Abstract
This study investigated if the use of the pesticides cyprodinil and fludioxonil produced an inhibitory effect on the bovine liver catalase (CAT) activity. It was documented that the activity of the enzyme decreased with increasing concentrations of cyprodinil and fludioxonil from 0 to 500 ppm. At pesticide concentrations of 250 and 500 ppm, the activity of CAT remained unchanged and passed to a steady state. The exposure to cyprodinil in concentrations of 10, 50, 100, 250 and 500 ppm, led to a decrease in the per cent of the CAT enzyme activity calculated as 45.4, 68.0, 73.0, 77.8 and 77.4, respectively. Similarly, the exposure to fludioxonil in concentrations of 10, 50, 100, 250 and 500 ppm, produced the following percentage decrease in the CAT enzyme activity: 20.0, 30.8, 42.8, 46.3 and 45.9, respectively. Cyprodinil inhibited CAT competitively, whereas the mechanism of fludioxonil inhibition over the enzyme was non-competitive.
Introductıon
Catalase (CAT) is a general enzyme found in nearly all living organisms exposed to oxygen. CAT is an enzyme with antioxidant properties. It catalyzes the degradation of hydrogen peroxide to water and oxygen.[Citation1] The molecule of CAT is made of a tetramer of four polypeptide chains, each over 500 amino acids long. CAT contains four porphyrin heme (iron) groups that permit the enzyme to react with hydrogen peroxide.[Citation2] Hydrogen peroxide itself is a harmful co-product of many general metabolic processes. In order that damage to cells and tissues is avoided, the produced hydrogen peroxide must be immediately converted into other, less-reactive substances. For this purpose, CAT is often used by cells to quickly catalyze the degradation of hydrogen peroxide into less-reactive oxygen and water molecules.[Citation3]
The fungicides cyprodinil and fludioxonil are active ingredients of the product Switch 62.5WG. It contains 37.5% cyprodinil and 25.0% fludioxonil.[Citation4] The product is used for the control of Botrytis cinerea (Grey Mold). Cyprodinil, is a widespread pyrimidinamine fungicide, with a worldwide agricultural usage for protection of fruit plants, vines, cereals and vegetables from a large number of pathogens.[Citation5] Fritz et al.,[Citation6] Kanetis et al.,[Citation7] Masner et al. [Citation8] and Mindt [Citation9] reported that cyprodinil acts by inhibiting the biosynthesis of methionine and other thionic amino acids in the fungi. Fludioxonil, is a phenylpyrrole antifungal compound derived from the antibiotic pyrrolnitrin.[Citation10] It has a wide antifungal spectrum and is now used to control a variety of important plant-pathogenic fungi. It is a unique antifungal compound that targets signal transduction.[Citation11]
In this study, the optimal temperature of CAT was determined and the inhibition of CAT by the two pesticides, cyprodinil and fludioxonil, was observed.
Materials and methods
Chemicals
CAT from bovine liver (C-1345) was obtained from Sigma. Fludioxonil (4-(2,2-difluoro-1,3-benzodioxol-4-yl) pyrrole-3-carbonitrile) (46102) and cyprodinil (4-cyclopropyl-6-methyl-N-phenylpyrimidin-2-amine) (34389) were obtained from Fluka Riedel-de Haen. All other used chemicals were of analytical grade.
CAT activity measurement
CAT activity was measured according to the methods described by Lartillot et al.[Citation12]
Protein measurement
The protein concentration was determined using the method of Lowry et al.[Citation13]
Effects of pesticides on enzyme activity
A total of 5000 ppm cyprodinil and fludioxonil were prepared by us and dissolved in 2 ml ethyl alcohol. Following, aliquots were arranged to match 10, 50, 100, 250 and 500 ppm, and then each of these was mixed with 700 µL enzyme solution. The final volume of enzyme solution plus ethyl alcohol and pesticide was 1 ml. The mixture was incubated at room temperature for an hour and then the activities of CAT were measured.
Temperature optimum
Activities of CAT were measured at temperatures between 5 and 50 °C and the optimal temperature was determined.
pH optimum
A phosphate buffer (50 mM, pH 7.5) was used according to Tukel and Alptekin in order to determine the optimal pH for the reaction.[Citation14]
Data analysis
Data were presented as mean ± standard error. For the statistical analyses, one-way analysis of variance was used, followed by the Student Newman–Keul's test using the SPSS version 21 statistical software (SPSS Inc., Chicago, IL, USA). Differences were considered significant, if P < 0.05.
Results and discussion
Temperature optimum
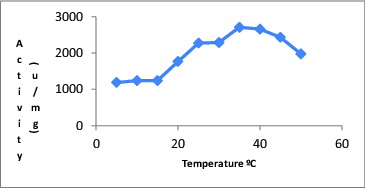
The maximal activity of CAT was determined at 35 °C, which is also presented in .
