Abstract
Ruscus aculeatus L. is a perennial semi-shrub with distinctive leaf-like branches (cladodes). Rhizomes and roots contain steroidal saponins (ruscogenins) that are used in medicine and cosmetics for their anti-inflammatory, venotonic and antihaemorroidal activity. Problematic cultivation of the species causes in many countries unsustainable over-collection from the wild. Tissue culture propagation of R. aculeatus was carried out for conservation and propagation purposes. The impact of the clonal origin (genotype) on the ruscogenin biosynthesis, genome-size stability and propagation traits and morpho-physiological response to long-term cultivation in vitro was studied. Production of ruscogenins in fully developed regenerants was quantified by high-performance liquid chromatography (HPLC). Genome-size stability of the clones was assessed by flow cytometry. Slow growth and prolonged lag-phase were characteristic for the whole propagation cycle. Produced plantlets with well-defined organs were suitable for direct ex vitro planting. Genome DNA content of all clones was stable and comparable to native plants. Ruscogenin biosynthesis was clone-specific, presenting distinctive profiles of the cultures. Our results imply that clone origin and culture type might influence saponin biosynthesis in Ruscus. These traits should be considered in the ex situ conservation of the genetic diversity of this species and by production of planting material as well.
Introduction
In vitro techniques are a powerful tool for ex situ plant conservation. They offer convenient storage in small space and controlled environment for valuable plant species that are hampered to propagate by seeds or by vegetative material. Adequate conservation practices require verification of the genetic conformity and normal physiology of the obtained plants.[Citation1,2] Clonal origin (genotype) is considered as a crucial factor for the culture performance in many species. Its impact is very important especially for conservation programmes since the protocols are generally based on one or few donor individuals. The effectiveness could be limited by species- and clone-specific traits causing recalcitrance of the cultures.[Citation3] The in vitro storage procedure itself can cause unnatural variation or occurrence of rare variants, especially when dedifferentiation stage is used (reviewed in [Citation4–9]).
Ruscus aculeatus L. (Ruscaceae) is a slowly growing perennial semi-shrub, naturally distributed in the Mediterranean, South Central Europe, Caucasus and Crimnea.[Citation10] Ruscus aculeatus is introduced as a garden and house plant in Britain and North America, as the evergreen stems with typical leaf-like flattened branches (cladodes) are prized as ornamental greenery. Rhizomes and roots contain steroidal saponins (ruscogenins) that are used in medicine and cosmetics for their anti-inflammatory, venotonic, antihaemorroidal activity.[Citation11–14] The herbage, as greenery, and the pharmaceutical raw material are collected mainly from the wild.[Citation15,16] Complicated cultivation of Ruscus species causes their unsustainable over-collection from the natural populations and management of wild resources remains an unresolved issue in some countries.[Citation7,Citation17–19] Slow plant growth and development associated with limited growth of the aboveground organs and pollination failure makes the species vulnerable to distress in the natural population.[Citation20] Conservation efforts, so far, have relied on limitation or prohibition of gathering together with cultivation trials with partial success.[Citation18,Citation21,Citation22] In vitro cultivation of R. aculeatus was carried out to meet the limitations of the conventional propagation (rhizome splitting). Research teams, mostly, in Mediterranean and Balkan countries have developed propagation protocols with donor material from wild populations, but none has reached commercial stage yet.[Citation23–26] Presented results are often partial, presenting different gaps in the initiation or propagation stages. Problems were related to decontamination, slow growth and lack of shoot regeneration. However, the limited data does not allow defining the production ‘bottle-necks’.
This work evaluates significance of the clonal origin on performance of the R. aculeatus cultures by assessment of the ruscogenin biosynthesis, genome-size stability, propagation rate and morpho-physiological response to long-term cultivation in vitro.
