Abstract
Over the past decade new ecological indices based on phytoplankton and macrophytes were developed as part of the tools for assessment of the ecological status of water bodies. This study demonstrates the applicability of two of them (Assemblage index /Q/ and Algae Group Index /AGI/) for evaluation of water bodies from a lake type L4 as well as their comparability. Assessment of the ecological status of two lake ecosystems was performed in order to ensure successful protection, enhancement and management of lowland and semi-mountain lakes in Bulgaria. Data on the aquatic flora from Golyamo Skalensko Lake and Malko Skalensko Lake over a period of two years were used to assess their ecological status. In addition, the toxic potential of the established dominant cyanoprokaryotic species was also evaluated. Phytoplankton- and macrophyte-based metrics resulted in complementary evaluation of temporary and long-term environmental conditions. Despite the hydraulic connection and proximity between the two lakes, Golyamo Skalensko Lake and Malko Skalensko Lake appear as completely different ecosystems, according to the phytoplankton structure (species composition, number of species, abundance, seasonal succession), macrophytes and ecological status.
Introduction
In the EU Water Framework Directive (WFD), phytoplankton and macrophytes are identified as two of the biological quality elements (BQEs) which are required to assess the ecological status of lakes.[Citation1] According to WFD, phytoplankton assessment should include the following metrics: taxonomic composition, species abundance or biomass, and frequency and intensity of phytoplankton blooms.[Citation2] In the past decade, new ecological indexes based on phytoplankton were developed in many European countries as part of the tools for assessment the ecological status of water bodies.[Citation3]
Aquatic macrophytes have also been widely used as indicators in lakes over the past decades.[Citation4] They react progressively to environmental alterations such as nutrient loadings, but slowly, unlike phytoplankton.[Citation5] Thus, they can be used as long-term indicators. According to WFD, ecological assessment has to be based on type-specific reference conditions and on aquatic flora composition and abundance.
Eutrophication is the most widespread pressure in European lakes, together with hydromorphological pressure.[Citation6] In this context, it is necessary to identify which metrics are least affected by natural and methodological variation, and thus best reflect the most widespread pressures affecting the lakes.
In many aquatic systems Cyanobacteria play a major role as primary producers and may have a significant influence on other organisms in the ecosystem.[Citation2] Under certain conditions, such as grading eutrophication of water bodies, higher water temperature, duration of sunlight and low values of the total nitrogen to total phosphorus (TN/TP) ratio,[Citation7] Cyanobacteria could cause intense blooms. Most of these blooms are concomitant with the release of a large amount of cyanotoxins. Depending on their concentration in the water pool, these natural toxins could cause acute intoxication, could exert chronic effects or accumulate and pass through the food chain. That is why, these toxins and the species which produce them are focus of active research worldwide.[Citation8]
This study included Skalenski Lakes, located in the Eastern Stara Planina Mountain. They are unique aquatic ecosystems that have not been investigated previously. The anthropogenic impact in these lakes is very weak and there are practically no sources of pollution. Our aims were (1) to study the phytoplankton, macrophytes and toxic potential of the established blue–green algae in the lakes; (2) to test the applicability of the Assemblage index for ecological assessment of semi-mountain natural lakes; (3) to perform ecological assessment of the lakes by using either the phytoplankton or macrophytes as indicators and to compare the two evaluations.
Materials and methods
Study area
Both Skalenski lakes fall within the catchment area of the river Luda Kamchia, Eastern Stara Planina Mountain (). According to the adopted lake typology they belong to lowland or semi-mountain natural lakes and swamps in Pontic province (Lake Type L4).[Citation9]
Golyamo Skalensko Lake (Reservoir Skala 1) is a natural semi-mountain lake, which is partially hydromorphologically modified and is rarely used for irrigation. Malko Skalensko Lake (Reservoir Skala 2) is highly modified and may be considered as an artificial water body. It was built in 1971 by pumping water from the river Luda Kamchia. Both lakes are near each other (500 m distance) and there is a hydraulic connection between them. The morphometric characteristics of Skalenski Lakes are given in .
Table 1. Morphometric, physical and chemical parameters of the studied lakes.
Sampling procedure
Physicochemical parameters (temperature, pH, conductivity, transparency, dissolved oxygen and oxygen saturation) of lake water were measured in situ. Additionally, NH4-N, NO2-N, NO3-N, PO4-P, turbidity, total nitrogen and phosphorus, COD, and BOD5 were analysed immediately after sampling by using a NOVA 60 spectrophotometer (MERCK) following adopted standards: ammonium nitrogen (ISO 7150/1), nitrite and nitrate nitrogen (EN 26777 and ISO 7890-1), total nitrogen (EN ISO 11905-1), phosphate phosphorus (EN ISO 6878), total phosphorus (EN ISO 6878), turbidity (ISO 7027), biochemical oxygen demand (EN 1899-1,2) and chemical oxygen demand (ISO 15705).
Phytoplankton sampling was performed twice (July and September) in 2011 and 2012. Integrated samples were taken from the euphotic zone in the deepest part of the lake by a barometer type Ruttner (volume 2 dm3). The depth of the euphotic zone was defined as 2.7 times the Secchi depth.[Citation10] Phytoplankton samples were preserved in formalin (4% final concentration). At the same time, non-preserved water samples were also collected and analysed for presence of cyanotoxins.
