Abstract
The pathways for synthesis of 5-aminolevulinic acid (5-ALA) use either succinyl-CoA and glycine (C-4 pathway), or glutamate (C-5 pathway). Although Rhodobacter sphaeroides synthesizes 5-ALA through the C-4 pathway, it also has the genes coding for the enzymes of the C-5 pathway, except for glutamyl-tRNA reductase. The glutamyl-tRNA reductase gene was cloned from Rhodospirillum rubrum and expressed in R. sphaeroides; thus, the C-5 pathway was enabled to function upon assembling all the required genes. Consequently, a new and unique bacterial strain producing more 5-ALA was developed. Biohydrogen was also produced in the same bioprocess within a biorefinery approach using sugar beet molasses as substrate. The amount of 5-ALA produced by the modified strain was 25.9 mg/g dry cell weight (DCW), whereas the wild-type strain produced 12.4 mg/g DCW. In addition, the amount of H2 generated by the modified and wild-type cells, respectively, was 0.92 L/L culture and 1.05 L/L culture.
Introduction
Purple non-sulfur (PNS) photosynthetic bacteria such as Rhodobacter sphaeroides (R. sphaeroides) have the ability to produce a number of value-added products; for instance, 5-aminolevulinic acid (5-ALA), hydrogen, vitamin B12, coenzyme Q10, poly-β-hydroxybutyrate (PHB) and carotenoids. What makes PNS bacteria of particular interest is the possibility to employ them for production of different products in the same bioprocess.
One such value-added product is 5-ALA, which is the precursor of tetrapyrrole porphyrins, vitamin B12 and chlorophyll and has multiple applications in medicine, agriculture and biotechnology. Specifically, it can be used as an anticancer agent, a growth promoter in plants and a biodegradable herbicide and insecticide.[Citation1] However, it does not have widespread usage, since its chemical synthesis is expensive and includes many complex steps.[Citation2] Nevertheless, it is more likely to achieve biosynthesis of 5-ALA at higher quantities in a sustainable bioprocess. A wide range of substrates have been tested for the production of 5-ALA and production of up to 27.5 mmol/L of 5-ALA was achieved with R. sphaeroides.[Citation3] In addition to R. sphaeroides, several studies have been conducted with green algae [Citation4] and cyanobacteria [Citation5] for the production of 5-ALA in cost-effective bioprocesses. However, the production of 5-ALA needs to be further improved and sustainable and cost-effective bioprocesses need to be developed with the use of renewable substrates instead of pure and synthetic carbon sources.
The pathways for synthesis of 5-ALA use either succinyl-CoA and glycine (C-4 pathway), or glutamate (C-5 pathway).[Citation1] R. sphaeroides synthesizes 5-ALA through the C-4 pathway upon condensation of glycine and succinyl CoA by ALA synthetase (ALAS; succinyl-CoA:glycine C-succinyltransferase (decarboxylating); [EC:2.3.1.37]). On the other hand, it also has the first enzyme (glutamyl-tRNA synthetase, RSP_0797, NCBI-GeneID: 3718415, [EC:6.1.1.17]) and the third enzyme (glutamate-1-semialdehyde aminotransferase, RSP_1569, NCBI-GeneID: 3718596, [EC:5.4.3.8]) of the C-5 pathway. However, interestingly, the second enzyme, glutamyl-tRNA reductase, is absent in this bacterium. In the present study, the gene coding for glutamyl-tRNA reductase (the second enzyme) was cloned from Rhodospirillum rubrum (Rsp. Rubrum, DSM 467, ATCC 11170) and expressed in R. sphaeroides O.U.001. In this way, in addition to the C-4 pathway, the C-5 pathway was enabled to function upon assembling all the necessary genes.
