed in CM compared with ST (10.3% of the plants measured for CM and 3.1% for ST).
All ecological variables analysed in our study had a statistically significant effect on plant size, as well as the interactions between geomorphology and substrates and between geomorphology and habitat significantly affected plant size (p < 0.001; Table ); in particular, larger plants were found in areas with the following ecological features: presence of structured soil, on the slopes, in the maquis habitat (Table ) and in areas with intensive human trampling (Figure ). In contrast, smaller plants were observed in areas without human trampling (5.55 ± 13.08 dm3).
Table 4. Generalized linear models results: effects of locality and ecological parameters on plant size (plant volume).
Reproductive traits
Flowering season occurs from March to late May, but isolated flowers were present until mid-June. In CM, the flowering season ranged from March to mid-May, and peak of flowering was recorded in mid-April. The flowering season in ST ranged from early April to mid-May, and peak flowering was recorded in early May. Generally, the fruiting season lasts 1 month; here, it lasted from April to July, and the peak occurred from mid-May to mid-June in both localities.
The mean percentage of reproductive plants was 76.72%, ranging from 64.86% (CM) to 88.08% (ST). The mean number of flowers per plant was 33.23 ± 46.98, ranging from 1 to 327. Notably, the number of ST flowers per reproductive plant (39.01 ± 44.52) was higher than CM (30.42 ± 48.00).
The mean number of fruits per reproductive plant was 58.03 ± 86.47 and it was higher in ST than in CM (78.49 ± 107.15 and 36.69 ± 49.43, respectively). All ecological variables analysed had a statistically significant effect on the number of fruits per plant, as well as the interactions between geomorphology and habitat significantly affected plant size (p < 0.001; Table ); plants displayed a higher mean number of fruits in deep and structured soil and in lowland areas, preferably in the garrigue and maquis habitats (Table ). The mean number of fruits per plant increased as human trampling intensified, with mean values ranging from 50.33 ± 71.17 (absent human trampling) to 113.18 ± 190.62 (intense human trampling; Figure ).
Table 5. Generalized linear models results: effects of locality and ecological parameters on fruit output per plant.
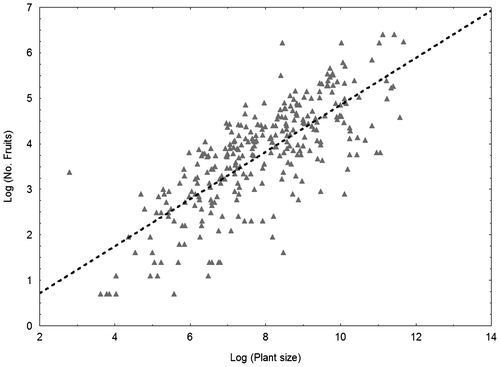
A positive correlation between number of fruits and plant size was observed (r = 0.76, r2 = 0.57 and p < 0.001; Figure ).
Fig. 3. Relationships between plant size (plant volume) and reproductive capacity (number of fruits per plant) in Helianthemum caput-felis. The following equation was used: No. of fruits = – 0.320 + 0.517 × plant volume; r2 = 0.57; r = 0.76; p < 0.001.
Overall, approximately 20% of the fruits were empty, ranging from 17.5% for CM to 22.5% for ST. The mean number of seeds per fruit was 4.29 ± 1.39, ranging from 1 to 7 seeds per fruit; the fruits collected in CM had a higher mean number of seeds per fruit (4.31 ± 1.40) compared with those in ST (4.27 ± 1.37), but this difference was not statistically significant (Mann–Whitney U-test, p > 0.05).
IUCN assessment at regional level
The same threats were detected in all localities where H. caput-felis was found, including areas with scattered plants growing in the southern part of the Sinis Peninsula. The major threat is tourism and other outdoor activities (such as human trampling), followed by the expansion of agricultural activities (such as agriculture and wood plantations) and the invasion of exotic plants. All of these pressures result in reduced population size due to habitat loss and fragmentation. Stochastic environmental events (e.g. landslides) pose a significant potential threat. All of these threats could result in the disappearance of small localities with a consequent reduction in EOO and AOO values, as well as in the number of localities. Additionally, continuous, human-induced habitat degradation will continue to occur in a predictable manner in the future.
