Abstract
Floodplain landscape is the result of man’s presence over a long period of time. This has caused the degradation, or even loss, of several habitats, especially wetlands, which frequently preserve rare biodiversity, even though they are subjected to intense human presence. Human activities can be of value, but can also cause problems in species preservation. The protected area “Ansa e Valli del Mincio” (northern Italy) is an outstanding case study in this respect: it is located in a densely urbanized context, but has great value from a conservation standpoint. This paper presents the results of 10 years of monitoring (2003–2012) of the spatial distribution of two native species (Trapa natans and Nymphaea alba) and the invasive Nelumbo nucifera. Data collected by volunteers from the protected area were processed in GIS and analysed using a number of configurational landscape metrics. Nymphaea alba and N. nucifera became more widespread; T. natans underwent a serious decline, mostly due to the interference of N. nucifera. An oscillating trend of this species is visible until 2008, followed by substantial stability. Furthermore, the potential area of each species was calculated. On the whole, citizen science is a valuable tool to enhance biodiversity knowledge and safeguarding, especially in wetlands that are used for tourism and surrounded by residential areas. As in other contexts, the volunteer contribution was particularly helpful in data collection on a local scale, over a considerable time span.
Introduction
All over the world, the decline of biodiversity is leading to massive “biotic homogenization” (García-Llorente et al. Citation2008; Winter et al. Citation2009). Many areas, such as European flood plains, have undergone widespread industrialization, urbanization and other land-use changes, causing fragmentation, degradation and loss of certain habitats such as continental alluvial grasslands or riverine wetlands (Sather and Smith Citation1984; Wassen, Peeters, and Olde Venterink Citation2003; Eckstein, Hölzel, and Danihelka Citation2006; Šeffer, Janák, and Šefferová Stanová Citation2008; Danihelka, Niklfeld, and Šípošová Citation2009; Wärner et al. Citation2011). On a European scale, the destruction or the degradation of wetland habitats has been so intense that 66% of the extinctions of continental species involve wetlands (Denny Citation1994; Sager and Clerc Citation2006).
Degraded and polluted habitats are susceptible to rapid colonization by invasive alien species (IAS), with unpredictable effects, which are difficult to manage and which result in a general loss of ecosystems (Perrings, Williamson, and Dalmazzone Citation2000; Pimentel et al. Citation2000; Duncan et al. Citation2004). Most of the IAS are responsible for the direct loss of biological diversity, causing numerous extinctions on a local or even global scale (Davis Citation2009), often resulting in irreversible landscape transformation. The habitats most subject to plant invasion are riverine and riparian wetlands (Pyšek and Prach, Citation1994; Pyšek, Prach, and Mandak Citation1998). In Italy, 163 plant species (i.e. 16% of alien taxa) are invasive (Blasi et al. Citation2010). More than half of the 12 locally invasive species are aquatic, some of them recently introduced and widely spreading throughout the territory. Sometimes, IAS provide benefits to the population (Kendle and Rose Citation2000; Baskin Citation2002): in these cases, people can be opposed to any limitation of their use, even if governments pursue a containment strategy for socio-economic and ecological sustainability (Bardsley and Edwards-Jones Citation2007). For these reasons, examining how local people perceive an invasive species is fundamental in designing effective environmental policies (Bardsley and Edwards-Jones Citation2007; García-Llorente et al. Citation2008). Various IAS were first introduced for ornamental purposes and people do not see them as a danger because of their high aesthetic value (McNeely Citation2001). In Italy, the economic development of the Po Plain caused many marsh areas to disappear, leaving a landscape with only a few relatively natural surviving parts in a matrix almost completely altered by human-induced transformations (Buldrini, Dallai, and Torri Citation2013). Especially from the 1920s onwards, large unhealthy, swampy areas were made habitable by drainage, satisfying man’s needs but leaving the pre-existing environmental and biological diversity greatly impoverished (Dallai et al. Citation2014). Human activities have led to the progressive decrease and fragmentation of habitats suitable for many hydro-hygrophilous species, confining them to marginal zones and significantly reducing their populations (e.g. Buldrini et al. 2013), with the irreversible loss of local phytogenetic features. Among these species, Trapa natans L. and Nymphaea alba L. are today characterized by the above-mentioned problems. Trapa natans is a palaeotemperate-subtropical therophyte, typical of lentic or stagnant eutrophic water (Tutin et al. Citation1968: 303). This species is generally considered a weed because of its very abundant growth in suitable ecological conditions (Groth, Lovett-Doust, and Lovett-Doust Citation1996). Nymphaea alba is a Eurasian radicant hydrophyte, typical of stagnant, fresh and oligotrophic water bodies (Tutin et al. Citation1964: 205), widespread in parks and gardens of many parts of the world because of its ornamental value. At a European and Italian level, both species are included in Red Lists of threatened species, albeit in different categories (Schnittler and Günther Citation1999; Bilz et al. Citation2011; Rossi et al. Citation2013).