Materials and methods
Plant material and culture conditions
Seeds were collected from sites in Strandzha Mt. (SE Bulgaria)–A (1–3) and Stara Planina Mt. (West Bulgaria)–B (1–4) as part of the Millennium Seed Bank Partnership collection missions. Voucher specimens were deposited in the Herbarium of Institute of Biodiversity and Ecosystem Research (SOM). Shoot cultures were initiated from rhizome segments of seedlings by a previously described technique.[Citation27]
Seven seed-derived clones from Bulgarian wild populations were compared. Cultures were maintained continuously on Murashige & Skoog (MS) media [Citation28] with 1 mg/L 6-Benzylaminopurine (BAP), 0.5 mg/L α-Naphthaleneacetic acid (NAA), 0.8 mg/L Plantagar (all supplied by Duchefa, Netherlands) and 30 g mg/L commercial sugar. All media were adjusted to pH 5.75 prior to sterilization and autoclaved at 121 °C/1 atm for 20 minutes. The cultures were maintained in VitroVent® containers (with 125 mL medium, at 16/8 h photoperiod and 23 ± 1 °C). Cultures were transferred onto fresh media every eight weeks and every shoot with minimal height of 0.5 mm was detached and grown separately on the same medium. Propagation rate, shoot growth and appearance were recorded for at least 18 months.
Genome-size stability
DNA content of the cultures from all clones was measured using flow cytometry with propidium iodide staining, using Partec CyFlowR SL and following the protocol provided by Partec (Sysmex Partec GmbH, Germany). Pisum sativum cv. Kleine Rheinländerin (2С = 8.84 pg) was used as internal standard. Native R. aculeatus plants from Stara Planina Mt. were used as reference control. Fresh cladode samples were collected and genome size was measured in three replicates, each run with 5000 counts. Data were analysed statistically for variation using Duncan's multiple range test.
Ruscogenin quantification
Production of ruscogenins in shoots, rhizomes and roots of fully developed regenerants was determined by HPLC following adapted procedure.[Citation29] Fine powdered samples (50 mg) of at least 50 fully developed in vitro plants were prepared by drying at 35 °С for 48 h. Extraction was done twice with 4 mL 50:50 aqueous methanol for 24 h, followed by evaporation under vacuum (Heidolph Laborota 4003 Rotary Evaporator, at 40 °С). Dry extracts were hydrolyzed for 4 h at 80 °C with 4mL n-butanol, 1 mL deionized water and 0.55 mL 37% HCl. Samples were neutralized with 5% NaHCO3 solution and washed. Butanolic fractions were filtered (0.20 μm PTFE syringe filters, DISMIC, Denmark) and evaporated to dryness. Residues were dissolved in 200 μL methanol (GC-grade, Leda) and filtered (Waters syringe filters, 0.45 μm, PTFE) before loading in autosampler vials (Waters 717plus). Chromatographic system: column: Phenomenex Synergi MAX-RP 80A 4 μm, 150 × 4.6 mm; pre-column: Synergi MAX 4 × 3.0 mm; detector: Waters M 996 Diode Array Detector PDA Max plot 180–800 nm; pump: Waters 600E. Chromatographic conditions: injection volume = 10 μL; V = 1 ml/ml, T = 25 °C. Elution gradient: А (acetonitrile:water) 65:35; B (acetonitrile:water) 50:50 ().
Table 1. Elution conditions for ruscogenin HPLC quantification.
Quantification was done against standards of the sapogenins, ruscogenin and neoruscogenin and two desmosides of the neoruscogenin:ruscin and desglucoruscin (butchers broom Ruscus aculeatus root VBRM ChromaDex®, USA). Calibration curves were set between 0.2 and 4 μg/0.01 mL.