Based on the EOO (3.99 km2), AOO (16 km2, i.e. four cells of 2 × 2 km), decline rate and number of locations (one, sensu IUCN Citation2014a), we propose that H. caput-felis should be included on the Red List categorization of Critically Endangered at the regional level, based on the following formula: B1ab(i,ii,iii,v) + 2ab(i,ii,iii,v).
Discussion
Our study highlighted important information regarding the distribution pattern, reproductive traits and ecological requirements of H. caput-felis, which are relevant issues for developing future conservation measures for this species in Sardinia. To the best of our knowledge, no exhaustive studies have been performed on the central populations of H. caput-felis, and the present study is the first investigation to analyse the population traits of this threatened species under natural conditions.
In the Sardinian population, density did not vary between localities and ecological features (geomorphology, substrate and habitat type), suggesting the absence of strong differences in ecological stress among different local conditions. The actual distribution of H. caput-felis in Sardinia is restricted in the two main localities, which constitute “ecological islands” (acting as local refuges) in coastal areas strongly modified by human activity. Hence, H. caput-felis could represent a “refuge-model” plant in Sardinia, with a range that simply occupies a reduced fraction of a wider habitat from which it is excluded by intensive human-induced habitat degradation, similar to several endemic species in the Mediterranean basin (Thompson Citation2005). In this restricted ecological context, H. caput-felis represents one of the principal species among coastal vegetation types.
Our data detected a significant effect of topography, landform and habitat type on plant fitness. Helianthemum caput-felis prefers lowland areas with deep, structured soil due to the amount of water and resources available and morphological stability. Moreover, as highlighted by the mean number of fruits per plant, H. caput-felis was ecologically optimum in garrigues, whereas it produced few fruits in micro-forests.
Considering previous studies (Tébar, Gil, and Llorens Citation1997; Rodriguez-Pérez Citation2005), the phenological pattern of H. caput-felis is similar in the Sardinian and Balearic populations. Information on the reproductive biology of endangered plants is crucial for predicting their survival capacity and establishing the appropriate measures for their conservation (e.g. Schemske et al. Citation1994; Cogoni, Fenu and Bacchetta Citation2015). However, despite the ecological importance of the Cistaceae in the Mediterranean Basin, few studies have been carried out on this topic for this family (Herrera Citation1992; Talavera, Gibbs, and Herrera Citation1993; Rodríguez-Pérez Citation2005; Guzmán, Narbona, and Vargas Citation2011). Local studies have been carried out on Helianthemum species (Tébar, Gil, and Llorens Citation1997; Rodriguez-Perez Citation2005; Aragón and Escudero Citation2008), but it remains one of the genera for which reproductive biology remains less documented.
The H. caput-felis population in Sardinia is mainly composed of reproductive plants (78.31%), while the percentage of saplings and seedlings is relatively low; this result, together with the high number of seeds produced each year, suggests that seedling establishment represent the main critical stage for this plant. However, further studies are needed to understand whether this is related to marginalization of the population or the lack of suitable micro-sites for plant establishment. In fact, in the case of locally restricted and threatened species, many seeds may end up in unsuitable areas depending on their peculiar ecological requirements (e.g. Cogoni et al. Citation2012). The lowest percentage of reproductive plants was observed in CM (two-thirds of the total), suggesting a greater rate of recruitment, whereas in ST, four-fifths of the plants were reproductive. The similar number of empty fruits per plant and number of seeds per fruit between the two localities (lower values in ST) could be related to the impact of agricultural activities that determine habitat fragmentation. However, at the population level, multiplying the number of viable seeds per fruit per the estimated number of reproductive plants per locality by the mean number of fruit per plant gives approximately 75 million viable seeds for CM and 137 million for ST; hence, seed production is not a limiting factor for this plant.