The protected area of “Ansa e Valli del Mincio” is one of the most important wetland areas of all northern Italy (Persico and Truzzi Citation2008). It is characterized by notable naturalistic and environmental value, but suffers due to its proximity to a densely inhabited urban area. This causes a number of ecological and conservation problems, but it also allows intense human presence for leisure activities and tourism. Hence the biodiversity hosted in the protected area must be considered as a value in itself and in relation to the perception that people have of the plant species living there. In addition, the area is interesting also for many volunteers, involved in various activities such as data collection and environmental education. This paper considers the eastern part of the “Ansa e Valli del Mincio”, where the high presence of N. alba and T. natans corresponds to a high presence of the IAS Nelumbo nucifera Gaertn. In particular, the results of 10 years of observation (2003–2012) by protected area volunteers are presented, to show the spatio-temporal dynamics of these three species. This paper assesses the usefulness of data obtained by volunteers (i.e. “citizen scientists” sensu Silvertown Citation2009) after data processing and harmonization, in view of an increasing “democratization of the environment” (sensu Carolan Citation2006). The final goal is to suggest some actions to improve area management so as to better preserve its characteristic native plant species.
Materials and methods
Study area
The study area is the eastern part of the “Ansa e Valli del Mincio” (45°9′46" N, 10°44′24" E, extent 15.170 km2; Figure ). For its conservation value, it is protected on a local, national and continental level: it is part of the Natura 2000, an EU-wide network for nature protection (Habitat Directive 92/43/EEC; Bird Directive 79/409/EEC) and is a Wetland Site of International Importance according to the Convention of Ramsar (code 3IT037). However, due to its location very close to densely urbanized sites and with a high level of soil sealing, the area is subject to many anthropic pressures (i.e. rubbish dumping, pollution, eutrophication, noise caused by boats and ferry boats) to which an intense tourist presence must be added.
Fig. 1. Spatial localization of the study area “Ansa e Valli del Mincio” (45°9′46" N, 10°44′24" E). (A) Map of the study area (the small arrows indicate the direction of the river current). (B) Location of the study area in Italy. (C) A summer aspect of the wetland area (photograph taken by F. Buldrini, July 2011).
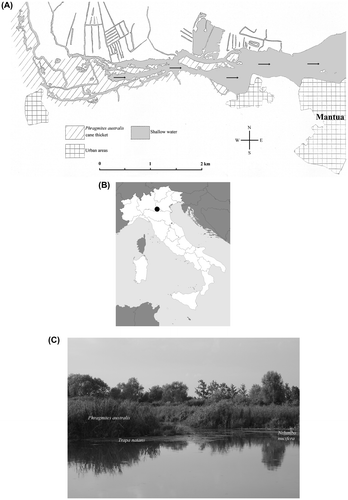
The present landscape is the result of medieval hydraulic works, dating back to the late twelfth century (Marani Citation1984). Vegetation is made up of Phragmites australis (Cav.) Trin. ex Steud. thickets and large alluvial meadows, dominated by Carex elata All. and Molinia caerulea (L.) Moench., where many protected species can grow, such as Gentiana pneumonanthe L. and various orchid species (Persico and Truzzi Citation2008). Shallow waters are characterized by reduced hydrodynamism, high rates of submerged and emerging macrophytic primary production, fine sediment with high organic load and intense bacterial processes of mineralization (Telò et al. Citation2007). They host a vegetation formed by populations of floating-leaved plant species, especially N. alba and T. natans.
Both species, T. natans and N. alba, are locally quite abundant, while in other Italian wetlands they are rare or absent. In the area, the presence of T. natans has always been part of the heritage of popular local tradition. However, nowadays its value has been progressively forgotten. In contrast, N. alba is probably the best-known aquatic species (Persico and Truzzi Citation2008), but its aesthetic impact is notably less than the flowering of the IAS N. nucifera. The latter is naturalized, forming large populations because of its vigorous vegetative propagation (the rhizome grows up to 20 m per year; see Scoppola and Avena Citation1987). This species was first introduced in 1921 to obtain starch from the rhizomes. As this experiment did not succeed, its cultivation was soon abandoned (Pignatti Citation1982). In the whole protected area, the first containment interventions were carried out in 1997–1998, but a real management plan was introduced only in 2004, consisting of at least one mowing action per year. Furthermore, because of the great aesthetic impact of its flowers, N. nucifera is widely appreciated by the local population and is also a tourist attraction (Martignoni and Persico Citation2001). Hence, the present floristic features of the territory are also due to the different perception of the importance of autochthonous and exotic plant species in zones that are densely frequented by man.
In this context, the work of the volunteers, who are part of an environmental association linked to the protected area, is of fundamental importance. They are assiduously present in the area and are actively involved in environmental data collection and environmental education of tourists, school pupils and the local population. All of this can help to improve the management of this protected zone.