Another interesting value-added product that can be produced by PNS bacteria is hydrogen. It can be produced by PNS bacteria under anaerobic conditions in the presence of a light source, using various substrates like organic acids and sugars. Hydrogen is actually the by-product of the enzyme nitrogenase, whose primary function is to reduce molecular nitrogen to ammonium. In the absence of N2 and ammonia, however, nitrogenase acts as an adenosine triphosphate (ATP) dependent hydrogenase and all the electrons and ATP molecules are used for hydrogen production.[Citation6] There are many reports on hydrogen production by PNS bacteria in different bioprocesses using various substrates. For instance, R. sphaeroides ATCC 17023 can produce 2.31 L H2/L culture and 1.62 L H2/L culture by using lactate or glucose, respectively.[Citation7] In another study, 13.7 mol H2/mol sucrose was obtained by a sequential dark and photofermentation process from molasses.[Citation8] Nevertheless, the processes of biohydrogen production still need to be enhanced with an effective use of feedstock, since most biohydrogen production studies typically use pure and synthetic substrates and the hydrogen production yields are below commercial usage.
In this context, the aim of the present study was to use sugar beet molasses as a renewable and sustainable substrate for the production of biohydrogen and 5-ALA in the same bioprocess. Consequently, a unique and more feasible bioprocess by which considerable amounts of 5-ALA and biohydrogen were produced was obtained using a cost-effective substrate in the context of a biorefinery.
Materials and methods
Bacterial strains, plasmids and culture conditions
The bacterial strains and plasmids used in this study are listed in and are briefly described below. Wild-type R. sphaeroides O.U.001 (DSM 5864, DSMZ GmbH, Germany) was used for the production of 5-ALA and biohydrogen. The second gene (glutamyl-tRNA reductase, Rru_A0749, EC:1.2.1.70) in the C-5 pathway was taken from Rsp. rubrum (DSM 467, ATCC 11170, DSMZ GmbH, Germany) and expressed in R. sphaeroides. Both bacteria were maintained according to the supplier's recommendation (Rhodospirillaceae medium No:27). Escherichia coli XL1 Blue (Stratagene) was used as a general plasmid host. E. coli S17-1 (λ pir) is a special strain used as plasmid donor in conjugation or diparental mating.[Citation9] Here, it was used to deliver the construct to R. sphaeroides by conjugation. pBBR1MCS2 [Citation10] was used for the cloning and heterologous expression of the glutamyl-tRNA reductase gene in R. sphaeroides. E. coli strains were maintained in Luria–Bertani (LB) medium supplied with antibiotics in the following concentrations: kanamycin (25 μg/mL) and tetracycline (10 μg/mL).
Table 1. Plasmids and bacterial strains used in this study.
In order to develop a cost-effective process, sugar beet molasses was supplied from a sugar factory (Konya Şeker, Turkey) and used as substrate. It is the viscous dark brown by-product of the refining of sugar beets and contains 50% (w/w) sugar, mainly sucrose. Sugar-containing medium with sucrose concentration of 28 g/L was used based on our previous results [Citation11] that in this medium, 5-ALA (35 mg/g dry cell weight (DCW)) and H2 (1.01 L/L culture) can be obtained using molasses. Fifty-five milliliter glass bioreactors with a final culture volume of 50 mL were used. After 10% inoculation, the anaerobic cultures made by flushing the bioreactors with argon for 3 min were incubated at 29 °C without shaking under 200 Watt/m2 illumination provided by 100 W incandescent light bulbs.
Cloning and expression of the glutamyl-tRNA reductase gene
The enzymes used, including kinase, phosphatase, ligase, DNA polymerases and restriction endonucleases, were supplied from Thermo Scientific Inc. (Germany).