Reproductive limitations were not detected for this species (i.e. fruit and seed set, pollination service and seedling survival on natural populations) in previous studies (e.g. Rodríguez-Pérez Citation2005); considering our findings on the high seed output, the increasing rarity of this species is probably a direct result of the destruction of its natural habitat.
Habitat fragmentation or destruction caused by human disturbance is currently considered one of the main factors responsible for reducing population viability and increasing the extinction risk of rare plants and/or of marginal and small populations (Schemske et al. Citation1994; Holsinger Citation2000; Schleuning and Matthies Citation2009). As a consequence, many threatened or rare plants are confined to naturally fragmented habitats or ecological islands that might be separated by large inhospitable areas, as noted in this study.
Among other factors, human trampling is an important threat for threatened endemic species in Sardinia (Quilichini and Debussche Citation2000; Fenu, Mattana, and Bacchetta Citation2011; Fenu et al. Citation2013; Rossi et al. Citation2015). A consistent reduction in reproductive traits was observed in plant populations subjected to human trampling (e.g. Rossi et al. Citation2006; Fenu et al. Citation2013), resulting in a serious threat to the persistence of the population. Accordingly, in the present study, a negative effect of human trampling was observed on plant density. Surprisingly, human trampling enhanced the plant size and the rate of fruit production, suggesting that the reproductive plant of H. caput-felis is tolerant to direct damage and probably benefits from the reduction of inter-specific and intra-specific competition. However, considering the critical limitation in seedling recruitment, as suggested by the negative effect on plant density, human trampling should be considered a significant threat to the persistence of the Sardinian population.
Red lists highlight the most pressing issues in biodiversity conservation matters, representing taxa that are closer to extinction (Rossi et al. Citation2013, Citation2015). In this context, Red lists may be policy-relevant for promoting conservation efforts, but they cannot be considered policy-prescriptive (Bilz et al. Citation2011; IUCN Citation2014a). Very little attention has been given to border populations in the application of the IUCN criteria at the regional level (IUCN Citation2003; Miller et al. Citation2007) and only recent research assigned some Italian marginal populations of widespread species to high-risk categories (e.g. Gargano et al. Citation2007; Del Vecchio et al. Citation2012; Rossi et al. Citation2015). This study confirms this trend for H. caput-felis, and it allows us to raise the risk category at the regional level for this species from Lower Risk (LR; Conti, Manzi, and Pedrotti Citation1997) to Critically Endangered (CR). Although criterion B is biased by restricted range and could overestimate the extinction risk, it is strongly supported by the population decline observed in Sardinia. However, to confirm or reject the assumed worse performance and higher vulnerability of border populations, an extensive and integrative approach that compares all population over a wide temporal context is needed; in addition, the effect of population attributes (e.g. population size or structure) or the particular conditions where populations occur (e.g. human trampling intensity analysed in this study) should be taken into account to separate the role of local and positional factors that drive populations (Garcia, Goñi, and Guzmán Citation2009). This approach is essential for all plants at the margin of their distribution range to plan appropriate conservation measures to reduce the extinction risk of these populations.
Implication for conservation
According to the European Strategy for Plant Conservation (Planta Europa Citation2008), populations at the border of their distribution area, such as H. caput-felis in Sardinia, should be considered of high interest, with a need for urgent conservation measures. In situ and ex situ (e.g. seed conservation in seed banks, cultivation in botanical gardens) conservation efforts should be improved. In particular, greater emphasis should be given to minimizing the range of negative impacts, including unsustainable tourism and recreation use (Fenu, Mattana, and Bacchetta Citation2011; Ballantyne and Pickering Citation2013; Fenu et al. Citation2013; Rossi et al. Citation2015). Therefore, touristic and recreational activities should be regulated in known localities, and no new pathways should be opened. A management strategy should exclude trampling in different portions of the population to facilitate the plant recruitment process and population renewal.