Methods
A georeferenced 10-year series of data (2003–2012) has been put together and analysed for N. alba, T. natans and N. nucifera, on the basis of paper maps reporting the patches of each plant species for each year. These data were spontaneously provided by the volunteers of the association linked to the protected area. Figure shows the workflow.
Fig. 2. Workflow of the present study. Activities carried out by volunteers (pale grey) and by university researchers (dark grey) are shown.
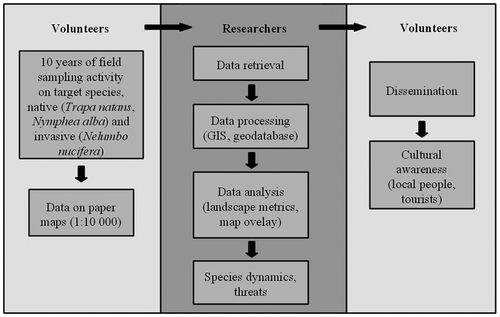
Data collection by volunteers
To obtain spatial data on the species studied, volunteers carried out surveys when the plants were at their maximum annual vegetative development: June to July for N. alba and N. nucifera, August to September for T. natans. All the surfaces with a plant cover of each species ≥ 40% were recorded. This threshold was considered to allow a clear detection of every single species patch. In the field, each patch was measured in length and width from a little boat and a visual assessment of the cover was carried out. Every year of survey took a total of 15 days of field activity. On each day of field sampling at least two people were involved. In the whole period (2003–2012) a total of four people were involved: two of them, who have a degree in Natural Sciences, collected data on plant species while the other two collaborators assisted them (i.e. by driving the boat). All the data collected were subsequently reported on a topographic paper map (1 : 10,000 scale) by one of the volunteers, trained by an expert in cartographic outputs.
Data processing
Data handling was carried out using Quantum GIS 2.8.1 (QGIS; www.qgis.org). Each map was scanned at 600 dpi and subsequently geo-referenced using a number of Ground Control Points (GCPs) recognizable on an already available geo-referenced topographic map. Selected GCPs produced a co-registration error of about 2 m; bilinear transformation was used as a re-sampling method. In QGIS, a vector layer was created for each of the 10 maps by manually tracing the limit of each patch.
Spatial data of the 10 layers were analysed by using Fragstats 4.2.1 (McGarigal, Cushman, and Ene Citation2012) to derive several landscape metrics at class level (i.e. every single species type):
| • | class area (CA): sum of the areas belonging to a species patch type | ||||
| • | number of patches (NP): sum of the patches belonging to a species patch type | ||||
| • | largest patch index (LPI): percentage of the landscape composed of the largest patch | ||||
| • | mean shape index (SHAPE_MN): average patch shape of a particular species patch type. | ||||
Full descriptions and algorithms of these metrics are provided in McGarigal, Cushman, and Ene (Citation2012).
Furthermore, the potential area of each species was derived by cumulating the area occupied by each single species in each year of observation, and the subsequent potential patch number was obtained. Then, the difference between the current and potential areas and the difference between the current and potential numbers of patches were calculated. Finally, interactions among species were evaluated by overlaying pairs of layers to detect the overlapping area. The Intersect geoprocessing tool of QGIS was used.
Results
Figures and 4, and Table show the results of change detection for the two native species T. natans and N. alba and the IAS N. nucifera for the 2003–2012 period.
Fig. 3. Trend (2003–2012) in the extent and patchiness of Trapa natans, Nymphaea alba and Nelumbo nucifera within the study area.
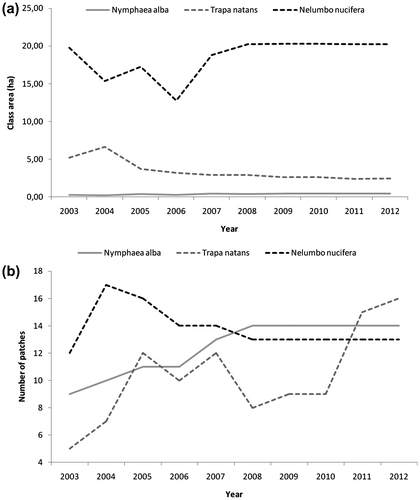
Fig. 4. Trend (2003–2012) in mean shape index of Trapa natans, Nymphaea alba and Nelumbo nucifera within the study area.
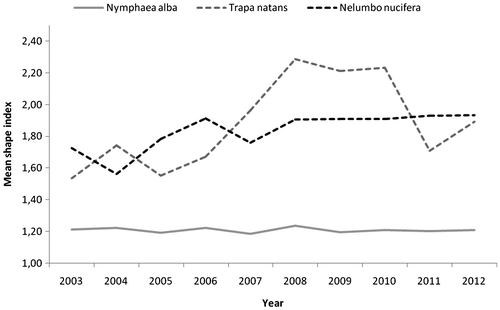
Table 1. List of landscape metrics calculated for the three studied species at each temporal step.
