Abstract
An F1 population was created by the cross ‘87-1’ × ‘9-22’. The female parent ‘87-1’ was an extremely early maturing cultivar with strong flavour. The male parent was an excellent breeding line producing large berries maturing late. The mapping population included 149 randomly chosen individuals. Molecular genetic map for each parent and the consensus map were constructed using simple sequence repeat and sequence-related amplified polymorphism markers by software JoinMap 3.0. The ‘87-1’ map covers a total length of 1272.9 cM distributed in 21 linkage groups and consists of 163 molecular markers with an average distance between adjacent markers of 8.9 cM. The ‘9-22’ map covers a total length of 1267.4 cM distributed in 20 linkage groups and consists of 158 molecular markers with an average distance between adjacent markers of 9.1 cM. The consensus map covers a total length of 1537.1 cM distributed in 21 linkage groups and one doublet and consists of 217 molecular markers with an average distance of 7.8 cM between adjacent markers. The length of the linkage groups is 69.8 cM on average. The map covers the 19 chromosomes of the Vitis genome and can lay a solid foundation for further studies such as quantative trait loci (QTL) mapping of correlated traits and marker-assisted selection.
Keywords:
Introduction
Grapevine is an old and important economic crop, and is cultivated throughout the world. Up to 2011, the global harvested area of grapevine reached 7.0 million hectares, and the yield exceeded 69 million tons. As a major fruit crop, grape has been causing widespread concern in cultivar modification in many countries. However, grapevine has a series of characters including complicated genetic background, large plant body and long generation cycle, etc., which leads to the restriction of grape breeding. The construction of genetic linkage maps is a major part of genome research and is necessary for gene mapping, gene cloning and genome structure and function research. The construction of high-density genetic map assists the genetic analysis of important agronomic traits at the molecular level, and as a matter of course, could lay the foundation for marker-assisted selection.
With the development of molecular marker technology and the application of ‘Double Pseudo-Test Cross Theory’ in pomology genetic research, genetic-map construction studies have been experiencing great progress. Since the first Vitis genetic map was released,[Citation1] many Vitis maps, including maps of V. vinifera,[Citation2–4] V. rupestris,[Citation5] V. riparia [Citation6] and V. champini [Citation7,8] have been constructed by grape researchers.
Previous maps were mainly based on dominant markers, such as random amplified polymorphic DNA (RAPD) and amplified fragment length polymorphism (AFLP).[Citation1,Citation9] The co-dominant simple sequence repeat (SSR) marker for Vitis was successfully developed in 1993,[Citation10] and its conservatism and generality was then verified in 2000.[Citation11] Subsequently, a lot of maps based on SSR markers have been successfully constructed.[Citation3–8,Citation12–17] SSR markers became one of the main molecular markers of Vitis genetic maps. What is more, a number of Vitis genetic maps were based on SSR marker together with other markers, such as Sequence Characterized Amplified Regions (SCAR),[Citation18–20] expressed sequence tag-simple sequence repeat (EST-SSR) [Citation21,22] and single nucleotide polymorphism (SNP).[Citation16,Citation22–25] And the combination of different markers enhanced the density and genome coverage of the maps.
The sequence-related amplified polymorphism (SRAP) marker is a type of dominant marker, which targets the coding sequences in the genome, especially the open reading frames (ORFs), so as to increase the relationship between amplification results and phenotype.[Citation26] And SRAP has already been used in genetic diversity analysis,[Citation27–29] genetic-map construction [Citation8,Citation30–32] and QTL detection.[Citation33,34] A V. amurensis map based on SSR marker along with SRAP marker was constructed by Liu et al., [Citation8] who found that SRAP markers could well fill the gaps between SSR markers and could also increase the genome coverage of the map. Here, we use both SSR and SRAP markers to construct a V. vinifera genetic linkage map, aiming to provide support for QTL mapping and marker-assisted selection in grape.
Materials and methods
Plant material
F1 population was obtained from the intraspecific cross ‘87-1’ × ‘9-22’. The female parent ‘87-1’, which is peculiar to China, is an extremely early maturing cultivar with strong flavour. Cultivar ‘9-22’ is a complex hybrid containing the genetic background of Italia, Rizamat and Moscato Blanco. The parent ‘9-22’ produces large berries with fragile pulp and high soluble solid content, but without any aroma. We randomly chose 149 individuals from the F1 population as the mapping population, along with the two parents, to perform molecular marker analysis and genetic-map construction. The experiment was carried out in the Molecular Biology Laboratory, Pomology, Shenyang Agricultural University. The mapping population and parents were all grown in the grape breeding nursery in Shenyang Agricultural University.