In addition, an ex situ conservation strategy must be implemented and the seeds collected could be used for future reinforcement or reintroduction of this species in suitable areas, as realized in Spanish regions or following low-cost programmes carried out in Sardinia (Cogoni et al. Citation2013). These actions may be extremely important for conservation in a changing climate (Sala et al. Citation2000; Godefroid et al. Citation2011). Moreover, because many threats will affect plant species over the next few decades (e.g. climate change, biological invasions), long-term monitoring programmes must be developed to reveal changes in the species conservation status (Balmford, Green, and Jenkins Citation2003; Fenu et al. Citation2015), as well as monitoring both the vegetation and the human threat dynamics.
Acknowledgements
The authors thank Sergio Sulis for his help with the fieldwork. We thank Elsevier Language Editing Services for linguistic revision (Project nr: 47013).
References
- Abeli, T., R. Gentili, G. Rossi, G. Bedini, and B. Foggi. 2009. “Can the IUCN criteria be effectively applied to peripheral isolated plant populations?” Biodiversity and Conservation 18: 3877–3890.
- Agulló, J. C., A. Juan, A. Guilló, M. Á. Alonso, and M. B. Crespo. 2011. “Genetic diversity and phylogeographical assessment of Helianthemum caput-felis Boiss. (Cistaceae) based on AFLP markers.” Fitosociologia 48: 21–29.
- Aragón, C. F., and A. Escudero. 2008. “Mating system of Helianthemum squamatum (Cistaceae), a gypsophile specialist of semi-arid Mediterranean environments.” Botanica Helvetica 118: 129–137.
- Arrigoni, P. V. 1971. “Helianthemum caput-felis Boiss. (2n=24) nuovo reperto per la flora italiana [Helianthemum caput-felis Boiss. (2=24) new report for the Italaian flora].” Webbia 26: 237–243.
- Balmford, A., R. E. Green, and M. Jenkins. 2003. “Measuring the changing state of nature.” Trends in Ecology and Evolution 18: 326–330.
- Ballantyne, M., and C. M. Pickering. 2013. “Tourism and recreation: A common threat to IUCN red-listed vascular plants in Europe.” Biodiversity and Conservation 22: 3027–3044.
- Bilz, M., S. P. Kell, N. Maxted, and R. V. Lansdown. 2011. European Red List of Vascular Plants. Luxembourg: Publications Office of the European Union.
- Cogoni, D., G. Fenu, and G. Bacchetta. 2015. “Reproductive biology of the narrow endemic Anchusa littorea Moris (Boraginaceae), an endangered coastal Mediterranean plant.” Turkish Journal of Botany, in press. doi:10.3906/bot-1403-16.
- Cogoni, D., G. Fenu, E. Concas, and G. Bacchetta. 2013. “The effectiveness of plant conservation measures: the Dianthus morisianus reintroduction.” Oryx 47: 203–206.
- Cogoni, D., E. Mattana, G. Fenu, and G. Bacchetta. 2012. “From seed to seedling, a critical stage for the psammophilous species Dianthus morisianus.” Plant Biosystems 146: 910–917.
- Comor, V., J. Orgeas, P. Ponel, C. Rolando, and Y. R. Delettre. 2008. “Impact of anthropogenic disturbances on beetle communities of French Mediterranean coastal dunes.” Biodiversity and Conservation 17: 1837–1852.
- Conradt, L. 2001. “Ecological and evolutionary processes at expanding range margins.” Nature 411: 577–581.
- Conti, F., A. Manzi, and F. Pedrotti. 1997. Liste Rosse Regionali delle Piante d’Italia [Regional Red Lists of Italian Plants]. Camerino: WWF Italia, Società Botanica Italiana.
- Davenport, J., and J. L. Davenport. 2006. “The impact of tourism and personal leisure transport on coastal environments: A review.” Estuarine, Coastal and Shelf Science 67: 280–292.