In 2012, T. natans had an extent of 2.45 ha. This was the result of a continuous decrease between 2003 and 2012 (– 2.72 ha). It should be noted that in 2004 T. natans reached its maximum extent (6.62 ha), whereas the historic lows were in 2011 (2.36 ha); from 2011 onwards there was a very weak trend reversal. This was related to some phases of fragmentation followed by a reduction and loss of patches. However, there was a general increase in the number of patches (from 5 to 16) between 2003 and 2012. From 2011, there was a very low increase in patch number. In the first years of observation (2003–2006) there was a patch whose largest patch index was quite high (> 10% until 2006): its extension varied from 10 to 20% of the total area covered by T. natans. In subsequent years, this patch underwent considerable reduction, so that the largest patch index in 2012 was only 4%. Concerning mean shape index, from 2003 to 2010 an increase was observable, especially between 2008 and 2010; a decrease followed in subsequent years. Overall, T. natans showed discrete instability both in patch shapes and in patchiness.
By contrast, N. alba extent was low throughout the study period: in 2012, the area it occupied was 0.40 ha. This was the result of a general increasing trend, notwithstanding the oscillating trend from 2003 to 2007 (+ 0.17 ha), which corresponded with an increase in the number of patches until 2008 (from 9 to 14). From 2009, some stabilization occurred. Given that the total extent increase was globally low, the main cause of this increase was a slight increase in the dimensions of the existing patches. The average shape of the patches did not show particular variations, ranging from 1.19 to 1.24.
The IAS N. nucifera was the most widespread among the three species under consideration. In 2012 it covered 20.27 ha: this surface was almost equivalent to the area occupied in 2003, although there was a slight increase from the first to the last record. However, two trends could be distinguished: oscillation in extent until 2008, then substantial stability. The same trend was also visible both in mean shape index and in largest patch index. The class area was influenced by one patch that covered about 60% of the total, whose historic lows in 2006 (46%) are correlated with the historic lows in extent (13 ha).
The potential extent of N. alba, T. natans and N. nucifera is reported in Table . When comparing the real occupied area to the potential one for every species and each year under consideration, all three species were always quite far from their potential extents. N. alba, despite the increase in its occupied area, never passed 52.6% of the potential extent; T. natans, after the 2004 peak (63%), stabilized at about 23%; N. nucifera, notwithstanding its oscillations in the 2004–2007 period, remained at around 64%. By intersecting the potential extent occupied by T. natans with the potential extent occupied by N. nucifera, an overlapping zone of 1.6 ha was found. However, this interference is present only in a single place of the whole study area. By contrast, no overlap between N. nucifera and N. alba was recorded. As to the number of patches, it was almost equivalent to the real value for N. alba and N. nucifera, whereas it was considerably different for T. natans, as a consequence of the fragmentation that this species underwent over the years.
Table 2. Potential extent and number of patches of the three studied species. For each year of observation, the difference between the potential and the real class area (CA) and the difference between the potential and the real number of patches (NP) are provided.
Discussion
The activities of volunteers have allowed a 10-year series (2003–2012) of spatial data to be available for three species (two native species, N. alba and T. natans, and one IAS, N. nucifera), which are of interest both for biological conservation and aesthetic value. Nymphaea alba is the least extended species.