The taxonomic composition and abundance of aquatic macrophytes were recorded as biological metrics in the normative definitions of the ecological status classification in the WFD, Annex 1.2.[Citation11] Sampling was carried out following the recommendations of Schaumburg et al.[Citation12] The macrophyte surveys were carried out once during the main vegetation period (end of June until September). In each sampling site belt transects of 20–30 m width orthogonal to the shoreline and positioned within an ecologically homogenous section of the littoral were surveyed. The transect numbers were in correlation to the lake size. Species, their abundance and additional relevant parameters were recorded for the defined depth zones (0–1; 1–2; 2–4; and >4 m). The taxonomy of vascular plants followed Flora Europaea.[Citation13,14] The abundance of each species was noted on a five-degree scale (1 = very rare, 2 = infrequent, 3 = common, 4 = frequent, 5 = abundant, predominant) according to Kohler.[Citation15]
Phytoplankton analysis
The taxonomic composition of the phytoplankton was determined by using an Amplival light microscope (400×). Live and preserved specimens were analysed. For taxonomic analyses additional live specimens were collected using a plankton net (35 μm). Guides and floras well established in the practice were used for identification of the taxa.[Citation16,17,Citation18,19,Citation20,21,Citation22]
Counting of the phytoplankton was performed by using an inverted microscope.[Citation23] For numerous species, at least 100 specimens were counted.[Citation24] The algal biovolume was calculated by using the formulas for geometric shapes.[Citation25] Each translation between biovolume and fresh biomass was performed according to Wetzel and Likens.[Citation26]
The assessment of the ecological status was made on the basis of five main and three additional metrics as follows. Main metrics: total biomass (mg L−1), Algae Group Index (AGI, Catálan Index), Assemblage index (Q), transparency according to Secchi (m) and Chlorophyll a (μg L1); additional metrics: percentage (%) of Cyanobacteria (towards total biovolume); bloom (intensity) and presence of bloom toxic species (Microcystis, Aphanizomenon, Cylindrospermopsis and others). The intensity of the phytoplankton bloom was assessed on the basis of total biovolume on a 5-degree scale.[Citation27]
In calculating the percentage of Cyanobacteria, some species/genera for oligotrophic waters were excluded, focusing on toxic species and eutrophic indicators (according to WFD Intercalibration Technical Teport, Part 2-Lakes, Section 3-Phytoplankton biomass metrics Annexes).
AGI and Assemblage index (Q) were calculated as previously described.[Citation28,29]
Analyses for presence of cyanotoxins
ELISA analysis
Collected water samples were analysed by Ridascreen™ ELISA kit for detection of saxitoxins (R-Bopharm, Darmstadt, Germany), with a detection limit of 0.010 μg L−1; and by Microcystins ELISA kit (Abraxis LLC, Warminster, PA, USA) for detection of microcystins and nodularins, with a limit of detection of 0.10 μg L−1, according to the manufacturers’ instructions.
HPLC analysis
HPLC analysis was carried out using an ÄKTApurifier system (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and software UNICORN V5.11. An analytical column Discovery® C18 (5 mm × 4 mm, I.D. 5 μm) from Supelco (Bellafonte, PA, USA) was used. The mobile phase was a mixture of solution A (10 mmol L−1 ammonium acetate, pH 5.5) and solution B (10 mmol L−1 ammonium acetate:acetonitrile, 80:20, v/v) as follows: 0% B at 0 min, 100% B from 45 min to 65 min by using a linear gradient. The flow rate was 0.8 mL min−1 and ultraviolet detection was performed at 238 nm. All analyses were performed at room temperature. The column was equilibrated between the analyses with 8 mL of solution A. Each standard was assayed separately (nodularin 5 μg mL−1, MC-LR 5 μg mL−1, STX 40.5 pg mL−1, 200 μL injection volume), and then a mixture of all standards with the same concentrations (in 200 μL) was also analysed. The peaks of the samples were compared with those from the standards on the basis of the detection time of the peak.
Macrophytes analysis
Identified macrophyte species were allocated to the three different type-specific species groups: reference indicators, indifferent taxa, and degradation indicators (according to growth depth, some taxa are assigned to different groups). The Reference Index is an expression of the “plant quantity” ratio of type-specific sensitive taxa dominating at reference conditions compared to the “plant quantity” of insensitive taxa and is, therefore, a tool for estimating the deviation of observed macrophyte communities from reference communities. Calculated Reference Index [Citation12,Citation30] was transformed into Ecological Quality Ratio, where the value of “1” reflects the best possible ecological status.
Classification system for assessment of the ecological status of lakes
For evaluation of the ecological status of the lakes by using the phytoplankton and macrophyte metrics, we applied the classification system for a mesotrophic lake type in Bulgaria.[Citation9] The scale for AGI (Catálan Index) is a modification after WFD intercalibration technical report, Part 2-Lakes, Section 3-Phytoplankton composition metrics (2008). Scales for chlorophyll-a and transparency are according to Cardoso et al.[Citation31] The scale for Assemblage index (Q) follows Padisák et al.[Citation29] The class boundaries for Reference Index were defined according to the normative definitions and interpretations of the WFD according to Gecheva et al.[Citation30]
Results and discussion
Physicochemical parameters
The measured temperatures in the lakes () were typical for the summer. The pH values were slightly in the alkaline range, more pronounced in Golyamo Skalensko Lake. Nitrogen compounds were at the limit of the analytical method, which is typical for the epilimnion of stratified lakes during the summer According to the morphometric characteristics, Malko Skalensko Lake and Golyamo Skalensko Lake are small lakes of a medium depth, with pronounced summer stratification (). They are not subject to anthropogenic interference and should be expected to be in reference conditions.