- Del Vecchio, S., E. Giovi, F. Izzi, G. Abbate, and A. T. R. Acosta. 2012. “Malcolmia littorea: The isolated Italian population in the European context.” Journal for Nature Conservation 20: 357–363.
- Dominguez Lozano, F., D. G. Herbada, L. M. Rivero, J. C. Saiz, and H. S. Ollero. 1996. “Threatened plants in Peninsular and Balearic Spain: A report based on the EU Habitats directive.” Biological Conservation 76: 123–133.
- Eckert, C. G., K. E. Samis, and S. C. Lougheed. 2008. “Genetic variation across species’ geographical ranges: The central-marginal hypothesis and beyond.” Molecular Ecology 17: 1170–1188.
- Fenu, G., and G. Bacchetta. 2008. “La flora vascolare della penisola del Sinis (Sardegna occidentale) [The vascular flora of the peninsula del Sini (Western Sardinia)].” Acta Botánica Malacitana 33: 91–124.
- Fenu, G., D. Cogoni, T. Ulian, and G. Bacchetta. 2013. “The impact of human trampling on a threatened coastal Mediterranean plant: The case of Anchusa littorea Moris (Boraginaceae).” Flora 208: 104–110.
- Fenu, G., E. Mattana, and G. Bacchetta. 2011. “Distribution, status and conservation of a critically endangered, extremely narrow endemic: Lamyropsis microcephala (Asteraceae) in Sardinia.” Oryx 45: 180–186.
- Fenu, G., E. Sulis, D. Cogoni, and G. Bacchetta. 2012. “Schede per una Lista Rossa della Flora vascolare e crittogamica Italiana: Helianthemum caput-felis Boiss.” [Record for a Red List of Vascular and Cryptogamic Intalian Flora: Helianthemum caput-felis Boiss]." Informatore Botanico Italiano 44: 233–236.
- Fenu, G., D. Cogoni, M. S. Pinna, and G. Bacchetta. 2015. “Threatened Sardinian vascular flora: A synthesis of ten years of monitoring activities.” Plant Biosystems, in press. doi:10.1080/11263504.2014.1000424.
- Fois, M., G. Fenu, A. Cuena Lombraña, D. Cogoni, and G. Bacchetta. 2015. “A practical method to speed up the discovery of unknown populations using distribution models.” Journal for Nature Conservation 24: 42–48.
- Garcia, M. B., D. Goñi, and D. Guzmán. 2009. “Living at the edge: local versus positional factors in the long-term population dynamics of an endangered orchid.” Conservation Biology 24: 1219–1229.
- Gargano, D., G. Fenu, P. Medagli, S. Sciandrello, and L. Bernardo. 2007. “The status of Sarcopoterium spinosum (Rosaceae) at the western periphery of its range: Ecological constraints led to conservation concerns.” Israel Journal of Plant Sciences 55: 1–13.
- Godefroid, S., C. Piazza, G. Rossi, S. Buord, A. D. Stevens, R. Aguraiuja, C. Cowell, et al. 2011. “How successful are plant species reintroductions?” Biological Conservation 144: 672–682.
- Grammont De, P. C., and A. D. Cuarón. 2006. “An evaluation of threatened species categorization systems used on the American continent.” Conservation Biology 20: 14–27.
- Grant, M. C., and J. Antonovics. 1978. “Biology of ecologically marginal populations of Anthoxanthum odoratum. I. Phenetics and dynamics.” Evolution 32: 822–838.
- Guzmán, B., E. Narbona, and P. Vargas. 2011. “Similar reproductive success of the two petal colour polymorphisms of Cistus ladanifer (Cistaceae).” Plant Biosystems 145: 931–937.
- Guzmán, B., and P. Vargas. 2009. “Historical biogeography and character evolution of Cistaceae (Malvales) based on analysis of plastid rbcL and trnL-trnF sequences.” Organisms Diversity and Evolution 9: 83–99.