N. alba had an increase in extent, although maintaining a low extent and patches with small dimensions (0.04 ha on average). Disturbance caused by river current, ferry-boat swell and water eutrophication might have limited the spread of the species. In addition, nitrate and phosphate concentrations required by N. alba are considerably lower than those of the environments suitable for T. natans (Klosowski Citation1995), so it can be assumed that N. alba is, in some respects, disadvantaged compared with the other species. By contrast, T. natans underwent a strong decline and some “pulsations” (cyclic fragmentations) in the number of patches were observed. The decrease in extent mostly happened at the expense of the most extended patches. Such a decrease was not counterbalanced by the formation of some new patches in 2007 and is particularly evident if previous data in the literature are taken into account (Tomaselli, Gualmini, and Spettoli Citation2002). Probably the species decline was caused by human interference and especially by the competition with N. nucifera. Although T. natans shows efficient vegetative reproduction (Groth, Lovett-Doust, and Lovett-Doust Citation1996), the extremely rapid growth of N. nucifera inhibits any recovery of this species. In other wetlands, N. nucifera has caused the almost total disappearance of other hydrophytes, showing a marked tendency to spread out and acquire its own ecological niche (Scoppola and Avena Citation1987; Mastrantuono and Mancinelli Citation1999). Furthermore, the possibility of additional spread through rhizome fragment dispersal during mowing should not be underestimated (see Larson Citation2007). The extent and patch trend of T. natans can be justified also by the swell caused by boats and ferry-boats, which may cause the formation of new patches also due to the fragmentation of existing ones. A feature common to N. alba and T. natans is some surface stabilization since 2008, corresponding to a notable decrease (50%) of almost all T. natans patch cover, particularly evident from that year onwards. N. nucifera is the most abundant among the three species: such wide diffusion is especially due to the largest patch, which in 2012 corresponded to more than 59% of the total occupied area. The species, apart from some oscillations, remained almost constant in the occupied area, but the single patches varied over the years in width and position. There was no clear trend of the species during the observation period. This is probably due to the management activities (mostly mowing) being inconsistent between years. All three species are still quite distant from their potential extent. As to N. nucifera, its potential extent of about 31 ha is due solely to containment interventions. The first containment interventions carried out in the past in the whole “Ansa e Valli del Mincio” area gave valuable results. This means that continuation of containment activities is strongly recommended, so as to avoid the invasion of a large area of shallow waters. A notable overlapping zone between the potential surfaces of T. natans and N. nucifera is observable (equal to 15.3% of T. natans potential extent), but this interference is present in a single location of the study area, so that real contact between the two species seems to exist only in places. In contrast, no overlapping between N. nucifera and N. alba was detected, though this fact does not mean that N. alba is completely safe from competition with N. nucifera (some patches of N. alba are less than 100 m distant from the nearest ones of N. nucifera).
Given the strong decline of T. natans and the possible pressures on N. alba, some measures can be suggested: monitoring the growth rate of N. nucifera, containing its diffusion in open water zones; excavation and removal of sediments, also due to the high biomass production by N. nucifera, to avoid silting up of the water basins. Sediment excavation and removal generally gives good results in environment restoration (Tickner, Evans, and Blackburn Citation1991; Moss, Madgwick, and Phillips 1996) and permits low water eutrophy, but in this case care must be taken because there is a serious risk of removing and uprooting T. natans seedlings, if these interventions are carried out during spring, before the emergence of leaf rosettes of T. natans (see Galanti, Guilizzoni, and Libera Citation1990; Cozza et al. Citation1994; Poovey and Getsinger Citation2007). Otherwise, populations that are apparently in good condition and have expanded over the years must be monitored, because of the possible presence of threats that affect the site and neighbouring populations.
On the whole, human activities affect (both directly and indirectly) the native species and the problem of landscape perception when plants with a great aesthetic impact are present must not be underestimated. Flag species are crucial for ecotourism and of strategic importance because they capture people’s attention. In the studied area, there is the risk of wrongly interpreting N. nucifera at the expense of N. alba and T. natans. Some equilibrium can be achieved between native and alien species, but only if combined with adequate management strategies. The interventions proposed could allow the “Ansa e Valli del Mincio” to be a more suitable place for the preservation of its characteristic native plant species, which are the primary reason for the general conservation of this site, while not losing the historical–aesthetic aspect given by N. nucifera.
This study confirms that the contributions of volunteers are very helpful in monitoring the presence and distribution of species on a local scale and over a considerable time span, especially in protected zones close to cities and urban environments (Bonney et al. Citation2009; Crall et al. Citation2010). Indeed, it is well known that citizens can provide free skill and labour for professional scientists (Carr Citation2004; Cohn Citation2008; Silvertown Citation2009; Conrad and Hilchey Citation2011). In our case, the very small group of volunteers permitted a homogeneous data set over a quite long temporal series to be built up completely for free (despite the notable amount of effort). The good reliability of data was assured by the high educational level of the people involved in field samplings. From the volunteer standpoint, they obtained information for the dissemination of botanical culture and a deeper awareness of both invasive alien and natural plant species. This is particularly important as the potential audience in the study area (although this is restricted in terms of extent) can be estimated to reach 3000 people per year, by considering both the local population and tourists (Associazione Per il Parco, pers. comm.). In addition, it has to be noted that about 80% of them are students. This means that environmental education may have a great impact for increasing environmental awareness, particularly in younger generations, in view of better citizen involvement in local issues (Conrad and Hilchey Citation2011). To summarize, citizen science is a useful complement to official research and is a valuable tool for safeguarding wildlife (Cooper et al. Citation2007; Dickinson, Zuckerberg, and Bonter Citation2010).
Notes on contributors
Fabrizio Buldrini, Ph.D., collaborates in various fields of research carried out by the Botanical Garden of Modena University, mainly concerning plant diversity conservation (with particular regard to rare and endangered species and peripheral populations), archaeobotany and plant systematics. Contribution: maps digitization, data elaboration by landscape metrics, data interpretation.
Antinisca Simoncelli, naturalist, teacher and scientific educator, is a scientific guide for the Association “Per il Parco”. Contribution: data collection and interpretation.
