Abstract
In tissue engineering, the possibility of a comprehensive restoration of the tissue, structure or a portion of the organ is largely determined by the type of material used. A wide range of materials such as graphene and other carbon nanocompounds which have different physical and chemical properties can be expected to react differently upon contact with biomolecules, cells and tissues. This mini-review describes the current knowledge on biocompatibility of graphene and its derivatives with a variety of mammalian cells, such as osteoblasts, neuroendocrine cells, fibroblasts NIH/3T3 line, PMEFs (primary mouse embryonic fibroblasts), stem cells and neurons. The results from different studies give hope for the possibility of graphene to be used in the regeneration of almost all tissues, including neural tissue implants or in the form of neural chips, which may allow in the future treatment of degenerative diseases and injuries of the central nervous system.
Introduction
The intensive development in tissue engineering promotes the search for a suitable scaffold material for the somatic and/or stem cells employed in tissue regeneration. The possibility of a comprehensive restoration of the tissue, structure or a portion of the organ is largely determined by the type of material used for tissue engineering. This material should have the ability to perform specific biological functions without a cytotoxic and/or mutagenic effect on cultured cells. It must have the appropriate physicochemical properties which will provide a favourable environment for adhesion, proliferation and cell differentiation.[Citation1] So far in tissue engineering many kinds of natural and synthetic non-absorbable and bioresorable substances have been examined. Currently, it is believed that carbon nanotubes, graphene and graphene oxide (GO) may be a promising scaffold material for various cells.[Citation2–4] However, not all issues related to the potential toxicity of these materials have been elucidated. A wide range of materials such as graphene and other carbon nanocompounds which have different physical and chemical properties can be expected to react differently upon contact with biomolecules, cells and tissues which consist of different layers, have different dimensions and are also characterized by different hydrophilicity.[Citation5] In the studies on the above-mentioned compounds, there are two very important issues. First, there is the possible toxicity or biocompatibility of the carbon nanocompounds with different tissues and cells and, second, the possibility for these nanocompounds to be used for biomedical purposes. Materials based on carbon compounds such as carbon nanotubes and carbon nanocrystals have been widely tested for their toxicity, and also in terms of tissue engineering applications. There are still contradictory reports, showing a strong cytotoxic activity in some cases and ability to stimulate cell growth in other cases.[Citation6,Citation7] In the course of research conducted so far it has been shown, among others, that graphene has the ability to induce oxidative stress in cultured cells due to the formation of reactive oxygen species on its surface. However, in this type of toxicity, the key role is played by factors such as the duration of the culture and the graphene structure defined as the number of layers, shape, texture and its dimensions.[Citation5,Citation8] It has been found that the most crucial factors that play an important role in the cell culture are the changes in the hydrophilic/hydrophobic nature of the substrate and its roughness.[Citation7,Citation9–11]
With regard to the cytotoxic effects, what is of particular importance is the purity of the substrate, which is fundamentally different in products obtained from carbon nanocompounds. In the case of carbon nanotubes, the factors affecting the efficiency of the cell cultures are amorphous carbon impurities or residues of the catalytic particles. In addition, during purification of the carbon nanotubes, the substances which increase the cytotoxicity may remain in the resulting material. Unlike carbon nanotubes, single-layer graphene has a much more simple structure and can also be produced as a relatively pure layer capable of maintaining conditions suitable for the growth and differentiation of cultured cells. For this reason, graphene appears to be an ideal substrate for conducting experiments with adherent cells such as mesenchymal stem cells (MSCs), neural stem cells (NSC) and osteoblasts. Results from studies involving the above-mentioned cell types demonstrate the positive impact of graphene on their adhesion, proliferation and differentiation. From the point of view of the cell culture and the biomedical applications, adhesion is a key factor in the subsequent cell activity, which should be understood as ability to proliferate and synthesize different proteins, and in the case of the osteoblasts, to form mineral deposits. Adhesion is strongly dependent on time, on the adhesive forces at the interface of the substrate and the cells and on the spatial layout (morphology) of the ground layer.