- Herrera, J. 1992. “Flower variation and breeding systems in the Cistaceae.” Plant Systematics and Evolution 179: 245–255.
- Holt, R. D., and T. H. Keitt. 2005. “Species’ borders: A unifying theme in ecology.” Oikos 108: 3–6.
- Holsinger, K. E. 2000. “Demography and extinction in small populations.” In Genetics, Demography and Viability of Fragmented Populations, edited by A.G. Young and G.M. Clark, 55–74. Cambridge: Cambridge University Press.
- IUCN. 2001. IUCN Red List Categories and Criteria: Version 3.1. IUCN Species Survival Commission. UK Gland, Switzerland and Cambridge: IUCN published.
- IUCN. 2003. Guidelines for application of IUCN Red List criteria at regional levels version 3.0. IUCN Species Survival Commission. UK Gland, Switzerland and Cambridge: IUCN published.
- IUCN. 2014a. Guidelines for application of IUCN Red List Criteria at regional levels: Version 11. IUCN Species Survival Commission. IUCN, Gland, Switzerland. Accessed February 20, 2015. http://www.iucnredlist.org/documents/reg_guidelines_en.pdf
- IUCN. 2014b. Threats classification scheme: version 3.2. Accessed February 20, 2015. http://www.iucnredlist.org/technical-documents/classification-schemes/threats-classification-scheme
- Kerbiriou, C., I. Leviol, F. Jiguet, and R. Julliard. 2008. “The impact of human frequentation on coastal vegetation in a biosphere reserve.” Journal of Environmental Management 88: 715–728.
- Kutiel, P., E. Eden, and Y. Zhevelev. 2000. “Effect of experimental trampling and off-road motorcycle traffic on soil and vegetation of stabilized coastal dunes, Israel.” Environmental Conservation 27: 14–23.
- Lesica, P., and F. W. Allendorf. 1995. “When are peripheral populations valuable for conservation?” Conservation Biology 9: 753–760.
- López González, G. 1992. “Apuntes para justificar el tratamiento del género Helianthemum Miller, s.l. (Cistaceae), en Flora Iberica.” [Notes to explain the treatment of the genus Helianthemum Miller, s.l. (Cistaceae), on Flora Iberica] Anales del Jardín Botánico de Madrid 50: 35–63.
- Miller, R. M., J. P. Rodríguez, T. Aniskowicz-Fowler, C. Bambaradeniya, R. Boles, M. A. Eaton, U. Gärdenfors, et al. 2007. “National threatened species listing based on IUCN criteria and regional guidelines: Current status and future perspectives.” Conservation Biology 21: 684–696.
- Pickering, C. M., and W. Hill. 2007. “Impacts of recreation and tourism on plant biodiversity and vegetation in protected areas in Australia.” Journal of Environmental Management 85: 791–800.
- Planta Europa. 2008. A sustainable future for Europe; the European strategy for plant conservation 2008–2014. Strasbourg: Plantlife International (Salisbury, UK) and the Council of Europe.
- Pouget, M., S. Youssef, J. Migliore, M. Juin, F. Médail, and A. Baumel. 2013. “Phylogeography sheds light on the central–marginal hypothesis in a Mediterranean narrow endemic plant.” Annals of Botany 112: 1409–1420.
- Próctor, M. C. F., and V. H. Heywood. 1968. “Helianthemum Miller.” In Flora Europaea, edited by T. G. Tutin, H. Heywood, N. A. Burges, D. M. Moore, D. H. Valentine, S. M. Walters, and D. A. Webb, 2: 286–292. Cambridge: Cambridge University Press.
- Quézel, P., and S. Santa. 1963. Nouvelle flore de l'Algérie et des régions désertiques méridionales. Volumen 2. Paris: Centre National de la Reherche Scientifique.
- Quilichini, A., and M. Debussche. 2000. “Seed dispersal and germination patterns in a rare Mediterranean island endemic (Anchusa crispa Viv., Boraginaceae).” Acta Oecologica 21: 303–313.