Phytoplankton
Taxonomic composition, species richness and biomass
The summer phytoplankton of Malko Skalensko Lake featured high species richness, low biomass and stability over time ((A)). Eighty-four species were identified in the samples collected in July 2011, and 66 species in those collected in September 2011. The species were distributed relatively evenly in eight taxonomic groups. The largest number of species in both months belonged to Chlorophyceae, 30.9% and 24.2%, respectively. In July, the second group in terms of species richness was Cyanobacteria (19.1%), followed by Bacillariophyceae (17.8%) and Zygnemaphyceae (10.7%). Towards the end of the summer, with the decrease in water temperature in September, the number of Chrysophyceae and Euglenophyceae species increased to 18.2% and 12.1%, respectively.
Figure 2. Relative abundance of the phytoplankton based on taxonomic groups. In parentheses: total species number and total biomass.
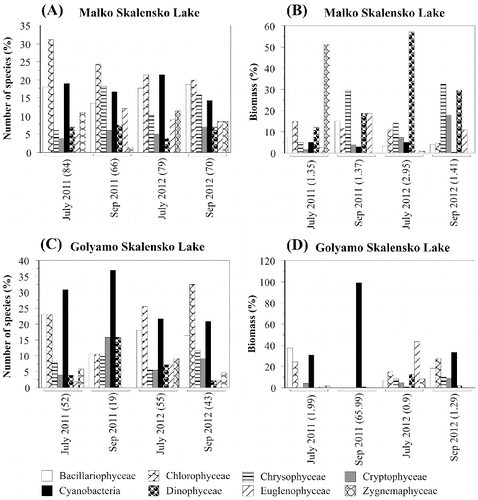
There were also a large number of species in the samples collected in 2012 (July and September), 79 and 70, respectively, belonging to eight taxonomic groups ((A)). Analogously to 2011, in July 2012, Chlorophyceae (21.5%), Cyanobacteria (21.5%) and Bacillariophyceae (17.7%) predominated, while in September the number of Chrysophyceae (18.6%) was increased. Both in 2011 and 2012, a small number of eutrophic and toxic Cyanobacteria species (Microcystis aeruginosa, Aphanizomenon flos-aquae) were observed as single specimens only in the quality samples.
The total biomass of the phytoplankton in Malko Skalensko Lake ranged from 1.35 to 2.95 mg L−l ((B)). In July 2011, Zygnemaphyceae (51.3%) dominated in the biomass, followed by Chlorophyceae (14.8%) and Dinophyceae (12.2%). In September 2011, other taxonomic groups were of paramount importance for the biomass: Chrysophyceae (29.4%) and Euglenophyceae (18.9%). In July 2012, algal bloom (1st degree) of the dinoflagellate alga Peridinium lomnickii was observed and it represented 57.1% of the total biomass. In September, the bloom was decreased and, similarly to 2011, an increase in the relative abundance of Chrysophyceae (32.6%) and Dinophyceae (29.7%) was registered. It is noteworthy that, both in 2011 and in 2012, Cyanobacteria were represented by few eutrophic and toxic species. The relative biomass of Cyanobacteria in Malko Skalensko Lake was very low and ranged from 0.4% to 5.0% ((B)), which corresponds to the absence of cyanotoxins in the analysed water samples.
The phytoplankton of Golyamo Skalensko Lake was characterized with lower species richness (from 19 to 55 species) and extraordinary dynamics in taxonomic composition and total biomass, without similarity between the months and years ((C) and 2(D)). In 2011, blue–green algae clearly dominated in number of species: Cyanobacteria (30.8%), Chlorophyceae (23.1%) and Bacillariophyceae (23.1%). In September 2011, the number of Cyanobacteria species increased even more (36.9%). In 2012, the same taxonomic groups dominated, but the largest number of species was that of Chlorophyceae: 25.5% (July) and 32.6% (September), respectively. In both years, eutrophic and toxic species such as Microcystis aeruginosa, Microcystis wesenbergii, Aphanizomenon flos-aquae and Anabaena spiroides were also identified.
The total biomass of the phytoplankton in Golyamo Skalensko Lake for the two studied years varied in a very large range: from 0.9 to 65.99 mg L−l. In July 2011 and September 2012, the total biomass was dominated by Cyanobacteria, Chlorophyceae and Bacillariophyceae, with approximately the same percentage distribution. In September 2011, Cyanobacteria were the only dominant group, representing 98.9% of the total biomass. During this period, there was an intense bloom of Anabaena spiroides f. crassa (65.33 mg L−l, third degree), which resulted in extremely high levels of total biomass (65.99 mg L−l). In July 2012, unlike other months, the total biomass was dominated by Euglenophyceae (43.7%) and Chlorophyceae (14.6%).
Main species and functional groups (FGs) of phytoplankton
The main phytoplankton species in Malko Skalensko Lake belonged to 11 functional groups: N, T, Lo, F, X3, X2, J, Y, W1, C and Ws (). Most of them inhabit pure oligo- and mesotrophic lakes. In July 2011, almost one-third of the total biomass (31.3%) in this lake was made up by functional group N, represented by the desmid alga Cosmarium depressum. Species from group N are known to grow during the summer in lakes of the temperate zone.[Citation32] These species are tolerant to nutrient deficiency.
Figure 3. Relative biomass (Bm, %) of the main phytoplankton species (>5% of the total biomass) with their taxonomic and functional groups in Malko Skalensko Lake.
