Abstract
Prototulbaghia is a genus within the Tulbaghieae in subfamily Allioideae (Amaryllidaceae) and comprises two species restricted to the northeastern escarpment of South Africa. The pollen morphology of these species is studied with the use of scanning electron and transmission electron microscopy. The pollen is compared with that of representatives from the sister genus Tulbaghia (Tulbaghia simmleri and Tulbaghia violacea) and sister tribe Leucocoryneae (Nothoscordum borbonicum). The pollen morphology of Prototulbaghia differs from other members of the Allioideae in both the shape of the pollen grains and the ornamentation of the sulcus membrane. The pollen grains of both Prototulbaghia species are oblate-spheroidal with a reticulate surface ornamentation, the sulcus membrane contains sexine elements (pila), and the wall is thick. Dehydrated grains of both Prototulbaghia species are also triangular in shape after folding due to the length of the sulcus and the thickening of sexine elements in the centre thereof. This type of folding is proposed as a taxonomic character to distinguish the dehydrated pollen grains of this genus from pollen grains of other members of the Allioideae.
Introduction
Prototulbaghia Vosa is a genus endemic to the northeastern escarpment of South Africa (Vosa Citation2007). The genus comprises two rare species with restricted distributions, Prototulbaghia siebertii Vosa (Vosa Citation2007), which is endemic to calcium-rich norite rock sheets, and an undescribed species (N.R. Crouch et al., in preparation) endemic to dolomite outcrops. The members of the genus are small, bulbous plants with thin, shiny, dark green leaves and white flowers that are open for a day (Vosa Citation2007). Prototulbaghia is classified within the tribe Tulbaghieae Endl. ex Meisn., subfamily Allioideae Herb. of the Amaryllidaceae J.St-Hil. (Vosa Citation2007; Chase, Reveal, and Fay Citation2009). It is closely related to Tulbaghia L., but differs from the latter in having a much reduced perigonal tube, a pseudo-corona and anthers arranged on the same plane at the top of the pseudo-corona (in Tulbaghia the anthers are arranged in two whorls, one whorl inserted on the perigonal tube just above the stigma and the second whorl near the mouth of the corona) (Siebert et al. Citation2008; Vosa, Siebert, and Van Wyk Citation2011).
Knowledge of the pollen morphology of this genus is based on a preliminary investigation of P. siebertii, which revealed the grains to have an unusual shape after folding when compared with other members of the Allioideae (Andriessen, Struwig, and Siebert Citation2013). To explain the triangular and disulcate appearance of the grains after desiccation, it was proposed that the grains fold along the breadth with the ends of the equatorial axis (opposed to the long sides of the sulcus) touching. However, this hypothesis does not hold for monosulcate pollen when the geometric principles of harmomegathy are considered (Katifori et al. Citation2010). This study therefore further investigates the morphology and folding of Prototulbaghia pollen by also including grains of the newly discovered, but undescribed, species in the genus. Shape and size of pollen grains show little variation within a genus, but often provide valuable characters to distinguish broadly between genera (Struwig, Siebert, and Jordaan Citation2013). The pollen morphology of Prototulbaghia will therefore be compared with representatives of its sister genus Tulbaghia (Tulbaghia simmleri Beauverd and Tulbaghia violacea Harv.) to assess the taxonomic value and significance of differential characters. Nothoscordum borbonicum Kunth is chosen as representative of the sister tribe Leucocoryneae Ravenna to allow for pollen comparison between tribes of the subfamily across continents (Sassone, Arroyo-Leuenberger, and Giussani Citation2014).
Material and methods
Plant material
Flowers were collected from live plants and fixed in Todd’s fixative (Todd Citation1986) and 70% ethanol. Three mature flowers per species were collected. Voucher specimens are listed in Table .
Table 1. Voucher specimens of species from which pollen were taken for the study.
Microscopy
For scanning electron microscopy, pollen grains fixed in 70% ethanol were dehydrated in an ethanol series and mounted on specimen stubs, then dried and sputter-coated with gold/palladium. Specimens were examined and micrographs were taken with an FEI Quanta 200 Environmental scanning electron microscope.
For transmission electron microscopy, pollen grains fixed in Todd’s fixative were rinsed in three changes of 0.05 m cacodylate buffer and post fixed in 1% osmium tetroxide (in cacodylate buffer) for 60 minutes, followed by three rinses of cacodylate buffer. Material was then dehydrated in a graded ethanol series and embedded in resin (L.R. White™; London Resin Company, London, UK). Embedded material was cut with a Reichert-Jung Ultracut E microtome and contrasted with 2% uranyl acetate (pH 2) for 5 minutes and lead citrate (Reynolds Citation1963) for 2 minutes. Sections were examined and micrographs were taken with an FEI Tecnai G2 20S-Twin 200 kV transmission electron microscope.