- Raynaud, C. 1999. “Cistaceae.” In Flore Pratique du Maroc. Manuel de détermination des plantes vasculaires, 1, s. Bot. 36, edited by M. Fennane, J. Ibn Tattou, A. Mathez, and J. Ouyahya El Oualidi, 302–326. Rabat: Travaux Institut Scientifique, Université Mohammed V.
- Rodríguez-Pérez, J. 2005. “Breeding system, flower visitors and seedling survival of two endangered species of Helianthemum (Cistaceae).” Annals of Botany 95: 1229–1236.
- Rodrigues, A. S. L., J. D. Pilgrim, J. L. Lamoreux, M. Hoffmann, and T. M. Brooks. 2006. “The value of the IUCN Red List for conservation.” Trends in Ecology and Evolution 21: 71–76.
- Rossi, G., C. Montagnani, T. Abeli, D. Gargano, L. Peruzzi, G. Fenu, S. Magrini, et al. 2013. “Are Red Lists really useful for plant conservation? The New Red List of the Italian Flora in the perspective of National Conservation policies.” Plant Biosystems 148: 187–190.
- Rossi, G., G. Parolo, L. A. Zonta, J. A. Crawford, and A. Leonardi. 2006. “Salix herbacea L. fragmented small population in the N-Apennines (Italy): Response to human trampling disturbance.” Biodiversity and Conservation 15: 3881–3893.
- Rossi, G., S. Orsenigo, C. Montagnani, G. Fenu, D. Gargano, L. Peruzzi, R. P. Wagensommer, et al. 2015. “Is legal protection sufficient to ensure plant conservation? The Italian Red List of policy species as a case study.” Oryx, in press. doi:10.1017/S003060531500006X.
- Sala, O. E., F. S. I. I. I. Chapin, J. J. Armesto, E. Berlow, J. Bloomfield, R. Dirzo, E. Huber-Sanwald, et al. 2000. “Global biodiversity scenarios for the year 2100.” Science 287: 1770–1774.
- Schleuning, M., and D. Matthies. 2009. “Habitat change and plant demography: Assessing the extinction risk of a formerly common grassland perennial.” Conservation Biology 23: 174–183.
- Schemske, D. W., B. C. Husband, M. H. Ruckelshaus, C. Goodwillie, I. M. Parker, and J. D. Bishop. 1994. “Evaluating approaches to the conservation of rare and endangered plants.” Ecology 75: 584–606.
- Sexton, J. P., P. J. McIntyre, A. L. Angert, and K. J. Rice. 2009. “Evolution and ecology of species range limits.” Annual Review of Ecology Evolution and Systematics 40: 415–436.
- Talavera, S., P. E. Gibbs, and J. Herrera. 1993. “Reproductive biology of Cistus ladanifer (Cistaceae).” Plant Systematics and Evolution 186: 123–134.
- Tébar, F. J., L. Gil, and L. Llorens. 1997. “Reproductive biology of Helianthemum apenninum (L.) Mill. and H. caput-felis Boiss. (Cistaceae) from Mallorca (Balearic Islands, Spain).” Acta Botánica Malacitana 22: 53–63.
- Thompson, J. D. 2005. Plant Evolution in the Mediterranean. Oxford: Oxford University Press.
- Villellas, J., J. Ehrlén, J. M. Olesen, R. Braza, and M. B. García. 2013. “Plant performance in central and northern peripheral populations of the widespread Plantago coronopus.” Ecography 36: 136–145.
- Villellas, J., W. F. Morris, and M. B. García. 2013. “Variation in stochastic demography between and within central and peripheral regions in a widespread short-lived herb.” Ecology 94: 1378–1388.
- Yu, S., D. Bell, and P. Kutiel. 2009. “Impact of microhabitats on the heterogeneity of seedling emergence in a Mediterranean coastal sand dunes community.” Ecoscience 16: 369–378.