Young leaves were collected for DNA extraction, according to the modified cetyltrimethyl ammonium bromide (CTAB) method.[Citation35]
SSR markers and progeny genotyping
The primer pairs flanking microsatellite loci were from marker sets VMC (Vitis Microsatellite Consortium, managed through AGROGENE, Moissy Cramayel, France), VVS,[Citation10] VVMD,[Citation36,37] VrZAG,[Citation38] VVI,[Citation39] UDV [Citation40] and Chr. [Citation7] In total 247 SSRs were used for map construction.
Polymerase chain reactions (PCR) were done in 16 μL mixtures containing 10 ng of template DNA, 2.0 mmol/L MgCl2, 100 μmol/L dNTPs, 0.3 μmol/L of each primer, 1 × PCR buffer and 0.8 U of Taq polymerase. Optimal annealing temperatures were optimized for each primer pair, using gradient annealing temperatures (from 50 to 63 °C) programmed by a S1000TM Cycler, keeping all other conditions of the amplification protocol constant (4 min at 94 °C followed by 25 cycles of 1 min at 94 °C, 1 min at optimized annealing temperature, 1 min at 72 °C followed by a final step of 7 min at 72 °C). Amplification products were separated by 5% native polyacrylamide gel electrophoresis (PAGE) and visualized by silver staining.
SRAP markers and progeny genotyping
SRAP primers were from Li and Quiros.[Citation26] A small population consisting of six randomly chosen individuals was used for the selection of 144 primer pairs (). Finally, 30 primer pairs, which produced stable, clear and polymorphic bands, were selected for the amplification of the mapping population.
Table 1. Forward and reverse primer sequences used in SRAP analysis.
The reaction system was 20 μL, including: 2.0 mmol/L MgCl2, 0.1 mmol/L dNTPs, 0.5 μmol/L primer, 10 ng of template DNA and 1.5 U of Taq DNA polymerase. The protocol for PCR amplification was: initial denaturation (5 min at 94 °C); denaturation (60 s at 94 °C) for five cycles; denaturation (60 s at 94 °C), annealing (60 s at 50 °C), extension (90 s at 72 °C), for 35 cycles; final extension (10 min at 72 °C). The amplification products were separated by electrophoresis in 7% polyacrylamide gels.
Segregation analysis and map construction
For co-dominant loci, fully informative markers with four (ab × cd) or three segregating alleles (ef × eg), double heterozygous markers (hk × hk) and markers only segregating in the female parent (lm × ll) were used to construct the female map. Fully informative markers, double heterozygous markers and markers only segregating in the male parent (nn × np) were used to build the male map. For dominant loci, markers segregating in both parents (hk × hk) and markers only segregating in the female parent (lm × ll) were used to construct the female map; markers segregating in both parents (hk × hk) and markers only segregating in the male parent (nn × np) were used to construct the male map. All marker sets together yielded the integrated map. Parental maps were created with JoinMap 3.0.
LOD value was set from 3.0 to 5.0, and the maximum recombination value was 0.4. The recombination rate was converted into map distance (cM) by the Kosambi function.[Citation41] Parental molecular genetic maps were drawn with the Mapchart 2.2 software.[Citation42] The linkage groups were numbered according to IGGP (http://www.vitaceae.org) reference map.[Citation12,Citation19]
Results and discussion
Analysis of SSR and SRAP markers
Among the 247 SSR primer pairs, 75 produced low-quality bands or no bands, and then were discarded. For the other 172 primer pairs, 17 yielded full co-dominant information exhibiting four alleles (ab × cd), and 40 primer pairs segregated with three alleles (ef × eg). Four primer pairs produced bands segregating from both parents with the same size scored as double heterozygous markers following the pattern (hk × hk). Twenty-five markers only segregated in the female parent (lm × ll), and 27 markers only in the male parent (nn × np) (). The remaining 59 pairs showed a homozygous profile in both parents, resulting in a heterozygous rate of 65.6%. Thirty SRAP primer pairs produced a total of 139 polymorphic markers, among which 53 separated only in the female parent (maternal specific), 47 separated only in the male parent (paternal specific) and 39 separated in both parents (common markers, theoretically according to the separation of 3:1).