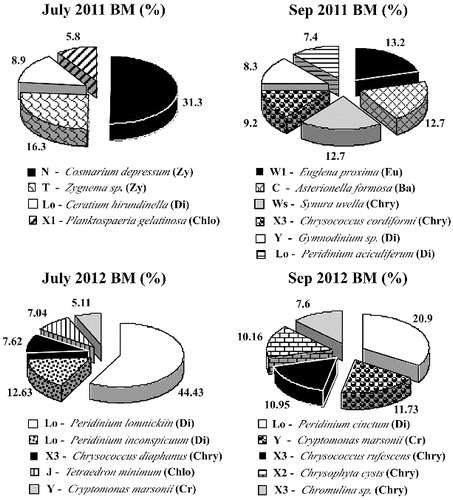
The second dominant species was Zygnema sp. (16.3%) from functional group T. This group inhabits swirling layers where light is a limiting factor.[Citation33] Ceratium hirundinella (Lo) was also a dominant species (8.9%). Group Lo (mostly dinoflagellates) are distributed in a wide range of habitats, reflecting their ability to live in almost all types of ecosystems.[Citation33] The motility of dinoflagellates allows efficient absorption of nutrients, and their mixotrophy and phagotrophy make them less dependent on the low levels of nitrogen and phosphorus.[Citation34] One of the dominants in Malko Skalensko Lake in July 2011 was Planktosphaeria gelatinosa (5.8%) from group F, which is also tolerant to low nutrients.[Citation32]
At the end of the summer, a change had taken place in the dominant functional groups in Malko Skalensko Lake (). In September 2011, there were three key functional groups with equal amounts of biomass: W1, Ws and C. The predominance of Ws (Synura uvella, 12.7%) indicates presence of organic matter derived from decomposition of plant materials.[Citation4,Citation35] Chrysophycean algae from genus Synura grow in small lakes rich in organic matter from decomposition of plant materials. Often, the water is coloured by humic substances. Probably this is the reason for the reduced transparency in Malko Skalensko Lake (1.42 m) at the end of the summer and lowest environmental assessment of this indicator – moderate status. In this type of lakes, euglenoids are often subdominants of Synura.[Citation35] This was also confirmed in Malko Skalensko Lake. In September, Synura uvella was associated with the euglenoid alga Euglena proxima (13.2%). The third dominant belonged to functional group C, which includes centric diatoms (Asterionella formosa, 12.7%). Species from group C are sensitive to silicon (Si) depletion and stratification.[Citation32] Therefore, we believe that the prevalence of Asterionella formosa in the late summer in Malko Skalensko Lake is an indicator of increased mixing of the water and its enrichment with Si. Other groups with low relative abundance in September 2011 included X3 (Chrysococcus cordiformis, 9.2%), Y (Gymnodinium sp., 8.3%) and Lo (Peridinium aciculiferum, 7.4%).
In July 2012, more than half of the phytoplanktonic biomass belonged to functional group Lo, represented by Peridinium lomnickii (44.43%) and P. inconspicuum (12.63%). The functional groups with less relative biomass were X3, J and Y. Group X3 inhabits shallow oligotrophic environments [Citation32] and was represented throughout the studied period by Chrysococcus diaphanous (7.62%), Chrysococcus rufescens (10.95%) and Chromulina sp. (7.6%). Representatives of functional group Y (mostly cryptomonads) inhabit a variety of aquatic systems, and they are sensitive only to the pressure of the zooplankton.[Citation33] From this group, Cryptomomas marsonii was identified in the samples from both months (July 5.11% and September 11.73%). Functional group J (Tetraedron minimum), which is distributed generally in shallow, enriched lakes,[Citation32] was found only in July 2012 in Malko Skalensko Lake (7.04%). Its co-existence with Peridinium lomnikii (which caused a first-degree bloom) is indication for a slight increase of the trophic level and good status in most phytoplanktonic indicators. At the end of the summer, in September, another dominant group was X2, represented by Chrysophyceae cysts (10.16%).
The phytoplankton in Golyamo Slakensko Lake differed considerably in the main phytoplankton species () and included five groups that were not found in Malko Skalensko Lake: H1, LM, М, B and W2. Assemblages H1, LM, M are typical for eutrophic habitats. In July 2011, eutrophic blue–green algae from functional group H1 dominated in biomass (Anabaena spiroides – 17.09%, Anabaena scheremetievi – 10.3%). This eutrophic assemblage comprises dinitrogen-fixing nostocaleans, which are tolerant to low nitrogen conditions.[Citation29,Citation32] Our results confirmed that the nitrogen compounds in Golyamo Skalensko Lake were on the limit of the analytical method (). Another dominant group were diatoms (C): Asterionella formosa (12.56%) and Cyclotella meneghiniana (12.06%). The colonial chlorococcalen alga Oonephris obesa (F) was also represented (15.58%). Other species with negligible biomass were from functional group LM: Ceratium furcoides (0.4%), Y: Cryptomonas marsonii (0.3%) and X2: Carteria multifilis (0.1%). Probably, due to increased nitrogen deficiency in the middle of September, A. spiroides (H1) reached bloom concentrations ( third degree, 65.3 mg L−1) and became monodominant with 98.99% of the total phytoplankton biomass (). According to Padisák et al.,[Citation35] monodominant blooms are often an indication for hypertrophic conditions.
Figure 4. Relative biomass (Bm, %) of the main phytoplankton species (>5% of the total biomass) with their taxonomic and functional groups in Golyamo Skalensko Lake.