The glutamyl-tRNA reductase gene was obtained by polymerase chain reaction (PCR) in a thermal cycler (Boeco, Germany) using the genomic DNA of Rsp. rubrum as template. The 1811 bp long DNA fragment including both the glutamyl-tRNA reductase gene and its upstream genetic elements was synthesized using designed primers (forward primer: 5′-GAATTCGTCACCACCGATCT-3′; reverse primer: 5′-GGCTCAGGTTCTCTTCCAAA-3′). The PCR programme was as follows: 30 s at 98 °C for pre-denaturation, 35 cycles of amplification step (10 s at 98 °C, 30 s at 55 °C and 45 s at 72 °C) followed by a final extension at 72 °C for 5 min. The reaction was performed in the presence of 3%, 5%, 7%, 9% or 11% (w/v) dimethyl sulphoxide (DMSO), using a high-fidelity DNA polymerase enzyme (Phusion, Thermo Scientific) in a total volume of 20 μL.
PCR products (250 μL) were precipitated with 3 mol/L sodium acetate (pH 5.2) and phosphorylated with T4 polynucleotide kinase, according to the manufacturer's protocol. The mixtures were then loaded into an agarose gel (1%) and purified using a gel extraction kit (Qiagen). The PCR products became ready to ligate into the vector. pBBR1MCS2, which can replicate in R. sphaeroides, was used to clone and express the glutamyl-tRNA reductase gene. The vector was cut with EcoRV, dephosphorylated by calf intestinal alkaline phosphatase (CIAP) to prevent self-ligation and purified with a gel extraction kit (Qiagen). The insert and vector were mixed in a 3:1 molar ratio to ensure successful ligation in the presence of T4 DNA ligase in a total volume of 20 μL. After 1-h incubation at 22 °C, a few microliters of ligation mixture were transformed to E. coli XL1Blue through CaCl2-mediated chemical transformation.
After transformation into E. coli XL1Blue, several white colonies were investigated to find the correct recombinant clone. For this purpose, plasmid isolations were done from these colonies and the plasmids were cut with HincII to confirm the cloning and find the orientation of the insert. Upon confirmation of the cloning, the correct recombinant vector carrying the glutamyl-tRNA reductase gene was denoted as pALA3.
For the delivery of the construct into R. sphaeroides, diparental mating (conjugation) was applied. The construct was first transferred into E. coli S17-1 (λpir) strain, which provides the transfer function by a tra gene on the chromosome. The mob region required for the transfer was provided by the construct. Fifty-milliliter cultures of E. coli S17.1 (λpir) containing the construct and 50 mL of wild-type R. sphaeroides O.U.001 were pelleted by centrifugation. The pellets were re-suspended and mixed in 5 mL of Biebl and Pfenning minimal medium.[Citation12] The cell mixture was re-pelleted and re-suspended in 1 mL of minimal medium and spotted onto a 0.45 μm pore size nitrocellulose filter that was previously placed on an LB plate without antibiotic. After 6-h incubation at 30 °C, the cell mixture was washed from the filter paper with 1 mL of minimal medium and collected into a tube. Then, several microliters of this mixture were spread onto selective minimal medium with kanamycin. Since the E. coli S17-1 (λpir) strain is a proline auxotroph, it was not able to form colonies on minimal medium but R. sphaeroides colonies containing the construct appeared on the selective medium within 2–3 days. R. sphaeroides colonies grown on the selective media with kanamycin were further cultured and plasmid isolation was performed from these cells to confirm the maintenance of pALA3 in R. sphaeroides. The vector isolated from R. sphaeroides was cut with KpnI and used as template in PCR to amplify the glutamyl-tRNA reductase gene. This proved that pALA3 was successfully delivered to R. sphaeroides by conjugation and it was stably maintained in the bacterium.