Table 2. Distribution of segregation types in the ‘87-1’ × ‘9-22’ population.
The X2 test showed that, for co-dominant markers, three paternal specific markers, two maternal specific markers and six common markers deviated from the Mendelian segregation ratio. The markers showing distorted segregation ratio were 9.7% of all co-dominant markers.
Genetic mapping
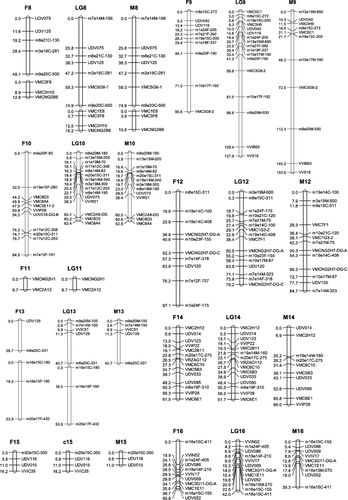
To construct the maternal map, 178 molecular markers containing 86 co-dominant ones and 92 dominant ones were used. One hundred and sixty-three markers (83 co-dominant, 80 dominant) formed the map with a total length of 1272.9 cM (, ). These markers were distributed into 21 linkage groups. The mean distance between adjacent markers was 8.9 cM. The mean length of the linkage groups was 60.6 cM. The largest group was LG4 (133.1 cM), consisting of seven SSRs and five SRAPs. Compared with the international reference map [Citation12,Citation19] (the same below), our LG8 and LG13 were split into fractions because of weak linkage. The largest gap of 40.7 cM was found in LG17.
Table 3. Main characteristics of linkage groups in the integrated, the maternal ‘87-1’ and the paternal ‘9-22’ map.
Figure 1. Linkage map of the integrated, the maternal ‘87-1’ and the paternal ‘9-22’ maps. The name of SRAP marker consists of three parts: ‘the combination of forward and reverse primers’ + ‘the origin of the amplified product’ + ‘the size of the product’. ‘F’, ‘M’ and ‘C’ denote that the product was from the female parent, the male parent or both parents, respectively, e.g. ‘m7e21F-121’ means a 121 bp product amplified in the female parent by primer combination me7em21.
The paternal map was constructed based on 174 molecular markers containing 88 co-dominant ones and 86 dominant ones. One hundred and fifty-eight markers (76 co-dominant, 82 dominant) formed the map with a total length of 1267.4 cM ( and ). These markers allocated into 20 linkage groups. The mean distance between adjacent markers was 9.1 cM. The mean length of the linkage groups was 63.3 cM. The largest group was LG9 (153.6 cM), consisting of six SSRs and five SRAPs. Compared with the international reference map, our LG8 was split into fractions because of weak linkage. The largest gap of 44.4 cM was found in LG18. And LG11 has not been constructed.
All markers (252 markers) were gathered together for integrated map construction. Finally, 217 molecular markers formed 20 linkage groups and a doublet. The whole length of the map was 1537.1 cM, with an average distance between adjacent markers of 7.8 cM, and a mean linkage group length of 69.8 cM. The largest group was LG9 (153.6 cM), containing six SSRs and five SRAPs. The smallest group was LG11 (9.7 cM), containing only two markers. Compared with the reference map, LG4, LG8 and LG13 were all hereby split into two groups. There were two large gaps in LG3, which were between UDV016 and m3e19F-15 (35.8 cM), and between m8e21F-280 and m8e21M-200 (39.4 cM). Most of the co-dominant markers had the same colinearity with the two reference maps, except for the following differences, due to the anomalous map location of VMC2G2 and VMC2H9 in LG6, VMC3H5 and VMC5C1 in LG9 and VMC1E11 and VVIV17 in LG17. In addition, VVIB63 and VVS16 existing in LG15 of the reference maps, appeared in LG9 of our map.