Exposure of CAT to cyprodinil
At different concentrations of cyprodinil mixed with the CAT enzyme, its activities were measured and were given in . As cyprodinil concentrations increased, CAT was inhibited and its activity decreased. There were statistically significant differences between all determined values for the inhibited CAT, which interacted with the cyprodinil at the mentioned concentrations (P < 0.05, n = 3), and the control activity. The calculated per cent decrease of CAT enzyme activity after exposure to cyprodinil at concentrations of 10, 50, 100, 250 and 500 ppm, were as 45.4, 68.0, 73.0, 77.8 and 77.4, respectively.
Table 1. Effect of cyprodinil concentrations on catalase activity.
Inhibition type of cyprodinil to CAT
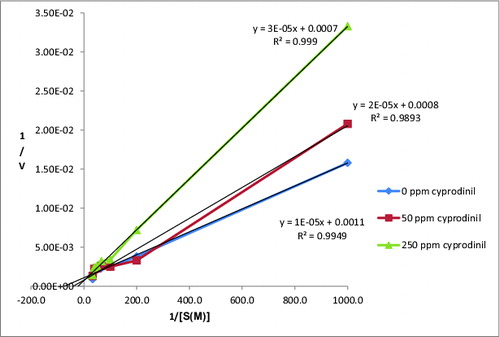
The activities of CAT were determined at different substrate concentrations of H2O2 (1, 5, 10, 15, 20, 25 and 30 mM) and at different concentrations of cyprodinil (50 and 250 ppm). Lineweaver–Burk graph of CAT was drawn by using the obtained results ().
According to , Vmax, Km and Ki (inhibition constant) of CAT at different concentrations of cyprodinil were calculated and were shown in . The Vmax values determined by us were near each other at the Lineweaver–Burk graph and we found increased Km values. Thus, it is suggested by us that cyprodinil acted on the activity of CAT via a competitive type of inhibition.
Table 2. Effect of cyprodinil and fludioxonil on the kinetic parameters of CAT.
Exposure of CAT to fludioxonil
At different concentrations of fludioxonil mixed with the CAT enzyme, its activities were measured and were given in . As fludioxonil concentrations increased, CAT was inhibited and its activity decreased. There were statistically significant differences between all determined values for the inhibited CAT, which interacted with the fludioxonil at the mentioned concentrations (P < 0.05, n = 3), and the control activity. The calculated per cent decrease of CAT enzyme activity after exposure to fludioxonil at concentrations of 10, 50, 100, 250 and 500 ppm, were as 20.0, 30.8, 42.8, 46.3 and 45.9, respectively.
Table 3. Effect of fludioxonil concentrations on catalase activity.
Inhibition type of fludioxonil to CAT
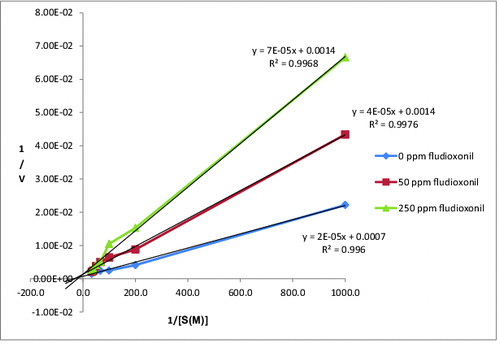
The activities of CAT were determined at different substrate concentrations of H2O2 (1, 5, 10, 15, 20, 25 and 30 mM) and at different concentrations of fludioxonil (50 and 250 ppm). Lineweaver–Burk graph of CAT was drawn by using the obtained results ().
According to , Vmax, Km and Ki (inhibition constant) of CAT at different concentrations of fludioxonil were calculated and were shown in . The Km values determined by us were near each other and we found decreased Vmax values at the Lineweaver–Burk graph. Thus, it is suggested by us that fludioxonil acted on the activity of CAT via a non-competitive type of inhibition.
The optimum temperature for CAT determined by us was 35 °C. Çetinus et al. [Citation15] reported same result for bovine liver CAT.[Citation15] However, Tukel and Alptekin [Citation14] indicated a maximum activity for CAT derived from the same source to be 25 °C.[Citation14]
Karadag and Bilgin [Citation16] treated cyprodinil and fludioxonil with a solution of copper-zinc superoxide dismutase (CuZnSOD), which was purified from human erythrocytes by diethylaminoethyl (DEAE)–cellulose chromatography and copper–iminodiacetic acid agarose chromatography.[Citation16] They reported similar results for CuZnSOD. Cyprodinil inhibited CuZnSOD competitively and fludioxonil inhibited CuZnSOD non-competitively. In addition to this, other studies also examined the inhibition of CAT by pesticides. Li et al. [Citation17] observed significant inhibition of CAT with prolonged exposure of propiconazole, a triazole fungicide, in the brain of the rainbow trout, Oncorhynchus mykiss.[Citation17] When, Radice et al. [Citation18] applied 0.3 and 0.4 mM, iprodione, a dicarboximide fungicide, to hepatocytes from rainbow trout (Oncorhynchus mykiss) they documented increasing both reactive oxygen species (ROS) and malondialdehyde (MDA) production and decreasing glutathione (GSH) content and CAT activity.[Citation18] Toni et al. [Citation19] applied different concentrations of the fungicide tebuconazole to common carp (Cyprinus carpio) and observed decrease in the activity of CAT at 1.19 mg/L.[Citation19] Rehman et al. [Citation20] studied the effect of deltamethrin in mice. They orally administered 2 doses of deltamethrin, 5.6 and 18 mg/kg body weight (bw), for 15 days. They observed suppressed CAT activity in liver and kidney of male Swiss albino mice.[Citation20]
Conclusions
According to our knowledge, this is the first study related to the effect of cyprodinil and fludioxonil on CAT enzyme activity. We observed inhibition of the CAT activity induced by these fungicides. We determined that cyprodinil inhibited CAT competitively and fludioxonil inhibited the enzyme non-competitively. Although cyprodinil molecules do not possess similarity to the molecules of hydrogen peroxide, we suggest that cyprodinil molecules generate radicals, which in turn compete with the hydrogen peroxide molecules to tie porphyrin heme (iron) groups.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Funding
This work was supported by the Research Foundation of Adiyaman University [project number FEFYL/2012-0013].