The transcription of the glutamyl-tRNA reductase gene in R. sphaeroides was investigated by reverse transcription PCR (RT-PCR). First, total RNA was isolated with TRI Reagent (Sigma), according to the manufacturer's instructions, using 4 mL of overnight R. sphaeroides culture containing pALA3. Then, c-DNA synthesis and subsequent PCR were done with specifically designed primers (GTR-1 forward: 5′-GCGTGGAGATCTTTGGTCAT-3′, GTR-1 reverse: 5′-TTGACCTGCCCCAAAATATG-3′ and product size: 211 bp). PCR was performed using 1 μL of cDNA with Taq DNA polymerase in a total volume of 25 μL. The PCR programme was as follows: 5 min at 95 °C for pre-denaturation, 30 cycles of amplification (30 s at 95 °C, 30 s at 51.5 °C and 30 s at 72 °C), followed by a final extension step at 72 °C for 5 min. Finally, PCR products were visualized by agarose gel (1%) electrophoresis.
Experimental design for biohydrogen and 5-ALA production
The experiments were done by using a metabolically engineered strain and a wild-type strain which was transformed with bare vector (pBBR1MCS2) as a control. Since molasses is a viscous and dark brown by-product, it was first diluted with distilled water to prepare the medium with 28 g/L of sucrose. In addition to 2 mmol/L of glutamate added as a nitrogen source, K2HPO4, MgSO4·7H2O, CaCl2·2H2O, FeSO4, MoO4·2H2O and vitamin solution (Thiamine, Niacin, Biotin) were included in the culture medium as described earlier.[Citation11] After 10% inoculation, the anaerobic cultures were incubated under the conditions defined above. During the bioprocess (circa the 90th hour), levulinic acid (1.74 g/L, 15 mmol/L) was added into the cultures to enhance 5-ALA production, as recommended by Choi et al. [Citation13]
The cell density of the cultures was monitored by measuring the absorbance of the cultures at 660 nm at specific time intervals. DCW was also calculated based on preliminary optical density (OD) experiments showing that one unit OD660 of R. sphaeroides O.U. 001 is equivalent to dry weight of 0.421 g/L. The pH changes in the cultures were also monitored and recorded. The evolved gas was collected in water-filled graduated tubes during the bioprocess and then the purity was determined at the end of the bioprocesses by gas chromatography (Shimadzu GC-2010 Plus, Japan) as described previously.[Citation11] The amount of hydrogen accumulation was given as the liters of hydrogen produced per liter of culture (L H2/L culture) and as the yield (mol H2/mol sucrose), which was calculated by taking the temperature to be 29 ºC and the pressure to be 101.3 kPa (i.e. 1 atm). After the completion of the batch processes, the cell-free culture media were lyophilized and re-suspended in 2 mL of distilled water. The amount of 5-ALA in this suspension was determined spectrophotometrically using a standard curve.[Citation14]
Statistical analysis
Hydrogen production experiments were independently repeated two times. Two-tailed t-test was used to determine the significant differences between mean values (p < 0.05). Each value in the graphs is the mean from two replicates (±standard deviation).
Results and discussion
Cloning and heterologous expression of the glutamyl-tRNA reductase gene
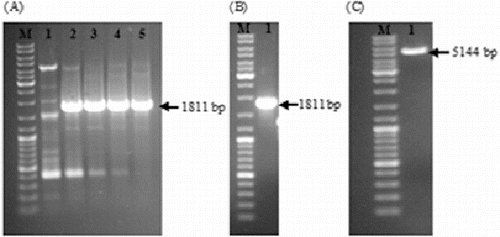
The 1811-bp long DNA fragment including both the glutamyl-tRNA reductase gene and its upstream genetic elements was successfully amplified by PCR ((A)). After tailoring the ends of the PCR product and subsequent purification by gel extraction kit, the amplified fragment became ready for ligation ((B)). Similarly, pBBR1MCS2 was cut with EcoRV, CIAP treated to prevent self-ligation and purified for ligation ((C)). Finally, the amplified DNA fragment was blunt-end cloned into pBBR1MCS2.