Final remarks
Genetic maps could help breeders to detect strong linkages and co-dominant inheritance, and could also affirm the number and position of genetic factors controlling quantitative characters.[Citation43] Up to now, a number of Vitis genetic maps have been reported, referring to the cross within V. vinifera,[Citation2,Citation4,Citation12,Citation16] interspecific hybridization[Citation6,Citation19,Citation13,Citation24,Citation44,45] and other subspecies.[Citation5,Citation7,Citation8] What is more, several QTLs of important traits have been mapped.
In this study, the intraspecific population was created using ‘87-1’ and ‘9-22’ which were originated in China. The female parent ‘87-1’ is extremely early maturing and with strong flavour. The male parent ‘9-22’ is mid-late maturing and with large berry of no flavour. We constructed molecular genetic maps of high density. Although containing several gaps, the maps we constructed cover the 19 chromosomes of the Vitis genome and can be used for QTL mapping of correlated characters.
Marker distortion is a common phenomenon in the mapping process.[Citation3,Citation6,Citation16] The discordance of parents, the chromosomal structure rearrangement, deletion, insertion and mutation could all lead to marker distortion in the next generation. In addition, other factors can also lead to marker distortion, such as a small mapping population as well as band missing as a result of PCR amplification or electrophoresis. The proportion of SSR markers with biased segregation observed in this study (9.7%) was lower than those reported by Grando et al. (22.4%) [Citation3], Lowe and Walker (16%), [Citation6] Troggio et al. (20.3%) [Citation16] and Blasi et al. (11.3%), [Citation7] but was almost the same with that reported by Doligez et al. (9.2%). [Citation12]
In some genetic mapping studies, markers without serious distortion or markers which did not affect marker order along the linkage group were used for map construction.[Citation2,Citation17] Bradshaw and Stettler [Citation46] found that markers with distortion and markers without distortion held the same mapping efficiency and that genes may lose the linkage relationship when discarding the distorted markers.
In our integrated map, distorted markers were mainly distributed in LG12. And one or two distorted markers existed in LG4, LG9, LG13 and LG17. These distorted markers did not affect the sequence of marker order and inversely played a good role in linking. Therefore, they were used for map construction.
Compared with co-dominant SSR markers, dominant markers are less capable of covering the map length.[Citation4] Here, we construct a V. vinifera genetic map based on SSR markers, and we also added 119 dominant SRAP markers into the V. vinifera genome. These SRAP markers, on the basis of SSR markers, not only lengthened the linkage groups, but also increased the marker density of the linkage groups and filled some gaps between SSR markers. We consider that SRAP markers play a role of supplementation in map construction. However, we did not obtain LG11 in the ‘9-22’ map, and LG4, LG8, LG13 were broken into two parts in the integrated map. Further research should focus on new developed EST-SSR and SNP markers from the 12 times grapevine genome sequence (http://www.genoscope.cns.fr/externe/Genome-Browser/Vitis/) and on increasing the number of SRAP markers, so as to improve the quality of the genetic map.
Conclusions
Here, we created an intraspecific Vitis vinifera cross population using ‘87-1’ and ‘9-22’. To construct the parental map and consensus map, which well covered the 19 chromosomes of the Vitis genome, 149 F1 individuals were used. Based on SSR markers, comparisons were made between this map and the reference maps. The V. vinifera map was complemented by using SRAP markers. This map could be used for QTL mapping, and could also lay the foundation for further studies such as marker-assisted selection.