References
- Chelikani P, Fita I, Loewen PC. Diversity of structures and properties among catalases. Cell Mol Life Sci. 2004;61(2):192–208.
- Boon EM, Downs A, Marcey D. Catalase: H2O2: H2O2 oxidoreductase. Catalase structural tutorial text [cited 2007 Feb 11]. Available from: http://biology.kenyon.edu/BMB/Chime/catalase/frames/cattx.htm
- Gaetani G, Ferraris A, Rolfo M, Mangerini R, Arena S, Kirkman H. Predominant role of catalase in the disposal of hydrogen peroxide within human erythrocytes. Blood. 1996;87(4):1595–1599.
- Switch 62.5 WG-Syngenta. 2011 [cited 2014 April 29]. Available from: http://www.syngenta.com/country/ke/en/products/fungicides/Pages/switch.aspx
- Ma ZH, Ye ZY. The new target fungicide-pyrimidine amines. World Pestic. 1997;3:12–13.
- Fritz R, Lanen C, Chapeland-Leclerc F, Leroux P. Effect of the anilinopyrimidine fungicide pyrimethanil on the cysthathionine beta-lyase of Botrytiscinerea. Pestic Biochem Physiol. 2003;77:54–65.
- Kanetis L, Forster H, Jones CA, Borkovich KA, Adaskaveg JE. Characterization of genetic and biochemical mechanisms of fludioxonil and pyrimethanil resistance in field isolates of Penicillium digitatum. Phytopathology. 2008;98:205–214.
- Masner P, Muster P, Schmid J. Possible methionine biosynthesis inhibition by pyrimidineamine fungicides. Pestic Sci. 1997;42:163–166.
- Mindt G. Novartis will Cyprodinil in Deutschland einführen. Anwendung in Getreide Gemüse, Obst und Wein. Gesunde Pflanzen [Healthy Plants]. 1997;49:193–198.
- Gehmann K, Nyfeler R, Leadbeater AJ, Nevill D, Sozzi D. A newphenylpyrrole fungicide for broadspectrum disease control. Brighton Crop Prot Conf Pests Dis. 1990;2:399–406.
- Kojima K, Bahn YS, Heitman J. Calcineurin, Mpk1 and Hog1 MAPK pathways independently control fludioxonil antifungal sensitivity in Cryptococcus neoformans. Microbiology. 2006;152:591–604.
- Lartillot S, Kadziora P, Athios A. Purification and characterization of new fungal catalase. Prep Biochem. 1988;18(3):241–246.
- Lowry OH, Rosebrough NJ, Farra NJ, Randall RJ. Protein measurements with the Folin phenol reagent. J Biol Chem. 1951;193:265–275.
- Tukel SS, Alptekin O. Immobilization and kinetics of catalase onto magnesium silicate. Process Biochem. 2004;39:2149–2155.
- Cetinus SA, Saraydın D, Sahin E. Preparation of Cu(II) adsorbed chitosan beads for catalase immobilization. Food Chem. 2009;114:962–969.
- Karadag H, Bilgin R. Effects of cyprodinil and fludioxonil pesticides on human superoxide dismutase. Asian J Chem. 2010;22:8147–8154.
- Li ZH, Zlabek V, Grabic R, Li P, Machova J, Velisek J, Randak T. Effects of exposure to sublethal propiconazole on the antioxidant defense system and Na+–K+-ATPase activity in brain of rainbow trout, Oncorhynchus mykiss. Aquatic Toxicol. 2010;98:297–303.
- Radice S, Ferraris M, Marabini L, Grande S, Chiesara E. Effect of iprodione, a dicarboximide fungicide, on primary cultured rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquatic Toxicol. 2001;54:51–58.
- Toni C, Ferreira D, Kreutz LC, Loro VL, Barcellos LJG. Assessment of oxidative stress and metabolic changes in common carp (Cyprinus carpio) acutely exposed to different concentrations of the fungicide tebuconazole. Chemosphere. 2011;83:579–584.
- Rehman H, Ali M, Atif F, Kaur M, Bhatia K, Raisuddin S. The modulatory effect of deltamethrin on antioxidants in mice. Clinica Chimica Acta. 2006;369:61–65.