Stefania Accordi, chairman of the Association “Per il Parco”, is a naturalist, scientific educator and hiking guide for the same association. Contribution: data collection and interpretation.
Giovanna Pezzi is researcher at the University of Bologna. Her studies concern multi-scalar and multi-temporal analyses of landscapes and habitats with a different degree of naturalness, image analysis and vegetation/land cover mapping. Contribution: maps digitization, data elaboration by landscape metrics, data interpretation.
Daniele Dallai is researcher at the University of Modena and Reggio Emilia. His studies concern the integrated in situ/ex situ conservation of rare and threatened hydro-hygrophilous plant species of the Po Valley and the Northern Apennines, with specific attention to the species living in conditions of range marginality. Contribution: coordination of the research.
Acknowledgements
We are very grateful to Prof. Carlo Del Prete, who critically read the manuscript, to Prof. Alessandro Chiarucci (Università degli Studi di Bologna), who gave us useful suggestions concerning data elaboration, and to Dr Giuseppina Ziccardi (Università degli Studi di Bologna) for technical advice in data analysis. Prof. Andrea Mary Lord (Università degli Studi di Modena e Reggio Emilia) kindly revised the original English text.
Additional information
Funding
References
- Bardsley, D. K., and G. Edwards-Jones. 2007. “Invasive species policy and climate change: social perception of environmental change in the Mediterranean.” Environmental Science & Policy 10: 230–242.
- Baskin, Y. 2002. A Plague of Rats and Rubbervines: the Growing Threat of Species Invasions. Washington, DC: Island Press.
- Bilz, M., S. P. Kell, N. Maxted, and R. V. Lansdown. 2011. European Red List of Vascular Plants. Luxembourg: Publications Office of the European Union.
- Blasi, C., L. Celesti-Grapow, F. Pretto, R. Accogli, A. Alessandrini, P. V. Arrigoni, et al. 2010. “Flora vascolare alloctona d’Italia” [Vascular alien flora of Italy], in Flora Vascolare Alloctona e Invasiva delle Regioni d’Italia [Vascular alien and invasive flora of Italian regions], edited by L. Celesti-Grapow, F. Pretto, E. Carli, and C. Blasi: 15–20. Roma: Casa Editrice Università La Sapienza.
- Bonney, R., C. B. Cooper, J. Dickinson, S. Kelling, T. Phillips, K. V. Rosenberg, and J. Shirk. 2009. “Citizen Science: A Developing Tool for Expanding Science Knowledge and Scientific Literacy.” BioScience 59: 977–984.
- Buldrini, F., D. Dallai, and P. Torri. 2013. “Can palynology contribute to plant diversity conservation activities? The wetland plants in southern Po plain as a case study.” Annali di Botanica (Roma) 2013(3): 245–254.
- Buldrini, F., L. Conte, D. Dallai, and C. Ferrari. 2013. “Genetic diversity of the rare and endangered meadow violet (Viola pumila Chaix) at the southern margin of its range.” Plant Biosystems 147(3): 563–572.
- Carolan, M. S. 2006. “Science, expertise and the democratization of the decision-making process.” Society and Natural Resources 19(7): 661–668.
- Carr, A. J. L. 2004. “Why do we all need community science?” Society & Natural Resources 17: 841–849.
- Cohn, J. P. 2008. “Citizen science: can volunteers do real research?” Bioscience 58: 192–197.
- Conrad, C. C., and K. G. Hilchey. 2011. “A review of citizen science and community-based environmental monitoring: issues and opportunities.” Environmental Monitoring and Assessment 176: 273–291.
- Cooper, C. B., J. L. Dickinson, T. Phillips, and R. Bonney. 2007. “Citizen Science as a Tool for Conservation in Residential Ecosystems.” Ecology and Society 12(2): 11. Accessed 25 February 2015. http://www.ecologyandsociety.org/vol12/iss2/art11/.
- Cozza, R., G. Galanti, M. B. Bitonti, and A. M. Innocenti. 1994. “Effect of Storage at low Temperature on the Germination of the Waterchestnut (Trapa natans L.).” Phyton 34(2): 315–320.
- Crall, A. W., G. J. Newman, C. S. Jarnevich, T. S. Stohlgren, D. M. Waller, and J. Graham. 2010. “Improving and integrating data on invasive species collected by citizen scientists.” Biological Invasions 12: 3419–3428.
- Dallai, D., F. Buldrini, E. Fanti, F. Tonelli, C. Zampighi, L. Conte, C. Ferrari, and A. Managlia. 2014. “L’erbario della Bonifica. Un progetto per la conoscenza del territorio e della biodiversità vegetale nei canali della Bonifica Burana (Emilia orientale).” [The Drainage Consortium herbarium. A project to know the territory and plant diversity in the canals of the Bonifica Burana]. Museologia Scientifica Memorie 11: 188–194.