Figure 1. Optimization of PCR amplification of the glutamyl-tRNA reductase gene by using various DMSO concentrations (A); Lane 1: 3% (w/v); Lane 2: 5% (w/v); Lane 3: 7% (w/v); Lane 4: 9% (w/v); and Lane 5: 11% (w/v). PCR product after tailoring the ends and purification by gel extraction kit (B). EcoRV cut, dephosphorylated and purified pBBR1MCS2 (C). DNA ladder (SM0331 Fermentas, M) was loaded into the first well.
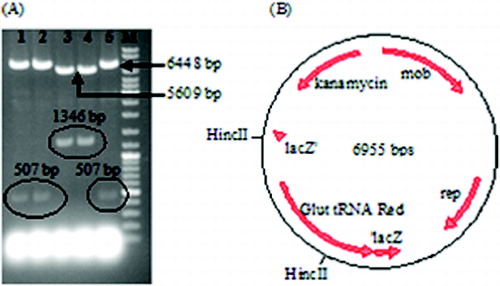
Following the transformation of E. coli XL1Blue, five white colonies were selected and investigated to determine if they were correct recombinant colonies. In this context, the plasmids were isolated from these colonies and they were cut with HincII. Two of the tested plasmids gave the expected DNA fragments (5609 and 1346 bp) and they were designated as pALA3 ((A)). In these two constructs, the transcriptional orientation of the glutamyl-tRNA reductase gene was the same as that of LacZ and the kanamycin resistance gene ((B)), avoiding the possibility for the transcription of one gene to decrease the transcription frequency of the neighboring one.
Figure 2. Selection of the correct recombinant vector by HincII digestion (A); Lanes 1–5: HincII digestion of five putative recombinant vectors isolated from five different colonies. Only the third and the fourth vector gave the expected bands. DNA ladder (SM0331 Fermentas, M) was loaded into the last well. Illustration of the correct recombinant vector designated as pALA3 (B).
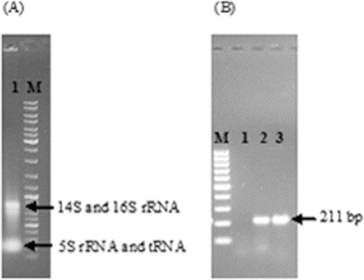
After the successful construction of the vector, it was first transferred to E. coli S17-1 (λpir) to mobilize the construct into R. sphaeroides by conjugation. Then, wild-type R. sphaeroides O.U.001 and E. coli S17.1 (λpir) containing the construct were mated and R. sphaeroides colonies transformed with pALA3 were selected on minimal medium with kanamycin. In order to ensure the maintenance and replication of the vector in R. sphaeroides, plasmid isolation was performed from these cells and they were cut with KpnI. The vector was linearized and the expected 6955 bp band was obtained ((A)). Next, the presence of the glutamyl-tRNA reductase gene in the vector was confirmed by PCR using these plasmids as a template and the expected 1811 bp DNA fragment was successfully amplified ((B)). Subsequent to the confirmation that pALA3 was successfully delivered to R. sphaeroides by conjugation and that it was stably maintained in the bacterial cells, the transcription of glutamyl-tRNA reductase gene was investigated by RT-PCR. In this context, total RNA isolation was first carried out using anaerobically grown R. sphaeroides containing pALA3 as shown in (A). 16S and 14S rRNA, which arise from the cleavage of 23S rRNA,[Citation15] were observed together with 5S rRNA and tRNA, without any contaminating DNA in the agarose gel. Following the cDNA synthesis, PCR was performed and the result is illustrated in (B). Thus, the transcription of the glutamyl-tRNA reductase gene in R. sphaeroides was demonstrated. It is known that transcription does not always guarantee successful translation or functional enzyme synthesis. For this reason, the effect of heterologous gene expression on the production of 5-ALA was investigated by comparing the amount of 5-ALA produced by the wild-type and the modified strain.