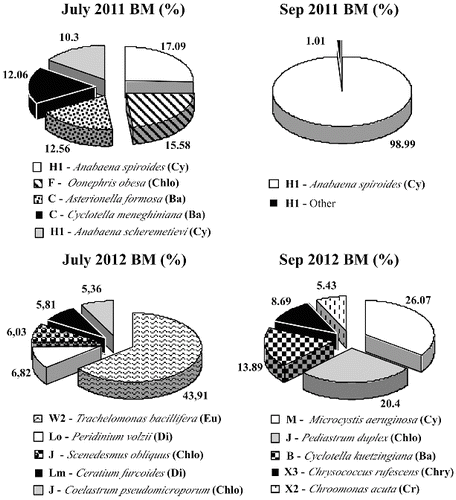
In 2012, the dominant functional groups in Golyamo Slakensko Lake were completely different from those in 2011. Most of the established functional groups were also typical for Malko Skalensko Lake (Lo, J, F, C, X2 and X3), which indicates an improvement in the ecological status. The eutrophic assemblage H1 was not dominant, but there still remained representatives of two other eutrophic groups in the phytoplankton (LM and M) as main species. In the middle of the summer, the euglenoid alga Trachelomonas bacillifera (W2) formed almost half of the total biomass; followed by functional group J: chlorococcal green algae Scenedesmus obliquus (6.03%) and Coelastrum pseudomicroporum (5.36%); Lo (Peridinium volzii – 6.82%); and LM (Ceratium furcoides – 5.81%). In September, the eutrophic functional groups M (Microcystis aeruginosa – 26.07%) and J (Pediastrum duplex – 20.4%) dominated. Functional group B (Cyclotella kuetzingiana – 13.89%), X3 (Chrysococcus rufescens – 8.69%) and X2 (Chroomonas acuta – 5.43%) were also observed.
Thus, the eutrophic state of Golyamo Skalensko Lake in 2011 remains unresolved, given the absence of significant anthropogenic intervention (occasional fish stocking and recreational fishing are the only human activities recorded). It is well known that the main reason for algal blooms is increased levels of total nitrogen and phosphorus [Citation36,37,Citation38] as a result of anthropogenic pressure. It could, therefore, be speculated that there was a sudden release of gases (hydrogen sulphide, etc.) from the cryptofault on which the lake is located. In this region of Stara Planina there have been found several similar faults, including the Skalenski cryptofault.[Citation39] So far, gas releases have been confirmed only in a small lake (Lokvata) located 3 km east of Golyamo Skalensko Lake. Morphometric data for the last 100 years confirm the rapid succession and gradual reduction of its area.[Citation40]
Presence of cyanotoxins
Taking into account the bloom of the blue–green alga Anabaena spiroides (third degree) observed in 2011, collected water samples were analysed for presence of cyanotoxins by HPLC and ELISA. The results were used for toxicological assessment of the state of both investigated lakes.
The HPLC analysis () revealed microcystins (hepatotoxins) in the water samples collected from Golyamo Skalensko Lake in July 2011 (peak at 47.99 min) and September 2011 (peak at 48 min). Nodularins were not detected in any of the samples but there were saxitoxins (peak at 14.47 min) in the sample collected in September 2011 ((C)). Taking into account the cyanoprokaryotic species identified in these periods, the producers of the detected microcystins were most likely Anabaena spiroides (65.33 mg L−l biomass), Aphanizomenon flos-aquae (0.01 mg L−l biomass), Microcystis aeruginosa (0.05 mg L−l biomass) and M. wesenbergii (0.03 mg L−l biomass), which have been repeatedly proven as producers of cyanotoxins.
Figure 5. HPLC chromatogram. Standards of cyanotoxins (A); water sample collected in July 2011 from Golyamo Skalensko Lake (B); water sample collected in September 2011 from Golyamo Skalensko Lake (C).
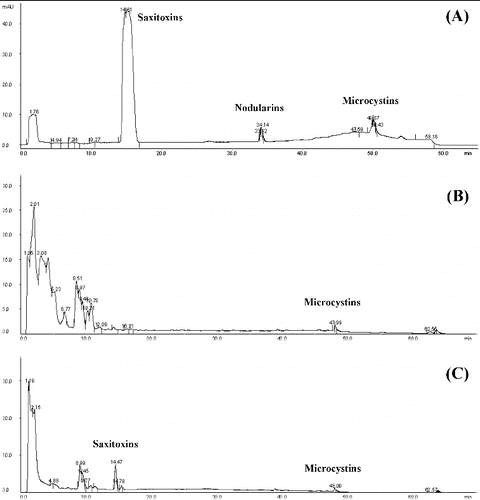
In the water samples from Golyamo Skalensko Lake collected in 2012 no cyanotoxins were detected. The quantitative development of blue–green algae in 2012 was insufficient ((D)). Their relative abundance was from 0.8% to 33.1%, but with lower absolute values of 0.007 mg L−l (July) and 0.43 mg L−l (September), respectively.
As expected, for the entire studied period cyanotoxins were not detected in Malko Skalensko Lake. In the species composition of the phytoplankton, the potential producers of cyanotoxins (Microcystis aeruginosa and Aphanizomenon flos-aquae) were found only as single specimens in the qualitative samples.
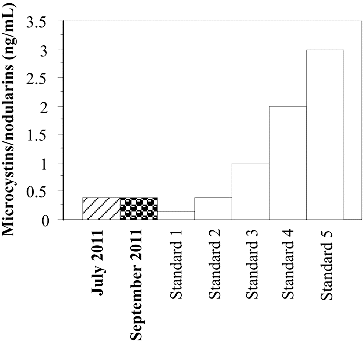
The ELISA results confirmed the presence of microcystins/nodularins in the water samples from Golyamo Skalensko Lake collected in July and September 2011 (). The detected cyanotoxins were at the level of the second standard (0.4 ng mL−l). In the water samples from Malko Skalensko Lake cyanotoxins were not detected either in 2011, or in 2012.