Results and discussion
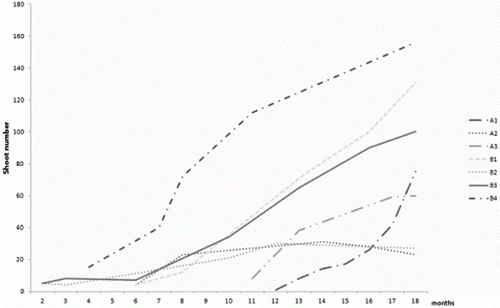
Dormancy in R. aculeatus seeds is considered to be morpho-physiological and spans between 2 months and year and a half.[Citation10,Citation30] Germination rates were described as variable depending on the seed lot, age and origin of the seeds.[Citation23,Citation26,Citation31] Seedlings had short rhizomatous stem and usually one shoot. Slow growth was characteristic for all R. aculeatus clones (). Seedlings presented a rather variable mode of growth and performance under controlled conditions. In the propagation stage, new shoots were induced directly on the surface of the rhizome explants in the limited proliferative area around the apical and adventitious buds. Only three of the tested clones showed propagation rates above 100 regenerants over the period of 18 months. All clones produced shoots with upright aerial stem, rarely branched, having 3–11 elliptical cladodes. Cultures tended to develop rooted shoots suitable for direct ex vitro planting. Limited shoot number could be related to apical dominance, suppressing new shoot development as shown in other rhizomatous species.[Citation32–34] Nevertheless, prolonged initiation phase and reduced shoot number was reported for R. aculeatus, even by authors using callus cultures.[Citation23,Citation25] Delayed regeneration in the closely related Asparagus officinalis is associated with continual alternation in sensitivity to growth regulators.[Citation35] Similar tendency was distinctive within three years of R. aculeatus cultivation. Raise in the propagation rate was linked to changed sensitivity to BAP in the media (data not shown). This habit was considered as positive for longer ex situ storage as most of aged cultures tend to worsen their vitality with time.[Citation2,Citation36] Differential requirement of growth regulators due to genotypic differences was shown as an important factor for micropropagation of Curcuma cultivars.[Citation37–39]
Figure 1. Propagation rate of R. aculeatus clones for 18 months. A (1–3): seed-derived clones from Strandzha Mt.; B: seed-derived clones from Stara Planina Mt.
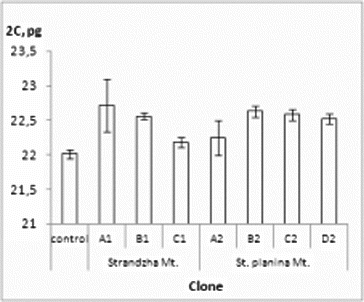
Flow cytometry is one of the convenient and quick methods to test genome stability of in vitro obtained plants and was readily applied to variety of species in the recent years.[Citation41–43] Present evaluation confirmed genome-size stability of all clones (). 2C-values ranged from 22.00 to 22.71 pg. The average genome DNA (2C = 22.49 pg) was slightly higher than the native control and close to previously reported values.[Citation40] Statistically significant differences were not observed.
Figure 2. Genome size of the R. aculeatus in vitro clones. Values in pg ± SE. Control – native R. aculeatus plants. Internal standard – P. sativum.
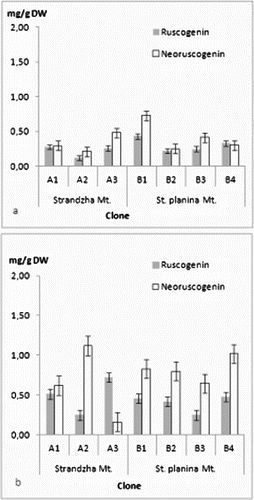
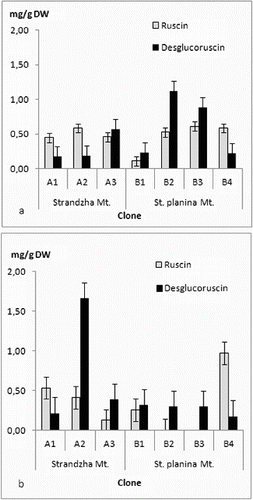
The four ruscogenins were determined in all in vitro clones ((a) and (b) and (a) and (b)). There was no clear tendency for grouping by geographical basis. Even more every clone had its own specific profile. Current ratios for ruscogenin and neoruscogenin were similar to those reported for Bulgarian populations.[Citation44] Quantitatively contents were similar to those reported for Romanian callus-derived clones.[Citation45] Highest amounts in shoots were recorded for the clone B1 – neoruscogenin (0.73 mg/g DW) and ruscogenin (0.43 mg/g DW). These were about the average for the rhizome and roots, where most productive for neoruscogenin and ruscogenin were clones A2 and A3, respectively (1.12 mg/g DW and 0.71 mg/g DW). All clones had about twice time lower production of ruscogenin than neoruscogenin in the underground organs. In vitro (callus) cultures were reported to have limited biosynthetic abilities with increase of the neoruscogenin and ruscogenin contents following the organogenesis.