Figure 3. Confirmation of the replication of pALA3 in R. sphaeroides by KpnI digestion (A); Lane 1: digestion of the vector isolated from R. sphaeroides and Lane 2: digestion of pALA3 as control. Confirmation of the presence of glutamyl-tRNA reductase gene in the isolated vector, using the plasmid as template in PCR (B); Lane 1: PCR product obtained by using vector isolated from R. sphaeroides and Lane 2: PCR product obtained by using pALA3 as control. DNA ladder (SM0331 Fermentas) was designated as M.
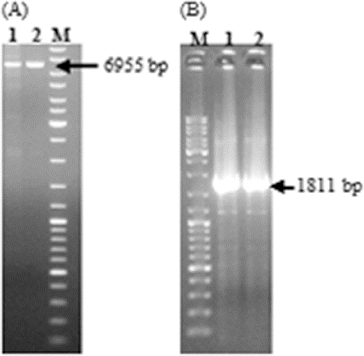
Figure 4. Total RNA isolation from R. sphaeroides transformed with pALA3 (A). Expression analysis of glutamyl-tRNA reductase gene by RT-PCR (B); Lane 1: RT− (without reverse transcriptase); Lane 2: RT+ (with reverse transcriptase); and Lane 3: positive control (with pALA3). DNA ladder (100 bp) was loaded (M).
Production of biohydrogen and 5-ALA
5-ALA and biohydrogen production studies were carried out with both the metabolically engineered strain and the wild-type R. sphaeroides which was transferred with a bare vector as a control. The pH of the medium was initially buffered to 6.8 and was not controlled during the process. The pH changes of the cultures are illustrated in (A). Both the modified and the wild-type strain followed almost the same pH pattern. Initially, the pH increased up to about 9.2 between 120 and 145 h and then returned back to about 7.3. This rise in pH to about 9.2 did not have any negative effects on the growth and hydrogen production of R. sphaeroides because substantial amounts of growth and hydrogen production were observed after this point of the cultivation process ((B) and (C)). Similar pH changes were observed in our previous studies where different concentrations of sugar were tested using molasses.[Citation11] As a whole, consistent results were obtained in both studies with slight variations. Likewise, the growth of wild-type and modified R. sphaeroides were monitored by measuring the absorbance at 660 nm ((B)). Both the wild-type and modified strains reached almost the same high cell densities (OD660 of 7.94 and DCW of 3.34 g/L). This result affirms that sugar beet molasses supports the growth of photosynthetic bacterium R. sphaeroides significantly, which is probably due to the rich composition of molasses. The defined media with organic acid also sustain the growth of R. sphaeroides well but at relatively lower cell densities, e.g. OD660 was 1.9 (DCW of 0.8 g/L) and 3.4 (DCW of 1.43 g/L), using malate and acetate, respectively.[Citation16,17] The production of a considerable amount of PHB (70.4%, w/w) by R. sphaeroides was reported with the use of sugar refinery wastewater.[Citation18] Based on these data, sugar beet molasses can be considered to serve as an excellent carbon, electron and energy source for various metabolic activities.
Figure 5. pH values (A); optical density (OD) measured at 660 nm (B); and hydrogen production (C) during the growth of wild-type and modified R. sphaeroides strain. Values are means from two replicates (±standard deviation).