Macrophytes
A total of 14 macrophyte species were recorded. Malko Skalensko Lake supported richer macrophyte diversity (11 taxa, ) with Potamogeton natans and Myriophyllum spicatum as dominant and deepest species. The shallowest depth zone (0 m to 1 m) supported the highest diversity. Tall reeds such as Phragmites australis, Typha angustifolia, T. latifolia and Scirpus lacustris were the most frequent emergent species (helophytes) on the shores. Three woody species were observed: willows (Salix fragilis L., S. triandra L.) and poplar (Populus tremula L.). Six species were registered in Golyamo Skalensko Lake. The species-poor macrophyte community was dominated by Elodea species.
Table 2. Macrophyte species and vegetation limits, 2011–2012.
Assessment of the ecological status based on phytoplankton
The Factor number (F), which is needed for calculating the Q index, was determined based on the reports of Padisák et al.,[Citation29] Becker et al.,[Citation41,42] Crossetti and Bicudo [Citation43] and expert knowledge. The specific values of F for all functional groups that were found in both lakes are listed in .
Table 3. Factor number (F) to the phytoplankton functional groups.
The assessment of the ecological status () of the two lakes showed that, based on the phytoplankton, Malko Skalensko Lake was on the border of the reference conditions in 2011 and 2012. In July 2011, it was within the reference status for all metrics, except the AGI and transparency, which indicated good status. In September 2011, all indicators indicate again high status, except the Assemblage index (good) and transparency (moderate). In July 2012, the ecological status was slightly deteriorated due to the bloom-forming Peridinium lomnickii (first degree), leading to poorer evaluation on a number of metrics (biomass, transparency, chlorophyll-a, degree of bloom). Most major indicators showed good ecological status. In September 2012, Malko Skalensko Lake recovered the high status on all metrics, except transparency (good status).
Table 4. Ecological assessment based on phytoplankton and macrophytes.
The assessments for Golyamo Skalensko Lake varied to a high degree during the studied months and years (), which confirms the well-known fact that phytoplankton is the most dynamic component of the aquatic ecosystems. In 2011, due to the high percentage of Cyanobacteria (July) and the subsequent intense bloom of Anabaena spiroides (September), the assessment based on phytoplankton varied between moderate and bad. In 2012, the phytoplankton assessment was dramatically improved and the ecological status of the lake ranged between high (in July) and moderate (in September). This change could be explained by a decrease in the nutrient levels in the lake. Despite the low values of biomass (respectively chlorophyll-a) and the lack of blooms, the metrics that reflect the taxonomic composition of the phytoplankton (Q, AGI and percentage of Cyanobacteria) indicate worse ecological status: good to poor (). This is natural, since changes in phytoplankton nutrient levels are usually reflected first in changes in biomass and then in changes in species composition.[Citation3]
In the present study, we tested the feasibility of the Assemblage index for ecological assessment of semi-mountain natural lakes. The index is suitable for monitoring of a variety of ecosystems, as it is based on the concept of habitats (10, 31). According to the authors of the index, the values of the Factor number (F) are most critical for its application.[Citation29] They have to be determined for the functional groups of any particular lake type. Due to the lack of paleolimnological data for the phytoplankton of the two lakes, the F values determined by us () are based primarily on a comparison between the dominant functional groups in both Skalenski Lakes in 2011 ( and ). The phytoplankton status in these lakes showed two extreme scenarios (: Ecological status, 2011). We gave a maximum value (F = 5) for functional groups N, T, X3, E and Lo, which correspond to unaffected conditions. These groups include the dominant species found in Malko Skalensko Lake in 2011. Lower values (F = 3 or 4) were given to W1 (euglenoids) and WS (Synura), since they indicate loading of the water with organic matter of vegetable origin. We assigned the lowest value (F = 0) to functional groups H1, LM and M, since they were found only in Golyamo Skalensko Lake. Intermediate values (F = 2.5 and 3) were given to functional groups C and F, as these groups had dominant representatives in both lakes.
The Q and AGI indices give a similar assessment of the ecological status and fit in half of the cases (). In the other cases, a probable reason for the difference in the assessments is the fact that the formula for calculating AGI does not include some groups, such as Zygnemaphyceae and Euglenophyceae, which in some months had higher relative biomass. As compared to biomass assessment, the indices generally provide a lower ecological assessment (). This result is in agreement with the finding of Padisák et al. [Citation29] that the ecological status determined by the Q index is one class lower than that based on the classical biomass evaluation, because it is sensitive to the occurrence of new species.
Assessment of the ecological status based on macrophytes
Based on the Reference Index, the status of Malko Skalensko Lake was good, whereas that of Golyamo Skalensko Lake was poor (). There were degradation indicators and invasive species (Elodea canadensis) in both lakes. The monodominant macrophyte community at Golyamo Skalensko Lake indicated an eutrophication process.
Deep macrophyte stands of dominant perennial Potamogeton natans, P. Lucens and Myriophyllum spicatum in Malko Skalensko Lake illustrated good status. Nevertheless, development of the last two insensitive species could be related to an elevated level of primary production. M. spicatum is relatively tolerant to pollution, salinity and pH values, and was found to be positively correlated with segmentation of lakes.[Citation44]
Type-specific reference species
The proposed list of general type-specific reference species () for national L4 type does not reflect the actual species composition for all lakes in a high condition within the type. Instead, it provides an open “pool of species” with room for further additions in the course of increasing knowledge and number of studied lakes.
Table 5. Alphabetic list of preliminary type-specific taxa in semi-mountain natural lakes.