[Citation7,Citation12] The crucial role in this process is that of integrins, which are a widely distributed family of transmembrane receptors. Integrins are glycoproteins present in animal tissues and classified as animal cell adhesion proteins interacting with other membrane receptors. As heterodimers, they are composed of two non-covalently associated subunits. Integrins on the surface of a ligand bind to actin molecules of the cytoskeleton, which leads to formation of focal adhesions. These contain both structural and signalling molecules.[Citation13] They constitute the main components influencing the adhesion process as structural connections between the cytoskeleton and the extracellular matrix proteins, and mediate adhesion and migration. Focal adhesions, in combination with growth factor receptors, activate signalling pathways of the transcription factor governing the activity, direct cell growth and differentiation.[Citation14]
Biomaterials, graphene and its derivatives
The biomaterials used in tissue engineering, depending on the type of materials from which they are produced, are divided into polymeric, metallic and ceramic materials (carbides, glass, ceramic) and composites, which are a combination of at least two different types of materials. Presently, the biomaterials that are widely used are non-degradable polymers such as polyethylene, polysulfone, polypropylene, acrylic polymers and polycarbonates. Among bioresorbable materials, there can be distinguished polyesters of lactic acid (PLA), glycolic acid (PGA) and their co-polymers. The materials that are commonly used in tissue engineering are natural ones, such as collagen, cellulose and metallic materials: stainless steel, steel, cobalt alloys, titanium and composite materials (silica reinforced silicone rubber and carbon fibres).[Citation15]
Recently, particular attention has been paid to carbon nanomaterials, including graphene, a potential candidate for biomedical applications as a biosensor, antibacterial, antiviral and anticancer material, used in photothermal therapy and tissue engineering.[Citation2,Citation15–18] Graphene is composed of a single layer of carbon atoms which form a two-dimensional material. It was discovered in 2004 by Andre Geim and Konstantin Novoselov, who obtained exfoliated graphene.[Citation19,Citation20] Six years later they received the Nobel Prize in Physics for this discovery. After the report on the isolation of graphene molecules, works on methods of its production started to rapidly emerge in different areas. At the same time, Walt de Heer and Claire Berger from Georgia Tech in Atlanta developed a more sophisticated method of preparation of the exfoliated graphene. This method is used for epitaxial growth of graphene on a substrate of silicon carbide.[Citation21] A technology for epitaxial graphene growth by deposition from the vapour phase on a substrate of silicon carbide (CVD -- chemical vapour deposition) was patented by Polish scientists from the Institute of Electronic Materials Technology (ITME) in Warsaw (patent number EP 2392547 A2) [Citation22]. Graphene growth is carried out in an Aixtron VP508 CVD reactor. For this purpose Ar and H2 are used, while propane, methane or acetylene are a source of carbon.[Citation23] At ITME, a technology for production of graphene on metal substrates, especially copper foil (), was also developed.[Citation24]
In this technique, growth of graphene is conducted by CVD of volatile phases in a CVD reactor Aixtron VP508 and Black Magic Pro 6′′. It allows the produced graphene to be moved to various foreign media. Research on the use of graphene in tissue regeneration is carried out using graphene produced on copper substrate, and then transferred to cover slides. The method of electrochemical delamination is used in the transfer process.[Citation24] The method developed in ITME enables the production of graphene on an industrial scale, maintaining its high quality.
Graphene is over 200 times stronger than steel of the same thickness, it remains flexible, can be stretched by up to 20% and it is also a good conductor of heat (4840–5300 W/mK). This material absorbs only 2.3% of the light.[Citation25] Owing to these properties, it has gained much popularity with the possibility of wide application in various fields of science, including: physics, electronics, biotechnology and medicine.[Citation26–28] Other materials that are also very popular are, as specified by Sanchez et al. [Citation25], the ‘graphene-family nanomaterials’ such as few-layer graphene, ultrathin graphite, GO, reduced GO and graphene nanosheets. So far, however, there is only sparse data on the safety of these materials.[Citation28–31]