Additional information
Funding
References
- Lodhi MA, Daly MJ, Ye GN, Weeden NF, Reisch BI. A molecular marker based linkage map of Vitis. Genome. 1995;38:786–794. doi:10.1139/g95-100
- Adam-Blondon AF, Roux C, Claux D, Butterlin G, Merdinoglu D, This P. Mapping 245 SSR markers on the Vitis vinifera genome: a tool for grape genetics. Theor Appl Genet. 2004;109(5):1017–1027. doi:10.1007/s00122-004-1704-y
- Grando MS, Bellin D, Edwards KJ, Pozzi C, Stefanini M, Velasco R. Molecular linkage maps of Vitis vinifera L. and Vitis riparia Mchx. Theor Appl Genet. 2003;106:1213–1224. doi:10.1007/s00122-002-1170-3
- Riaz S, Dangl GS, Edwards KJ, Meredith CP. A microsatellite based framework linkage map of Vitis vinifera L. Theor Appl Genet. 2004;108:864–872. doi:10.1007/s00122-003-1488-5
- Doucleff M, Jin Y, Gao F, Riaz S, Krivanek AF, Walker MA. A genetic linkage map of grape, utilizing Vitis rupestris and Vitis arizonica. Theor Appl Genet. 2004;109:1178–1187. doi:10.1007/s00122-004-1728-3
- Lowe KM, Walker MA. Genetic linkage map of the interspecific grape rootstock cross Ramsey (Vitis champinii) × Riparia Gloire (Vitis riparia). Theor Appl Genet. 2006;112:1582–1592. doi:10.1007/s00122-006-0264-8
- Blasi P, Blanc S, Wiedemann-Merdinoglu S, Prado E, Rühl EH, Mestre P, Merdinoglu D. Construction of a reference linkage map of Vitis amurensis and genetic mapping of Rpv8, a locus conferring resistance to grapevine downy mildew. Theor Appl Genet. 2011;123(1):43–53. doi:10.1007/s00122-011-1565-0
- Liu Z-D, Guo X-W, Guo Y-S, Lin H, Zhang P-X, Zhao Y-H, Li K, Li C-X. SSR and SRAP marker based linkage map of Vitis amurensis Rupr. Pak J Bot. 2013;45(1):191–195.
- Dalbó MA, Ye GN, Weeden NF, Steinkellner H, Sefc KM, Reisch BI. A gene controlling sex in grapevine placed on a molecular marker-based genetic map. Genome. 2000;43:333–340. doi:10.1139/g99-136
- Thomas MR, Scott N. Microsatellite repeats in grapevine reveal DNA polymorphisms when analyzed as sequence-tagged sites (STSs). Theor Appl Genet. 1993;6:985–990. doi:10.1007/BF00211051
- Di Gaspero G, Peterlunger E, Testolin R, Edwards KJ, Cipriani G. Conservation of microsatellite loci within the genus Vitis. Theor Appl Genet. 2000;101:301–308. doi:10.1007/s001220051483
- Doligez A, Adam-Blondon AF, Cipriani G, Di Gaspero G, Laucou V, Merdinoglu D, Meredith CP, Riaz S, Roux C, This P. An integrated SSR map of grapevine based on five mapping populations. Theor Appl Genet. 2006;113:369–382. doi:10.1007/s00122-006-0295-1
- Fischer B, Salakhutdinov I, Akkurt M, Eibach R, Edwards KJ, Toepfer R, Zyprian EM. Quantitative trait locus analysis of fungal disease resistance factor on a molecular map of grapevine. Theor Appl Genet. 2004;108:505–515. doi:10.1007/s00122-003-1445-3
- Mandl K, Santiago JL, Hack R, Fardossi A, Regner F. A genetic map of Welschriesling × Sirius for the identification of magnesium-deficiency by QTL analysis. Euphytica. 2006;149:133–144. doi:10.1007/s10681-005-9061-8
- Schwander F, Eibach R, Fechter I, Hausmann L, Zyprian E, Töpfer R. Rpv10: a new locus from the Asian Vitis gene pool for pyramiding downy mildew resistance loci in grapevine. Theor Appl Genet. 2012;124(1):163–176. doi:10.1007/s00122-011-1695-4
- Troggio M, Malacarne G, Coppola G, Segala C, Cartwright DA, Pindo M, Stefanini M, Mank R, Moroldo M, Morgante M, Grando MS, Velasco R. A dense single-nucleotide polymorphism based genetic linkage map of grapevine (Vitis vinifera L.) anchoring Pinot noir bacterial artificial chromosome contigs. Genetics. 2007;176:2637–2650. doi:10.1534/genetics.106.067462
- Zhang J-K, Hausmann L, Eibach R, Welter LJ, Töpfer R, Zyprian EM. A framework map from grapevine V3125 (Vitis vinifera ‘Schiava grossa’ x ‘Riesling’) x rootstock cultivar ‘Borner’ (Vitis riparia x Vitis cinerea) to localize genetic determinants of phylloxera root resistance. Theor Appl Genet. 2009;119(6):1039–1051. doi:10.1007/s00122-009-1107-1
- Costantini L, Battilana J, Lamaj F, Fanizza G, Grando MS. Berry and phenology-related traits in grapevine (Vitis vinifera L.): from quantitative trait loci to underlying genes. BMC Plant Biol. 2008;8:38. doi:10.1186/1471-2229-8-38
- Di Gaspero G, Cipriani G, Adam-Blondon AF, Testolin R. Linkage maps of grapevine displaying the chromosomal locations of 420 microsatellite markers and 82 markers for R-gene candidates. Theor Appl Genet. 2007;114:1249–1263. doi:10.1007/s00122-007-0516-2
- Welter LJ, Göktürk-Baydar N, Akkurt M, Maul E, Eibach R, Töpfer R, Zyprian EM. Genetic mapping and localization of quantitative trait loci affecting fungal disease resistance and leaf morphology in grapevine (Vitis vinifera L.). Mol Breed. 2007;20:359–374. doi:10.1007/s11032-007-9097-7
- Peng FY, Reid K, Liao N, Schlosser J, Lijavetzky D, Holt R, Martinez Zapater JM, Jones S, Marra M, Bohlmann J, Lund ST. Generation of ESTs in Vitis vinifera wine grape (Cabernet Sauvignon) and table grape (Muscat Hamburg) and discovery of new candidate genes with potential roles in berry development. Gene. 2007;402:40–50. doi:10.1016/j.gene.2007.07.016.