- Danihelka, J., H. Niklfeld, and H. Šípošová. 2009. “Viola elatior, Viola pumila and Viola stagnina in Austria, Czechia and Slovakia: a story of decline.” Preslia 81: 151–171.
- Davis, M. A. 2009. Invasion Biology. New York: Oxford University Press.
- Denny, P. 1994. “Biodiversity & wetlands.” Wetlands Ecology and Management 3: 55–61.
- Dickinson, J. L., B. Zuckerberg, and D. N. Bonter. 2010. “Citizen Science as an Ecological Research Tool: Challenges and Benefits.” The Annual Review of Ecology, Evolution, and Systematics 41: 149–172.
- Duncan, C. A., J. J. Jachetta, M. L. Brown, V. F. Carrithers, J. K. Clark, J. M. DiTomaso, R. G. Lym, K. C. McDaniel, M. J. Renz, and P. M. Rice. 2004. “Assessing the Economic, Environmental, and Societal Losses from Invasive Plants on Rangeland and Wildlands.” Weed Technology 18: 1411–1416.
- Eckstein, R. L., N. Hölzel, and J. Danihelka. 2006. “Biological Flora of Central Europe: Viola elatior, V. pumila and V. stagnina.” Perspectives in Plant Ecology, Evolution and Systematics 8: 45–66.
- Galanti, G., P. Guilizzoni, and V. Libera. 1990. “Biomanipulation of Lago di Candia (Northern Italy): a three-year experience of aquatic macrophyte management.” Hydrobiologia 200–201: 409–417.
- García-Llorente, M., B. Martín-López, J. A. Gonzáles, P. Alcorlo, and C. Montes. 2008. “Social perceptions of the impacts and benefits of invasive alien species: Implications for management.” Biological Conservation 141: 2969–2983.
- Groth, A. T., L. Lovett-Doust, and J. Lovett-Doust. 1996. “Population density and module demography in Trapa natans (Trapaceae), an annual, clonal aquatic macrophyte.” American Journal of Botany 83(11): 1406–1415.
- Kendle, A. D., and J. E. Rose. 2000. “The aliens have landed! What are the justifications for ‘native only’ policies in landscape plantings?” Landscape and Urban Planning 47: 19–31.
- Klosowski, S. 1995. “Habitat conditions of the phytocoenoses of Trapetum natantis Müller et Görs 1960 in Poland.” Acta Botanica Gallica: Botany Letters 142(6): 555–562.
- Larson, D. 2007. “Growth of three submerged plants below different densities of Nymphoides peltata (S. G. Gmel.) Kuntze.” Aquatic Botany 86: 280–284.
- McGarigal, K., S. A. Cushman, and E. Ene. 2012. “FRAGSTATS v4: Spatial Pattern Analysis Program for Categorical and Continuous Maps.” Computer software program produced by the authors at the University of Massachusetts, Amherst. Available at http://www.umass.edu/landeco/research/fragstats/fragstats.html
- Marani, E. 1984. “Un ingegnere romanico: Alberto Pitentino.” [A Romanesque engineer: Alberto Pitentino]. Civiltà mantovana 1984(2): 1–8.
- Martignoni, C., and G. Persico. 2001. Piante Acquatiche [Aquatic Plants]. Mantova, Italy: Publi Paolini.
- Mastrantuono, L., and T. Mancinelli. 1999. “Long-term changes of zoobenthic fauna and submerged vegetation in the shallow Lake Monterosi (Italy).” Limnologica 29(2): 160–167.
- McNeely, J. A. (ed). 2001. The Great Reshuffling. Human Dimensions of Invasive Alien Species. Gland: IUCN.
- Moss, B., J. Madgwick, and G. Phillips. 1996. A Guide to the Restoration of Nutrient-Enriched Shallow Lakes. Norwich, Norfolk, USA: Broads Authority.
- Perrings, C., M. Williamson, and S. Dalmazzone (eds). 2000. The Economics of Biological Invasions. Northampton: Edward Elgar.
- Persico, G., and A. Truzzi. 2008. Manuale per lo studio della flora e della vegetazione delle zone umide della pianura mantovana [Guide for the study of flora and vegetation of the wetlands of the Mantua plain]. Labter-CREA Mantova, Mantova, Italy: Publi Paolini.
- Pignatti, S. 1982. Flora d’Italia [Flora of Italy]. Bologna, Italy: Edagricole.
- Pimentel, D., L. Lach, R. Zuniga, and D. Morrison. 2000. “Environmental and Economic Costs of Nonindigenous Species in the United States.” BioScience 50: 53–65.
- Poovey, A. G., and K. D. Getsinger. 2007. “Subsurface Application of Triclopyl and 2,4-d amine for Control of Water Chestnut (Trapa natans L.).” Journal of Aquatic Plant Management 45: 63–66.