In vitro biocompatibility testing
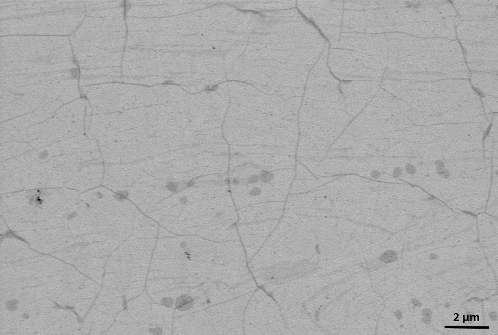
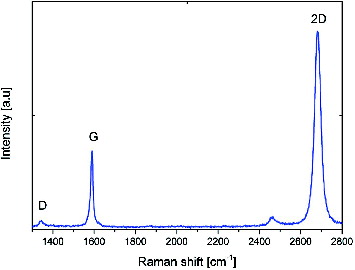
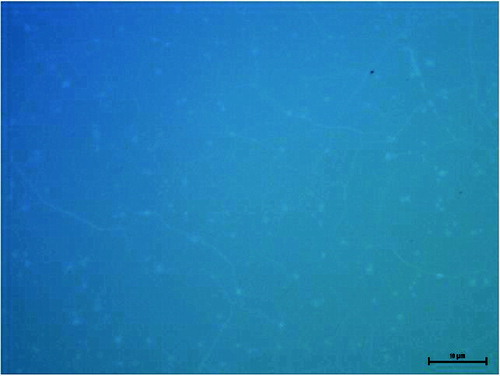
The interaction of cells with the biomaterial surface involves several steps: adsorption of proteins on the surface of the biomaterial, adhesion, proliferation, differentiation, and, in cases of extreme toxicity, even cell death. The phenomenon that occurs at the interface (solid–liquid, solid–gas) is determined by the properties of the biomaterial surface. Therefore, recent studies on the biocompatibility of implant materials extend the notion of a set of physical and chemical characteristics that describe the surface of the material, i.e.: roughness, surface charge, quantity and quality of chemical groups, microstructure of the surface topography and surface energy.[Citation15] Information about the type of surface parameters that determine the cellular response can be obtained by atomic force microscopy (AFM), scanning electron microscopy (SEM) and Raman spectroscopy in a simple and fast way.[Citation3,Citation15,Citation18,Citation32] The response of cells to synthetic surfaces is the basis for modelling and designing of biocompatible plastic implants. shows an SEM image of graphene on a glass surface; , Raman spectrum with characteristic peaks marked; , an optical microscope picture of graphene on a cover glass. The images show a graphene layer transferred to a foreign substrate and its uniform single layer.
Biocompatibility is a key factor in determining the applicability of graphene in tissue engineering. Materials with high biocompatibility should be characterized by the absence of toxicity, the lack of effect on the immune system and lack of haemolysis.[Citation33] Some studies indicate biocompatibility of graphene and its derivatives with a variety of mammalian cells, such as osteoblasts,[Citation34,Citation35] neuroendocrine cells,[Citation34] fibroblasts NIH/3T3 line,[Citation2] PMEFs (primary mouse embryonic fibroblasts),[Citation18] and stem cells [Citation3,Citation36]. Another important role is that of the different properties of the surface and the form used in the study of graphene and its derivatives. It is available in the form of flakes, solutions, powders and sheets. Based on data in the available literature and the Polish standard PN-EN ISO 10993, in vitro biocompatibility evaluation is performed on the basis of examination of the morphology of the cells, their viability using the method of trypan blue (TB), which is the most commonly used vital staining method, and the WST-8 mitochondrial activity test.[Citation33] The results of Liao et al. [Citation32] suggest that the MTT assay is unsuitable for cells cultured on graphene, because it gives false positive results. Direct contact testing and the comet assay are used to evaluate the cytotoxicity in vitro (ISO-10993). The degree of toxicity of biomaterials in the direct contact test is evaluated based on changes in the morphology of the cells, their viability and proliferative capacity, according to the criteria given in .
Table 1. Degrees of direct contact toxicity test [ISO 10993].
To measure the number of damaged nucleotides in the DNA of the pool of cells, two types of approaches are used: chemical and biological approaches. The chemical ones are based mainly on methods such as the Sanger dideoxy sequencing method [Citation37] and the method using the biological phenomenon of DNA fragmentation due to damage resulting from enzymatic digestion. The biological approaches for evaluation of the number of damaged nucleotides are less accurate and are based on estimating the number of defects, rather than on precise measurement. The most popular biological method is the comet assay, also known as single cell gel electrophoresis (SCGE), i.e. electrophoresis of individual cell nuclei in an agarose gel. During the analysis, the cells that are in direct contact with the biomaterial are immobilized on an agarose gel on microscope slides and then lysed with alkali, which leads to the release of DNA from the nucleus. The parameter directly measured in this method is the change of the electrophoretic properties of the modified nucleic acid. The last step is the microscopic analysis, in which a microscope image of the damaged cells is treated like a comet: i.e. ‘head’ corresponds to the place where the DNA has been immobilized before the lysis of the cell, and ‘tail’ are the loops and strands of DNA fragments released from the nuclear structure.[Citation38] To estimate the magnitude of damage of the genetic material after the comet assay, the method of Gedik is used.[Citation39] It is based on the classification of nuclear DNA damage into five classes. Microscopic image analysis via the Gedik comets method allows specification of the percentage of individual classes of defects (N, L, M, H, T) in the sample ().