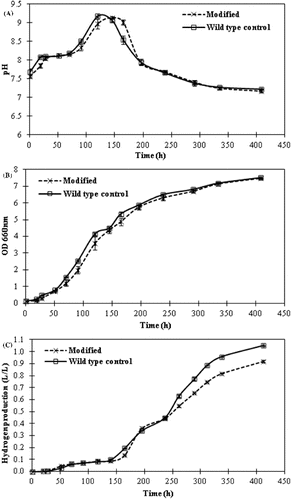
The gas chromatography results about the obtained amounts of pure hydrogen are illustrated in (C). While 1.05 L H2/L culture (0.59 mol H2/mol sucrose) was produced by the wild-type R. sphaeroides, 0.92 L H2/L culture (0.50 mol H2/mol sucrose) was produced by the modified strain. These results indicate that more energy and reducing equivalents were directed towards the nitrogenase enzyme and thus, a little more hydrogen accumulation was achieved with the wild-type cells. On the other hand, the modified cells allocated much of their energy and reducing equivalents to the 5-ALA production pathways and therefore relatively less hydrogen was obtained. These results are in agreement with our previous findings that 0.5 mol H2/mol sucrose was obtained under similar conditions,[Citation11] which suggests that heterologous expression of glutamyl-tRNA reductase gene in R. sphaeroides did not drastically lessen the amount of hydrogen production. There are, however, also reports for relatively higher amounts of hydrogen obtained in different bioprocesses. For example, 3.47 mol H2/mol sucrose was produced in a dark fermentation process using a mixed anaerobic culture.[Citation19] An even higher yield (13.7 mol H2/mol sucrose) was obtained in a sequential dark and photofermentation process with a mutant strain of R. capsulatus.[Citation8]
In this study, we also aimed at improved production of 5-ALA by introducing a second 5-ALA production pathway (C-5 pathway) in addition to the existing C-4 pathway. In this context, the missing glutamyl-tRNA reductase gene was successfully expressed in R. sphaeroides. The concentrations of 5-ALA produced by the modified and the wild-type strain were 25.9 and 12.4 mg/g DCW, respectively. Thus, it was shown that R. sphaeroides produced more 5-ALA upon establishing a second 5-ALA production pathway. A wide range of 5-ALA quantities have been reported. For instance, 0.45–226 μg of 5-ALA/g DCW were obtained in different halotolerant strains of R. sphaeroides.[Citation20] In comparison with these, significant amounts of 5-ALA were produced with both modified and wild-type strains in this study. On the other hand, it was thought that the presence of antibiotic and vector exerted a negative effect on growth, hydrogen and 5-ALA production. For example, when comparing with the results from our previous study [Citation11] where antibiotic was not used, in this study either a delay or a decrease in cell density, hydrogen and 5-ALA productions occurred. For this reason, in the future, the glutamyl-tRNA reductase gene should be integrated into the chromosome to eliminate vector usage and the accompanying negative effects. Further studies for improvements in the gene expression system are underway.
Conclusions
In this study, a second 5-ALA production pathway (C-5 pathway), in addition to the C-4 pathway, was enabled by heterologous expression of the missing glutamyl-tRNA reductase gene in R. sphaeroides. Thus, a new and unique bacterial strain producing more 5-ALA was developed by implementing a novel idea. The gene expression system could be optimized in the future or the glutamyl-tRNA reductase gene could be integrated into the chromosome to eliminate vector usage and the accompanying negative effects. Biohydrogen was also produced in the same bioprocess within a biorefinery approach using sugar beet molasses as substrate. As a result, a unique and more feasible bioprocess was obtained.
Acknowledgements
We thank Prof. Dr Richard R. Burgess (University of Wisconsin–Madison) for his valuable comments and corrections.
Additional information
Funding
References
- Sasaki K, Watanabe M, Tanaka T, Tanaka T. Biosynthesis, biotechnological production and applications of 5-aminolevulinic acid. Appl Microbiol Biotechnol. 2002;58:23–29.
- Miyachi N, Tanaka T, Nishikawa S, Takeya H, Hotta Y. Preparation and chemical properties of 5-aminolevulinic acid and its derivatives. Porphyrins. 1998;7:342–347.
- Kamiyama H, Hotta Y, Tanaka T, Nishikawa S, Sasaki K. Production of 5-aminolevulinic acid by a mutant strain of a photosynthetic bacterium. Seibutsu Kogaku Kaishi. 2000;78:48–55.
- Ano A, Funahashi H, Nakano K, Nishizawa Y. Effect of glycine on 5-aminolevulinic acid biosynthesis in heterotrophic culture of Chlorella regularis YA-603. J Biosci Bioeng. 1999;88:57–60.