- Pyšek, P., and K. Prach. 1994. “How important are rivers for supporting plant invasion?”, in Ecology and Management of Invasive Riverside Plants, edited by L.C. de Waal et al.: 19–26. Chichester: Wiley.
- Pyšek, P., K. Prach, and B. Mandak. 1998. “Invasions of aliens plants into habitats of central European landscape: an historical pattern”, in Plant Invasions. Ecological Mechanisms and Human Responses, edited by U. Starfinger, K. Edwards, I. Kowarik, and M. Williamson: 23–32. Leida: Backhuys.
- Rossi, G., C. Montagnani, D. Gargano, L. Peruzzi, T. Abeli, S. Ravera, A. Cogoni et al. (eds.). 2013. Lista Rossa della Flora Italiana. 1. Policy Species e altre specie minacciate [Red List of the Italian Flora. 1. Policy Species and other threatened species]. Comitato Italiano IUCN e Ministero dell’Ambiente e della Tutela del Territorio e del Mare.
- Sager, L., and C. Clerc. 2006. “Factors influencing the distribution of Hydrocharis morsus-ranae L. and Rumex hydrolapathum Huds. in a mowed low-lying marshland, Réserve de Cheyres, lac de Neuchâtel, Switzerland.” Hydrobiologia 570: 223–229.
- Sather, J. H., and R. D. Smith. 1984. An overview of major wetland functions. U.S. Fish Wildlife Service FWS/OBS-84-18.
- Schnittler, M., and K. F. Günther. 1999. “Central European vascular plants requiring priority conservation measures – an analysis from national Red Lists and distribution maps.” Biodiversity and Conservation 8: 891–925.
- Scoppola, A., and G. Avena. 1987. “Indagini ecologico-faunistiche sulle zone umide interne del Lazio. 3: Variazioni cenologiche indotte da Nelumbo nucifera sulle comunità vegetali del Lago di Monterosi” [Ecological and faunistic investigations on the interior wetland areas of Latium]. Annali di Botanica (Roma) 45: 145–156.
- Šeffer, J., M. Janák, and V.Šefferová Stanová. 2008. Management models for habitats in Natura 2000 Sites. 6440 Alluvial meadows of river valleys of the Cnidion dubii. European Commission.
- Silvertown, J. 2009. “A new dawn for citizen science.” Trends in Ecology and Evolution 24(9): 467–471. doi:10.1016/j.tree.2009.03.017
- Telò, R., M. Pinardi, M. Bartoli, A. Bodini, P. Viaroli, E. Racchetti, D. Cuizzi, M. Vannuccini, and L. Previdi. 2007. Progetto “Da Agenda 21 ad Azione 21” - Caratterizzazione dello stato ambientale del fiume Mincio e analisi della strategia di riqualificazione integrata e partecipata - Relazione conclusiva anno 2007 [Project “From Agenda 21 to Action 21” - Characterization of the environmental state of river Mincio and analysis of the strategy of integrated and participated requalification - Conclusion report for 2007]. Mantova, Italy: Forum del Mincio.
- Tickner, M., C. Evans, and M. Blackburn. 1991. “Restoration of a Norfolk Broad: A case study at Strumpshaw Fen.” RSPB Conservation Review 5: 72–77.
- Tomaselli, M., M. Gualmini, and O. Spettoli. 2002. La vegetazione della Riserva Naturale delle Valli del Mincio [The vegetation of the Natural Reserve of the Valli del Mincio]. Parma, Italy: Collana Annali della Facoltà di Scienze Matematiche, Fisiche e Naturali, Università di Parma.
- Tutin, T. G., V. H. Heywood, N. A. Burges, D. H. Valentine, S. M. Walters, and D. A. Webb. 1964. Flora Europaea. vol. 1. Cambridge: Cambridge University Press.
- Tutin, T. G., V. H. Heywood, N. A. Burges, D. M. Moore, D. H. Valentine, S. M. Walters, and D. A. Webb. 1968. Flora Europaea. vol. 2. Cambridge: Cambridge University Press.
- Wärner, C., E. Welk, W. Durka, B. Wittig, and M. Diekmann. 2011. “Biological Flora of Central Europe: Euphorbia palustris L.” Perspectives in Plant Ecology, Evolution and Systematics 13: 55–69.
- Wassen, M. J., W. H. M. Peeters, and H. Olde Venterink. 2003. “Patterns in vegetation, hydrology, and nutrient availability in an undisturbed river floodplain in Poland.” Plant Ecology 165: 27–43.
- Winter, M., O. Schweiger, S. Klotz, W. Nentwig, P. Andriopoulos, M. Arianoutsou, C. Basnou, et al. 2009. “Plant extinctions and introductions lead to phylogenetic and taxonomic homogeneization of the European flora.” Proceedings of the National Academy of Sciences of the United States of America 106(51): 21721–21725.