For light microscopy, whole and sectioned (0.5-μm thick) pollen grains were mounted in glycerine jelly and sealed with entellan (Product 7961; E. Merck, Darmstadt, Germany) according to the method of Fripp (Citation1983). Material was studied with a Nikon Eclipse E800 microscope, and micrographs were taken with a Nikon digital camera DXM 1200 F.
A minimum of 10 pollen grains of each species were measured to calculate the length and breadth of the pollen grains, muri and lumina of the sexine, and the thickness of the exine including the columellae and footlayer, as well as the intine. Box-and-whisker plots show the distribution, central value and variability of measurements of the length and breadth of the pollen grains and measurements of the ornamentation of the sexine.
Pollen terminology follows Hesse et al. (Citation2009) and Punt et al. (Citation1999).
Results
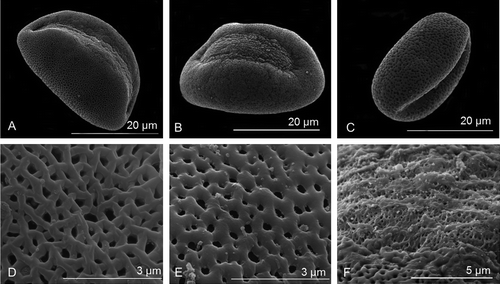
The pollen grains of N. borbonicum, T. simmleri and T. violacea are > 17 and < 20 μm in polar length and > 40 μm in equatorial width (Table ; Figure ). The shape of the grains are peroblate (Table : P/E <0.5) (Figure C) and the sexine ornamentation is reticulate and heterobrochate (Figure D, E). The sulcus is smooth with few sexine elements – simplisulcate type as defined by Halbritter and Hesse (Citation1993) (Table ; Figure F) and does not reach the proximal side of the pollen grain.
Table 2. Measurements of the characteristic features of hydrated pollen grains of the species studied with SEM (n = 10).
Figure 1. Box-and-whisker plots of the measured palynological data for the studied species as presented in Table (Noth bor, Nothoscordum borbonicum; Prot spn, Prototulbaghia sp. nov.; Prot sie, Prototulbaghia siebertii; Tulb sim, Tulbaghia simmleri; Tulb viol, Tulbaghia violacea).
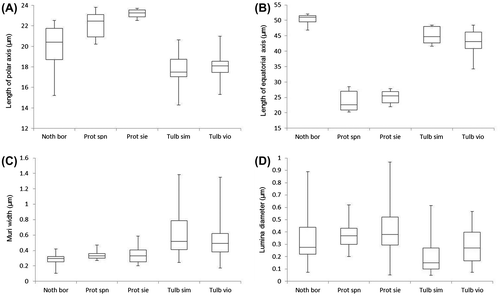
Figure 2. Scanning electron micrographs of the distal polar-equatorial view of (A) Nothoscordum borbonicum. (B) Tulbaghia simmleri. (C) Tulbaghia violacea. (D) Reticulate surface ornamentation of the sexine of N. borbonicum. (E) Reticulate surface ornamentation of the sexine of T. violacea. (F) Sulcus membrane of T. violacea showing no distinct ornamentation.
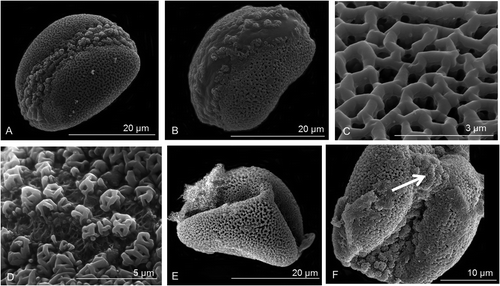
The pollen grains of Prototulbaghia species are 22.1–23.18 μm in polar length and 23.8–25.1 μm in equatorial width (Table ; Figure ). The grains are oblate-spheroidal in shape (Table : P/E 0.92–0.93) (Figure A, B) and the surface ornamentation is reticulate and heterobrochate (Figure C). The sulcus extends from the distal pole down the proximal sides of the grain up to one-third below the equatorial axis (Figure A, B, E, F). The sulcus has distinctive pila which can be either free-standing or their capita merged – insulae type as defined by Halbritter and Hesse (Citation1993) (Figure D). Grains appear triangular and disulcate after desiccation (Figure E) with the sulcus membrane collapsing inwards to expose an exine tip at the centre of the sulcus (Figure F). The muri of Nothoscordum and Prototulbaghia are relatively narrow (< 0.35 μm) compared with Tulbaghia (> 0.5 μm) (Table ; Figure ). Lumina are also much smaller in diameter for Tulbaghia (< 0.3 μm) compared with Nothoscordum and Prototulbaghia (> 0.35 μm) (Table ; Figure ).