- Pandey U, Pandey J. Enhanced production of δ-aminolevulinic acid, bilipigments, and antioxidants from tropical algae. Biotechnol Bioprocess Eng. 2009;14:316–321.
- Liang J, Burris HR. Hydrogen burst associated with nitrogenase-catalyzed reactions. Proc Natl Acad Sci USA. 1988;85:9446–9450.
- Hustede E, Steinbüchel A, Schlegel HG. Relationship between the photoproduction of hydrogen and the accumulation of PHB in non-sulphur purple bacteria. Appl Microbiol Biot. 1993;39:87–93.
- Özgür E, Mars AE, Peksel B, Louwerse A, Yücel M, Gündüz U, Claassen PAM, Eroğlu İ. Biohydrogen production from beet molasses by sequential dark and photofermentation. Int J Hydrogen Energy. 2010;35:511–517.
- Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Nat Biotechnol. 1983;1:784–791.
- Kovach EM, Elzer HP, Hill SD, Robertson TG, Farris AM, Roop MR, Peterson MK. Four new derivatives of the broad host range cloning vector pBBR1MCS, carrying different antibiotics-resistance cassettes. Gene. 1995;166:175–176.
- Kars G, Alparslan Ü. Valorization of sugar beet molasses for the production of biohydrogen and 5-aminolevulinic acid by Rhodobacter sphaeroides O.U.001 in a biorefinery concept. Int J Hydrogen Energy. 2013;38:14488–14494.
- Biebl H, Pfennig N. Isolation of member of the family Rhodosprillaceae. In: Starr MP, Stolp H, Trüper HG, Balows A, Schlegel HG, editors. The prokaryotes. Vol. 1. New York (NY): Springer; 1981. p. 267–273.
- Choi C, Hong BS, Sung HC, Lee HS, Kim JH. Optimization of extracellular 5-aminolevulinic acid production from Escherichia coli transformed with ALA synthase gene of Bradyrhizobium japonicum. Biotechnol Lett. 1999;21:551–554.
- Mauzerall D, Granick S. The occurrence and determination of δ-aminolevulinic acid and porphobilinogen in urine. J Biol Chem. 1956;219:435–446.
- Marrs B, Kaplan S. 23 s precursor ribosomal RNA of Rhodopseudomonas spheroides. J Mol Biol. 1970;49: 297–317.
- Kars G, Gündüz U, Yücel M, Türker L, Eroğlu İ. Hydrogen production and transcriptional analysis of nifD, nifK and hupS genes in Rhodobacter sphaeroides O.U.001 grown in media with different concentrations of molybdenum and iron. Int J Hydrogen Energy. 2006;31:1536–1544.
- Kars G, Gündüz U, Yücel M, Rakhely G, Kovacs K, Eroğlu İ. Evaluation of hydrogen production by Rhodobacter sphaeroides O.U.001 and its hupSL deficient mutant using acetate and malate as carbon sources. Int J Hydrogen Energy. 2009;34:2184–2190.
- Yiğit DÖ, Gündüz U, Türker L, Yücel M, Eroğlu İ. Identification of by-products in hydrogen producing bacteria; Rhodobacter sphaeroides O.U. 001 grown in the waste water of a sugar refinery. J Biotechnol. 1999;70:125–131.
- Guo WQ, Ren NQ, Wang XJ, Xiang WS, Meng ZH, Ding J, Qu YY, Zhang LS. Biohydrogen production from ethanol-type fermentation of molasses in an expanded granular sludge bed (EGSB) reactor. Int J Hydrogen Energy. 2008;33:4981–4988.
- Tangprasittipap A, Prasertsan P, Choorit W, Sasaki K. Biosynthesis of intracellular 5-aminolevulinic acid by a newly identified halotolerant Rhodobacter sphaeroides. Biotechnol Lett. 2007;29:773–778.