- Riaz S, Krivanek AF, Xu K, Walker MA. Refined mapping of the Pierce's disease resistance locus, PdR1, and Sex on an extended genetic map of Vitis rupestris × Vitis arizonica. Theor Appl Genet. 2006;113(7):1317–1329. doi:10.1007/s00122-006-0385-0
- Lamoureux D, Bernole A, LeClainche I, Tual S, Thareau V, Paillard S, Legeai F, Dossat C, Wincker P, Oswald M, Merdinoglu D, Vignault C, Delrot S, Caboche M, Chalhoub B, Adam-Blondon AF. Anchoring of a large set of markers onto a BAC library for the development of a draft physical map of the grapevine genome. Theor Appl Genet. 2006;113:344–356. doi:10.1007/s00122-006-0301-7
- Salmaso M, Malacarne G, Troggio M, Faes G, Stefanini M, Grando MS, Velasco R. A grapevine (Vitis vinifera L.) genetic map integrating the position of 139 expressed genes. Theor Appl Genet. 2008;116(8):1129–1143. doi:10.1007/s00122-008-0741-3
- Vezzulli S, Troggio M, Coppola G, Jermakow A, Cartwright D, Zharkikh A, Stefanini M, Grando MS, Viola R, Adam-Blondon AF, Thomas M, This P, Velasco R. A reference integrated map for cultivated grapevine (Vitis vinifera L.) from three crosses, based on 283 SSR and 501 SNP-based markers. Theor Appl Genet. 2008;117(4):499–511. doi:10.1007/s00122-008-0794-3
- Li G, Quiros CF. Sequence-related amplified polymorphism (SRAP), a new marker system based on a simple PCR reaction: Its application to mapping and gene tagging in Brassica. Theor Appl Genet. 2001;103:455–461. doi:10.1007/s001220100570
- Abedian M, Talebi M, Golmohammdi HR, Sayed-Tabatabaei BE. Genetic diversity and population structure of mahaleb cherry (Prunus mahaleb L.) and sweet cherry (Prunus avium L.) using SRAP markers. Biochem Syst Ecol. 2012;40:112–117. doi:10.1016/j.bse.2011.10.005.
- Uzun A, Yesiloglu T, Polat I, Aka-Kacar Y, Gulsen O, Yildirim B, Tuzcu O, Tepe S, Canan I, Anil S. Evaluation of genetic diversity in lemons and some of their relatives based on SRAP and SSR markers. Plant Mol Biol Rep. 2011;29(3):693–701. doi:10.1007/s11105-010-0277-y
- Yildiz M, Ekbic E, Keles D, Sensoy S, Abak K. Use of ISSR, SRAP, and RAPD markers to assess genetic diversity in Turkish melons. Sci Hortic. 2011;130(1):349–353. doi:10.1016/j.scienta.2011.06.048
- Lu J-J, Zhao H-Y, Suo N-N, Wang S, Shen B, Wang H-Z, Liu J-J. Genetic linkage maps of Dendrobium moniliforme and D. Officinale based on EST-SSR, SRAP, ISSR and RAPD markers. Sci Hortic. 2012;137(1):1–10. doi:10.1016/j.scienta.2011.12.027.