Figure 3. Scanning electron micrographs of Prototulbaghia. (A) Pollen grains of Prototulbaghia siebertii in its distal polar-equatorial view. (B) Pollen grains of Prototulbaghia sp. nov. in its distal polar-equatorial view. (C–F) Prototulbaghia siebertii. (C) Reticulate surface ornamentation of the sexine. (D) Pila visible on the sulcus membrane. (E) Dehydrated pollen grain. (F) Sulcus membrane forming the tip of the triangle (indicated by white arrow).
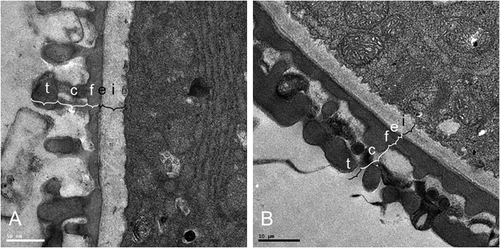
The exine of the pollen grains of N. borbonicum, T. simmleri and T. violacea are > 0.8 and < 0.9 μm thick and that of P. siebertii is 1.06 μm (Table ). The columellae of N. borbonicum, T. simmleri and T. violacea are short (< 0.35 μm) compared with P. siebertii (0.55 μm), and unbranched. The footlayer and endexine is continuous and thick in P. siebertii (0.42 and 0.52 μm, respectively), but thin in the other species studied (< 0.3 μm footlayer and < 0.5 μm intine) (Table ; Figure ).
Table 3. Pollen wall features and measurements of four of the species studied with transmission electron microscopy.
Discussion
The pollen grains of Prototulbaghia species differ from those of Nothoscordum and Tulbaghia with a longer polar length (> 20 μm) and narrower equatorial breadth (< 40 μm). The pollen shape differs in that Prototulbaghia grains are oblate-spheroidal and those of the other genera are peroblate. A major difference, which also influences shape of dehydrated pollen, is the extent of the sulcus. The sulcus of Prototulbaghia species extends from the distal pole down the proximal sides of the grains from above to one-third below the equatorial axis. In the other genera, the sulcus extends from the distal pole down the proximal sides of the grains, but never below the equatorial axis. Prototulbaghia grains have sexine elements present on the sulcus membrane, whereas the sulcus of the other genera is smooth. The surface ornamentation for all the genera studied is the same (reticulate and heterobrochate), although the lumina and muri of Prototulbaghia and Nothoscordum are similar in size. The pollen wall of Prototulbaghia is also thicker (2.5 μm) than that of the other genera (1.75 μm).
Pollen of both Prototulbaghia species displays a peculiar phenomenon in that the grains assume a triangular shape during harmomegathy. Rather than invaginating evenly along the aperture during desiccation, as is characteristic of monosulcate pollen grains (Katifori et al. Citation2010), the sulcus folding extends from the end points of the sulcus below the equatorial axis to the centre of the sulcus on the distal pole. The sexine elements are denser in this area of the sulcus and so prevent the pole from folding in the same plane, giving the dehydrated pollen a triangular appearance as the sulcus membrane folding occurs from both the proximal sides inward. The triangular shape is a direct consequence of the length of the sulcus and it being extended below the equatorial axis, which is not the case for the other genera. The mode of infolding can be used as a taxonomic character for a genus (Halbritter and Hesse Citation2004) and the triangular shape of the dehydrated grains of Prototulbaghia can certainly be used as such to distinguish it from Tulbaghia and Nothoscordum.
Conclusion
The pollen morphology Prototulbaghia sp. nov. confirms the previous characteristics identified for the genus. The pollen morphology of Prototulbaghia is distinct from that of the other members of the subfamily Allioideae in both the shape of the pollen grains and the ornamentation of the sulcus membrane. The pollen grains of Prototulbaghia are oblate-spheroidal with a reticulate surface and the sulcus membrane contains sexine elements. Dehydrated grains are triangular in shape, which can be applied as a taxonomic character to distinguish the pollen grains of this genus from pollen grains of the other members of the Tulbaghieae in southern Africa and the Leucocoryneae of South America.