[Citation29] Comparing all measured ruscogenins, it was evident that roots were more productive than shoots, contrary to in vitro cultures of Spanish origin.[Citation46] Neoruscogenin and ruscogenin in cladodes were mostly even. Neoruscogenin prevailed in rhizomes and roots. Only one of the clones (A3) showed ruscogenin domination. Studies in natural populations in Turkey and Romania revealed that neoruscogenin was higher in the shoots rather than in the roots, and for the ruscogenin the organ distribution was contrasting.[Citation28,Citation47] Ruscin and desglucoruscin were measured for the first time in tissue cultures, performing considerable amounts both in shoots and underground parts, similar to neoruscogenin. Desglucoruscin reached highest value in rhizomes and roots of clone A2 (1.66 mg/g DW) and for the shoots in clone B2 (1.12 mg/g DW). Ruscin was missing in underground parts of B2 and B3 clones. These results suggested that origin of the material on population as well as on individual level could be important factor for the saponin biosynthesis. Moreover, the obtained data does not support the hypothesis that ruscogenin biosynthesis occurs mainly in the aerial shoots.[Citation46] The differences could be related not only to genotype but also to culture type and conditions, explants source, regenerants age and sampling time, as the yield of secondary metabolites depends on multiple factors.[Citation48–50] The obtained results are particularly interesting, considering the limited number of seed collections of R. aculeatus,[Citation51] low germination rates [Citation21] and the recalcitrant micropropagation habit reported previously.[Citation23,Citation25,Citation27]
Conclusions
Considering the above-mentioned issues recovery of R. aculeatus populations and production of planting material by micropropagation could be greatly influenced by the choice of the source material. However, in vitro preservation is suitable for conservation of the species with minimal efforts as flow cytometric data confirm the stability of the genome size of the regenerants. Still clone-specific propagation rates and ruscogenin profiles necessitate large number of donor plants to be maintained so as to cover the genetic diversity of the species.
Funding
This work was supported by the Bulgarian Ministry of Education and Science [grant number BG051PO001/07/3.3-02/70].
Acknowledgements
The methodological advice and assistance of B. Pandova and St Yanev for HPLC analyses are gratefully acknowledged. The authors are thankful to RGB Kew Millenium Seed Bank staff and the coordinator for Bulgaria (C. Trivedi) for their support.
References
- Benson E, editor. Plant Conservation Biotechnology. London: Taylor & Francis; 1999.
- Engelmann F. Use of biotechnologies for conserving plant biodiversity. Acta Horticulturae. 2009;812:63–82.
- Benson E. In vitro plant recalcitrance: an introduction. In Vitro Cell Dev Biol Plant. 2000;36:141–148.
- Ahloowalia B. Limitations to the use of somaclonal variation in crop improvement. In: Semal J, editor. Somaclonal variations and crop improvement. The Hague: Martinus Nijhoff; 1985. p. 14–27.
- Bairu M, Adeyemi O, Van Staden J. Somaclonal variation in plants: causes and detection methods. Plant Growth Regul. 2011;63:147–173.
- Elmaghrabi A, Ochatt S. Isoenzymes and flow cytometry for the assessment of true-to-typeness of calluses and cell suspensions of barrel medic prior to regeneration. Plant Cell Tissue Organ Culture. 2006;85:31–43.
- Larkin PJ, Scowcroft WR. Somaclonal variation – a novel source of variability from cell cultures for plant improvement. Theor Appl Genet. 1981;60:197–214.
- Phillips RL, Kaeppler SM, Olhoft P. Genetic instability of plant tissue cultures: breakdown of normal controls. Proc Natl Acad Sci USA. 1994;91:522–526.
- Rani V, Raina S. Genetic fidelity of organized meristem-derived micropropagated plants: a critical reappraisal. In Vitro Cell Dev Biol Plant. 2000;36:319–330.