- Zhao Y-H, Guo Y-S, Hu Y-L, Zhang B, Liu R, Ouyang R, Fu J-X, Liu C-M. Construction of a genetic linkage map of litchi with RAPD, SRAP and AFLP. Acta Hortic Sinica. 2010;37(5):697–704.
- Xie W, Zhang X, Cai H, Huang L, Peng Y, Ma X. Genetic maps of SSR and SRAP markers in diploid orchardgrass (Dactylis glomerata L.) using the pseudotestcross strategy. Genome. 2011;54(3):212–221. doi:10.1139/G10-111
- Guo Y-S, Zhao Y-H, Liu C-M. QTLs analysis of several traits in Longan. Biotechnol Biotech Eq. 2011;25(1):2203–2209. doi:10.5504/bbeq.2011.0014
- Zhang F, Chen S, Chen F, Fang W, Chen Y, Li F. SRAP-based mapping and QTL detection for inflorescencerelated traits in chrysanthemum (Dendranthema morifolium). Mol Breed. 2011;27(1):11–23. doi:10.1007/s11032-010-9409-1
- Hanania U, Velcheva M, Sahar N, Perl A. An improved method for isolating high-quality DNA From Vitis vinifera Nuclei. Plant Mol Biol Rep. 2004;22(2):173–177. doi:10.1007/BF02772724
- Bowers JE, Dangl GS, Meredith C. Development and characterization of additional microsatellite DNA markers for grape. Am J Enol Vitic. 1999;50:243–246.
- Bowers JE, Dangl GS, Vignani R, Meredith C. Isolation and characterization of new polymorphic simple sequence repeat loci in grape (Vitis vinifera L.). Genome. 1996;39:628–633. doi:10.1139/g96-080
- Sefc KM, Regner F, Turetschek E, Glössl J, Steinkellner H. Identification of microsatellite sequences in Vitis riparia and their applicability for genotyping of different Vitis species. Genome. 1999;42:367–373. doi:10.1139/g98-168
- Merdinoglu D, Butterlin G, Bevilacqua L, Chiquet V, Adam-Blondon AF, Decroocq S. Development and characterization of a large set of microsatellite markers in grapevine (Vitis vinifera L.) suitable for multiplex PCR. Mol Breed. 2005;15:349–366. doi:10.1007/s11032-004-7651-0
- Di Gaspero G, Cipriani G, Marrazzo MT, Andretta D, Prado CMJ, Peterlunger E, Testolin R. Isolation of (AC)n –microsatellites in Vitis vinifera L. and analysis of genetic background in grapevines under marker assisted selection. Mol Breed. 2005;15:11–20. doi:10.1007/s11032-004-1362-4
- Kosambi DD. The estimation of map distances from recombination values. Ann Eugen. 1944;12:172–175. doi:10.1111/j.1469-1809.1943.tb02321.x
- Voorips RE. Mapchart: software for the graphical presentation of linkage maps and QTLs. J Hered. 2002;93:77–78. doi:10.1093/jhered/93.1.77
- Staub JE, Serquen FC. Genetic markers, map construction, and their application in plant breeding. HortScience. 1996;31(5):729–741.
- Marguerit E, Boury C, Manicki A, Donnart M, Butterlin G, Némorin A, Wiedemann-Merdinoglu S, Merdinoglu D, Ollat N, Decroocq S. Genetic dissection of sex determinism, inflorescence morphology and downy mildew resistance in grapevine. Theor Appl Genet. 2009;118:1261–1278. doi:10.1007/s00122-009-0979-4
- Moreira FM, Madini A, Marino R, Zulini L, Stefanini M, Velasco R, Kozma P, Grando MS. Genetic linkage maps of two interspecific grape crosses (Vitis spp.) used to localize quantitative trait loci for downy mildew resistance. Tree Genet Genomes. 2011;7:153–167. doi:10.1007/s11295-010-0322-x
- Bradshaw HD, Stettler RF. Molecular genetics of growth and development in Populus. A genetic linkage map of a hybrid poplar composed of RFLP, STS, and RAPD markers. Theor Appl Genet. 1994;89:167–178. doi:10.1007/BF00225137