Conclusions
Both Skalenski Lakes are not subjected to anthropogenic pressure, but have a different assessment of the ecological status. According to the phytoplankton assessment, Malko Skalensko Lake is on the border of the reference conditions, but in good status according to the macrophytes. Golyamo Skalensko Lake was assessed to be in poor status based on macrophytes. Phytoplankton metrics indicated moderate and bad status in 2011, and high and moderate status in 2012. Cyanobacterial blooms during the first year of observation cannot be related to anthropogenic pressure, but could rather reflect the influence of natural gases (hydrogen sulphide, etc.) coming from gas conductive faults of the East Stara Planina Mountain. Phytolpankton-based assessment showed temporary ecosystem alterations and different assessment results during the period 2011–2012 in response to nutrient levels. In addition, the macrophytes illustrated an eutrophication process, which should be monitored and managed in order to improve the lake potential. Toxicological assessment by HPLC and ELISA showed presence of hepatotoxins in Golyamo Skalensko Lake in 2011. Although the detected concentrations were lower than the limit levels (1 μg L−1), Cyanobacteria and the toxins produced by them have to be monitored in the lakes.
Two BQEs and eight phytoplankton metrics were applied, instead of only one (e.g. chlorophyll-a), to obtain a more complete picture of the pressures: a fast response of the phytoplanktonic biomass and composition illustrates the momentary status, while macrophytes provide information on long-term environmental alterations. Choosing the appropriate assessment metrics and providing standardized classification systems in compliance with the requirements of WFD depends on the specific and unique lake conditions. In general, establishment of natural variability of the lake reference conditions is necessary. This will provide important information for the lake management and successful restoration if needed.
Additional information
Funding
References
- EC Parliament and Council. Directive of the European parliament and of the council 2000/60/EC establishing a framework for community action in the field of water policy ( PE-CONS 3639/1/100 Rev 1). Luxembourg: European Commission; 2000.
- Pasztaleniec A, Poniewozik M. Limnologica Ecol Manag Inland Waters. 2010;40:251–259. Available from: http://dx.doi.org/10.1016/j.limno.2009.07.001
- Marchetto A, Padedda BM, Mariani MA, Lugliè A, Sechi N. J Limnol. 2009;68:106–121. Available from: http://dx.doi.org/10.4081/jlimnol.2009.106
- Pall K, Moser V. Hydrobiologia. 2009:633:83–104. Available from: http://dx.doi.org/10.1007/s10750-009-9871-0
- Melzer A. Hydrobiologia. 1999;395/396:181–190. Available from: http://dx.doi.org/10.1023/A:1017001703033
- Lyche-Solheim A, Feld CK, Birk S, Phillips G, Carvalho L, Morabito G, Mischke U, Willby N, Søndergaard M, Hellsten S, Kolada A, Mjelde M, Böhmer J, Miler O, Pusch MT, Argillier C, Jeppesen E, Lauridsen TL, Poikane S. Hydrobiologia. 2013;704:57–74. Available from: http://dx.doi.org/10.1007/s10750-012-1436-y
- Falconer IR. Cyanobacterial toxins of drinking water supplies: cylindrospermopsins and microcystins. Boca Raton (FL): CRC Press; 2005.
- van Apeldoorn ME, van Egmond HP, Speijers GJ, Bakker GJ. Mol Nutr Food Res. 2007;51:7–60. Available from: http://dx.doi.org/10.1002/mnfr.200600185
- Cheshmedjiev SD, Karagiozova TI, Michailov MA, Valev VP. Ecologia Balkanica. 2010;2:75–96.
- Cole GA. Textbook of limnology. Prospect Heights (IL): Waveland Press; 1994.
- European Union. Off J Eur Communities. 2000;L327:1–72.
- Schaumburg J, Schranz C, Stelzer D, Hofmann G. Action instructions for the ecological evaluation of lakes for implementation of the EU Water Framework Directive: Makrophytes and Phytobenthos. München, Germany: Bavarian Environment Agency; 2007.
- Tutin TG, Burges NA, Chater AO, Edmondson JR, Heywood VH, Moore DM, Valentine DH, Walters SM, Webb DA. Flora europaea. Vol. 5. Cambridge: Cambridge University Press; 1976.
- Tutin TG, Burges NA, Chater AO., Edmondson JR, Heywood VH, Moore DM, Valentine DH, Walters SM, Webb DA. Flora europaea. 2nd ed. Vol. 1. Cambridge: Cambridge University Press; 1993.
- Kohler A. Landschaft Stadt. 1978;10:73–85.
- Ettl H. Xanthophyceae, süßwasserflora von mittelueropa [Xanthophyceae, freshwater flora of central Europe]. Vol. 3/1. Stuttgart (NY): Gustav Fischer Verlag; 1978.
- Ettl H. Chlorophyta І. phytomonadina, süßwasserflora von mittelueropa [Chlorophyta y. phytomonadina, freshwater flora of central Europe]. Vol. 9. Jena: VEB Gustav Fischer Verlag; 1983.
- John DM, Whitton BA, Brook AJ. The freshwater algal flora of the British isles: an identification guide to freshwater and terrestrial algae. Cambridge: Cambridge University Press; 2003.
- Komárek J, Anagnostidis K. Cyanoprokaryota, 1. teil: chroococcales, süßwasserflora von mitteleuropa [Cyanoprokaryota, 1. chroococcales, freshwater flora of central Europe]. Vol. 19/1. Stuttgart (NY): Gustav Fischer Verlag; 1999.
- Komàrek J, Anagnostidis K. Cyanoprokaryota, 2. teil: oscillatoriales, süßwasserflora von mitteleuropa [Cyanoprokaryota, 2. oscillatoriales, freshwater flora of central Europe]. Vol. 19(2). Munich: Elsevier/Spektrum Akademischer Verlag; 2005.