- Yeo PF. Ruscus L. In: Tutin et al. editors. Vol. 5. Alismataceae to Orchidaceae (Monocotyledones). Cambridge: Cambridge University Press; 1980.
- Blumenthal M, Goldberg A, Brinkmann J, editors. Herbal medicine: expanded commission E monographs. Boston (MA): Integrative Medicine Communications; 2000.
- Capra C. Pharmacological and toxicological study on the constituents of Ruscus aculeatus L. Fitoterapia. 1972;43:99–113.
- De Combarieu E, Falzoni M, Fuzzati N, Gattesco F, Giori A, Lovati M, Pace R. Identification of Ruscus steroidal saponins by HPLC-MS analysis. Fitoterapia. 2002;73:583–596.
- Sparg S, Light M., Staden J. Biological activities and distribution of plant saponins. J Ethnopharmacol. 2004;94(2–3):219–243.
- Goi K, Fleurentin J, Todisco M. The market for medicinal plants in France. International trade in medicinal plants. France (Europe): TRAFFIC; 1997.
- Özhatay N, Koyuncu M, Atay S, Byfield AJ. The wild medicinal plant trade in Turkey. (Europe): TRAFFIC; 1997.
- Coskun M, Guvenc A, Kilic C, Anhan O. Ruscus aculeatus trade in Turkey: Is it sustainable? Planta Med. 2006;72: 298. doi:10.1055/s-2006-950098.
- Karlović K. Introduction of ornamental native plants into commercial production in Croatia. Acta Horticulturae. 2009;813:107–112.
- Lange D. Europe's medicinal and aromatic plants: their use, trade and conservation. France (Europe): TRAFFIC; 1998.
- Martínez-Pallé E, Aronne G. Pollination failure in Mediterranean Ruscus aculeatus. Bot J Linnean Soc. 2000;134:443–452.
- D’Antuono L, Lovato A. Germination trials and domestication potential of three native species with edible sprouts: Ruscus aculeatus L., Tamus communis L. and Smilax aspera L. Acta Horticulturae. 2003;598:211–218.
- Zistler C, Krafka O, Kranvogel A, Sonnenschein M. Cultivation experiments with butcher's broom (Ruscus aculeatus L.). Zeitschrift für Arznei Gewuerzpflanzen. 2008;13(3):114–116.
- Banciu C, Aiftimie-Păunescu A. In vitro propagation of rare species Ruscus aculeatus L. and histological peculiarities of the regenerants. Ann Oradea Univ, Biol. 2012;19(1):67–73.
- Banciu C, Mitoi M, Brezeanu A. Biochemical peculiarity of in vitro morphogenesis under conservation strategy of Ruscus aculeatus L. Ann For Res. 2009;52:109–116.
- Luz E, Mendes ML, Romano A. In vitro multiplication of Ruscus aculeatus L. Revista de Biologia (Lisbon). 2004;22:133–142.
- Moyano E, Montero M, Bonfill M, Cusidó RM, Palazón J, Piñol MT. In vitro micropropagation of Ruscus aculeatus. Biologia Plantarum. 2006;50:441–443.
- Ivanova T, Gussev Ch, Bosseva Y, Stanilova M, Stoeva T. In vitro regeneration of Ruscus aculeatus L. – effective micropropagation by shoot cultures. Propagation Ornamental Plants. 2008;8(1):39–41
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum. 1962;15:473–497
- Palazon J, Moyano E, Bonfill M, Osuna LT, Cusido R, Pinol T. Effect of organogenesis on steroidal saponin biosynthesis in calli cultures of Ruscus aculeatus. Fitoterapia. 2006;77(3):216–220.
- Komar G. Asparagaceae. In: Takhtajan AL, editor. Comparative seed anatomy. Vol. 1. Leningrad: Nauka; 1985. p. 99–103.
- Gosling P. Raising trees and shrubs from seed. Forestry Commission Practice Guide. Edinburgh: Forestry Commission; 2007.