Notes on contributors
Madeleen Struwig is a post-doctoral researcher in taxonomy at the University of Zululand, South Africa. Contribution: planned the study, conducted laboratory work, analysed the results and wrote the manuscript.
Stefan J. Siebert is a professor in botany at the North-West University, South Africa. Contribution: planned the study, contributed to analyses and co-wrote the manuscript.
Melissa Andriessen is a masters-degree student at the North-West University, South Africa. Contribution: conducted laboratory work, contributed to analyses and co-wrote the manuscript.
Anine Jordaan: is a senior subject specialist at the laboratory of electron microscopy at the North-West University, South Africa. Contribution: conducted laboratory work.
Acknowledgements
We are grateful to Dr L.R. Tiedt of the Laboratory of Electron Microscopy, North-West University for providing technical support and to Prof. L. du Preez from the School of Biological Sciences, North-West University for the use of a light microscope and digital camera.
References
- Andriessen, M., M. Struwig, and S. J. Siebert. 2013. “Stuifmeelmorfologie van Prototulbaghia Vosa: ’n Vergelykende palinologiese studie van die Alliaceae in Suider-Afrika. [Pollen morphology of Prototulbaghia Vosa: A comparative palynological study of the Southern African Alliaceae.].” Suid Afrikaanse Tydskrif vir Natuurwetenskap en Tegnologie 32: 1–5.
- Chase, M. W., J. L. Reveal, and M. F. Fay. 2009. “A subfamilial classification for the expanded asparagalean families Amaryllidaceae, Asparagaceae and Xanthorrhoeaceae.” Botanical Journal of the Linnean Society 161: 132–136.
- Fripp, P. J. 1983. “A method of preserving glycerine-jelly microscopic preparations.” South African Journal of Science 79: 228–228.
- Halbritter, H., and M. Hesse. 1993. “Sulcus morphology in some monocot families.” Grana 32: 87–99.
- Halbritter, H., and M. Hesse. 2004. “Principal modes of infoldings in tricolp(or)ate Angiosperm pollen.” Grana 43: 1–14.
- Hesse, M., H. Halbritter, R. Zetter, M. Weber, R. Buchner, A. Frosch-Radivo, and S. Ulrich. 2009. Pollen terminology: An illustrated handbook. New York, NY: Springer.
- Katifori, E., S. Alben, E. Cerda, D. R. Nelson, and J. Dumais. 2010. “Foldable structures and the natural design of pollen grains.” Proceedings of the National Academy of Sciences USA 107: 7635–7639.
- Punt, W., S. Blackmore, S. Nilsson, and A. Le Thomas. 1999. "Glossary of pollen and spore terminology." Laboratory of palaeobotany and palynology, University of Utrecht. http://www.pollen.mtu.edu/glos-gtx/glos-int.htm (accessed October 12 2015).
- Reynolds, E. S. 1963. “The use of lead citrate at high pH as an electron-opaque stain for electron microscopy.” Journal of Cell Biology 17: 208–212.
- Sassone, A. B., S. C. Arroyo-Leuenberger, and L. M. Giussani. 2014. “Nueva circunscripción de la Tribu Leucocoryneae (Amaryllidaceae, Allioideae).” Darwiniana 2 (2): 197–206.
- Siebert, S. J., C. Vosa, A.-E. Van Wyk, and H. Muller. 2008. “Prototulbaghia (Alliaceae), a new monotypic genus from Sekhukhuneland, South Africa.” Herbertia 62: 76–84.
- Struwig, M., S. J. Siebert, and A. Jordaan. 2013. “Pollen morphology of members of southern African Boerhavia L. and Commicarpus Standl.” Bothalia 43 (1): 15–22.
- Todd, W. J. 1986. “Effects of specimen preparation on the apparent ultrastructure of microorganisms.” In Ultrastructure techniques for microorganisms, edited by H. C. Aldrich and W. J. Todd, 87–100. New York, NY: Plenum Press.
- Vosa, C. G. 2007. “Prototulbaghia, a new genus of the Alliaceae family from the Leolo Mountains in Sekhukhuneland, South Africa.” Caryologia 60: 273–278.
- Vosa, C. G., S. J. Siebert, and A.-E. Van Wyk. 2011. “Alliaceae: Micromorphology and cytology of Prototulbaghia siebertii, with notes on its taxonomic significance.” Bothalia 41: 311–314.