- Komárek J, Fott B, Huber Pestalozzi G. Chlorophyceae (Grunalgen) ordnung: chlorococcales, die binnengewässer, band XVI, das phytoplankton des Süßwassers: systematik und biologie [Chlorophyceae (green algae) order: chlorococcales, the inland waters, band XVI, the phytoplankton of fresh water: systematics and biology]. Vol. 7(1). Stuttgart (NY): E. Schweizerbart'sche Verlagsbuchhandlung; 1983.
- Popovsky J, Pfiester LA. Dinophyceae (dinoflagellida), süßwasserflora von mitteleuropa [Dinophyceae (dinoflagellida), freshwater flora of central Europe] 6. Jena: Gustav Fischer Verlag; 1990.
- Utermöhl H. Mitteilungen der internationale vereinigung für theoretische und angewandte limnologie [Proceedings of the international association of theoretical and applied limnology]. Vol. 5. Schweizerbartsche, Stuttgart; 1958. p. 567–596.
- Lund JWG, Kipling C, Lecren ED. Hydrobiologia. 1958;11:143–170. Available from: http://dx.doi.org/10.1007/BF00007865
- Hillebrand H, Dürselen D, Kirschtel D, Pollingher U, Zohary T. J Phycol. 1999;35:403–424. Available from: http://dx.doi.org/10.1046/j.1529-8817.1999.3520403.x
- Wetzel RG, Likens GE. Limnological analyses. 3rd ed. New York (NY): Springer-Verlag; 2000.
- Belkinova D, Mladenov R, Cheshmedjiev S. In: Belkinova D and Gecheva G, editors. Biological analysis and ecological assessment of the surface water types in Bulgaria. Plovdiv: Plovdiv University Press; 2013. p. 55–95. Bulgarian.
- Agència Catalana de l’Aigua. Desenvolupament d’un índex integral de qualitat ecològica i regionalització ambiental dels sistemes loacustres de Catalunya [Development of a comprehensive index of environmental quality and ecological regionalization systems of lakes in Catalonia]. Generalitat de Catalunya: Department de Medi Ambient i Habitatge; 2003.
- Padisák J, Borics G, Grigorszky I, Soróczki-Pintér É. Hydrobiologia. 2006;553:1–14. Available from: http://dx.doi.org/10.1007/s10750-005-1393-9
- Gecheva G, Dimitrova-Dyulgerova I, Cheshmedjiev S. In: Belkinova D, Gecheva G, editor. Biological analysis and ecological assessment of the surface water types in Bulgaria. Plovdiv: Plovdiv University Press; 2013. p. 127–146. Bulgarian.
- Cardoso AC, Duchemin J, Magoarou P, Premazzi G. Criteria for the identification of freshwaters subject to eutrophication: their use for the implementation of the Nitrates and Urban Waste Water Treatment Directives (Report EUR 19810). European Commission, Water research and Monitoring Unit, JRC Ispra & Directorate General for Environment; 2001.
- Reynolds CS, Huszar VLM, Kruk C, Naselli-Flores L, Melo S. J Plankton Res. 2002;24:417–428. Available from: http://dx.doi.org/10.1093/plankt/24.5.417
- Padisák J, Crossetti LO, Naselli-Flores L. Hydrobiologia. 2009;621:1–19. Available from: http://dx.doi.org/10.1007/s10750-008-9645-0
- Kruk C, Huszar VLM, Peeters ETHM, Bonilla S, Costa L, Lürling M, Reynolds CS, Scheffer M. Freshwater Biol. 2010;55:614–627. Available from: http://dx.doi.org/10.1111/j.1365-2427.2009.02298.x
- Padisák J, Borics G, Fehér G, Grigorszky I, Oldal I, Schmidt A, Zámbóné-Doma Z. Hydrobiologia. 2003;502:157–168. Available from: http://dx.doi.org/10.1023/B:HYDR.0000004278.10887.40
- Albay M, Akçaalan R. Hydrobiologia. 2003;502:85–95. Available from: http://dx.doi.org/10.1023/B:HYDR.0000004272.38702.c3
- Conley DJ, Paerl HW, Howarth RW, Boesch DF, Seitzinger SP, Havens KE, Lancelot C, Likens GE. Science. 2009;323:1014–1015. Available from: http://dx.doi.org/10.1126/science.1167755
- Sun L, Jin XC, Zhong Y, Zhi YL, Li H, Zhou QX, Zhuang YY. Bull Environ Contam Toxicol. 2009; 82:543–548. Available from: http://dx.doi.org/10.1007/s00128-009-9655-2
- Paskalev M, Hristov V, Petrov P. In: Proc. National Conference GEOSCIENCES 2009, Bulgarian Geological Society; 2009; Sofia. p. 77–78. Bulgarian.
- Michev TM, Stoyneva MP. Inventory of Bulgarian wetlands and their biodiversity. Part 1: non-lotic wetlands. Sofia: Publishing House Elsi-M; 2007.
- Becker V, Caputo L, Ordóñez J, Marcé R, Armengol J, Crossetti LO, Huszar VLM. Water Res. 2010;44:3345–3354. Available from: http://dx.doi.org/10.1016/j.watres.2010.03.018
- Becker V, Huszar VLM, Crossetti LO. Hydrobiologia. 2009;628:137–151. Available from: http://dx.doi.org/10.1007/s10750-009-9751-7
- Crossetti LO, Bicudo CEM. Hydrobiologia. 2008;610:161–173. Available from: http://dx.doi.org/10.1007/s10750-008-9431-z
- Oťaheľova H, Oťaheľ J. Ekologia (Bratislava). 2006;25:398–411.