- Anisuzzaman M, Sharmin S, Mondal S, Sultana R, Khalekuzzaman M, Alam I, Alam M. In vitro microrhizome induction in Curcuma zedoaria (Christm.) Roscoe – a conservation prioritized medicinal plant. J Biol Sci. 2008;8(7):1216–1220.
- Bond S, Alderson P. The influence of apical dominance on the in vitro multiplication of the rhizome of Alstroemeria. J Hortic Sci. 1993;68:905–910.
- Pumisutapon P, Visser R, De Klerk G-J. Apical dominance in Alstroemeria cultured in vitro. Acta Horticulturae. 2009;829:145–148.
- Doré C. Asparagus anther culture and field trials of dihaploids and F1 hybrids. In: Bajaj Y, editor. Haploids in crop improvement. Biotechnology in agriculture and forestry. Vol. 12. Berlin: Springer-Verlag; 1990. p. 322–345.
- Cousins M, Adelberg J. Short-term and long-term time course studies of turmeric (Curcuma longa L.) microrhizome development in vitro. Plant Cell Tissue Organ Culture. 2008;93:283–293.
- Balachandran SM, Bhat SR, Chandel KPS. In vitro clonal multiplication of turmeric (Curcuma spp) and ginger (Zingiber officinale Rosc.). Plant Cell Rep. 1990;8:521–524
- Das A, Kesari V, Rangan L. Plant regeneration in Curcuma species and assessment of genetic stability of regenerated plants. Biologia Plantarum 2010;54:423–429.
- Nayak S. In vitro multiplication and microrhizome induction in Curcuma aromatica Salisb. Plant Growth Regul. 2000;32:41–47.
- Veselý P, Bureš P, Šmarda P, Pavlíček T. Genome size and DNA base composition of geophytes: the mirror of phenology and ecology? Ann Bot. 2012;109:65–75.
- Doležel J. Flow cytometric analysis of nuclear DNA content in higher plants. Phytochemical Anal. 1991;2:143–154.
- Loureiro J, Suda J, Doležel J, Santos C. FLOWer: a plant DNA flow cytometry database. In: Doležel J, Greilhuber J, Suda J, editors. Flow cytometry with plant cells. Weinheim: Wiley-VCH; 2007. p. 423–438.
- Sliwinska E, Thiem B. Genome size stability in six medicinal plant species propagated in vitro. Biologia Plantarum. 2007;51:556–558.
- Nikolov S, Joneid, M, Panova D. Quantitative determination of ruscogenin in Ruscus species by densitometric thin-layer chromatography. Pharmazie. 1976;31(9):611–612.
- Balica G, Vlase L, Deliu C, Tămaş M, Crişan G. Comparative phytochemical study on rhizome and tissue culture of Ruscus aculeatus L. Planta Med. 2007;73:226.
- Mangas S, Bonfill M, Osuna L, Moyano E, Tortoriello J, Cusid, RM, Piñol MT, Palazón J. The effect of methyl jasmonate on triterpene and sterol metabolisms of Centella asiatica, Ruscus aculeatus and Galphimia glauca cultured plants. Phytochemistry. 2006;67:2041–2049.
- Vlase L, Kiss B, Balica G, Tamas M, Crisan G, Leucuta SE. High-throughput LC/MS/MS analysis of ruscogenin and neoruscogenin in Ruscus aculeatus L. J AOAC Int. 2009;92(4):1055–1059.
- Canter P, Thomas H, Ernst E. Bringing medicinal plants in cultivation: opportunities and challenges for biotechnology. Trends in Biotechnol. 2005;23:180–185.
- Ramawat KG, Merillon JM, editors. Biotechnology-secondary metabolites. Enfield: Science Pub Inc.; 1999.
- Nayak S, Kaur T, Mohanty S, Ghosh G, Choudhury R, Acharya L, Subudhi E. In vitro and ex vitro evaluation of long-term micro propagated turmeric as analysed through cytophotometry, phytoconstituents, biochemical and molecular markers. Plant Growth Regul. 2011;64:91–98.
- EURISCO Catalogue. Available from: http://eurisco.ecpgr.org, (2013-04-28).