Table 2. Second class of nuclear DNA damage according to Gedik et al. [Citation39]
A more precise measurement requires the use of specialized software for analysis of comet images (where the lines are histograms of DNA content in the comet's head, tail and across the comet). The analysis allows the identification of fragments and more precise determination of the number and size of the DNA fragments tested. It can be used for example for the analysis of the nucleotide sequence of DNA or analysis of genotype.
Influence of graphene and its derivatives on cells cultured in vitro
Fibroblasts
The assessment of cytotoxicity and other biological tests is carried out using specific cell lines approved, registered and stored in the European Collection of Cell Cultures (ECACC) or the American Type Culture Collection (ATCC). Investigations of the effect of graphene and its derivatives on the morphology of fibroblasts have been carried out on human and animal cell lines, among others, human dermal fibroblasts on line CRL-2522,[Citation32] HDF,[Citation40] mature mouse fibroblasts line L-929 [Citation41] and embryonic line NIH/3T3, without inhibiting contact [Citation2] and Balb/3T3 line that was previously used to evaluate the cytotoxicity and morphological changes in the cells under the influence of nanomaterials [Citation42]. The sensitivity of the Balb/3T3 line and its reliability and reproducibility of the results make it one of the best options for testing the toxicity of nanoparticles.[Citation43] Research by Liao et al. [Citation32] showed the cytotoxic activity of graphene and its derivatives, determined on the basis of haemolysis of human erythrocytes and damage to human skin fibroblasts, using tetrazolium salts assays (WST-8 assay), trypan blue (TBDE assay) and reactive oxygen species (ROS assay). Wang et al. [Citation40] studied the biocompatibility of GO, using human fibroblasts (HDFs), and in vivo in mice. The authors have shown that a water-soluble dose of GO above 50 mg/mL causes considerable damage to fibroblasts in vitro. Doses below 20 mg/mL were non-toxic. In mice, dose-dependent toxicity on the basis of histological changes in the lungs and liver was also observed.[Citation40] In another study, Ryoo et al. [Citation2] demonstrated that NIH/3T3 fibroblast cells on supported thin films of graphene and carbon nanotubes grow well with different numbers and sizes of focal adhesions and gene transfection efficiency improved up to 250% compared with cells grown on a cover glass.
Stem cells and osteoblasts
Due to its physicochemical properties, graphene has become desirable in the reconstruction of tissues, particularly in reconstructive bone surgery. One of the main areas of interest in this branch of medical science is to assess the potential growth of osteoblasts on the surfaces of graphene. An important aspect is the assessment of the possibility of using graphene-coated implants in bones reconstruction. Promising results concerning osteoblasts cultured on graphene substrates were obtained by Kalbacova et al. [Citation7]. Human mesenchymal stem cells (hMSCs) obtained from patients as a result of diagnostic trephination biopsy and osteoblasts derivatives of the SAOS-2 cell line were used for cultivation. MSCs are derived from mononuclear cells of mature bone marrow. They have the ability to proliferate and further differentiate into various populations of cells, such as osteoblasts, adipocytes and chondrocytes, as a result of exposure to different combinations of growth factors [Citation44]. In the experiment of Kalbacova et al. [Citation7], significant differences were observed between the growth of the cells on a graphene substrate and silicon oxide. After 48 hours of incubation, the cells cultured on a uniform continuous graphene layer formed a continuous layer covering the entire substrate. The cells cultured on the silicon oxide formed separate ‘islands’ which were clusters of cells similar to those observed in the cell cultures on glass. The authors showed that both MSCs and osteoblasts clearly prefer graphene as a substrate and cover it with a uniform layer of spindle-shaped cells. The cells on silicon oxide had distinctly different morphology forming polygonal shapes. The studies conducted so far demonstrate that MSCs morphology is one of the main factors regulating biological processes such as cell proliferation and their further differentiation.[Citation7] Recent reports suggest that the main factor that determines cell differentiation is the cell shape.[Citation45,Citation46] Spindle-shaped MSCs and osteoblasts on graphene can show a higher proliferation rate comparing to polygonal cells cultured on silicon oxide.[Citation7] It can be considered that the probable causes of this morphological differentiation in cell populations are both the surface properties of graphene and the associated degree of hydration of the substrate. Similarly to many other carbon nanostructures, graphene has natural hydrophobic properties, but the hydration of the substrate is not only due to its chemical properties but also to physical characteristics such as roughness and shape of the surface. The presence of water on the surface of the cell in contact with the ground layer has a considerable influence on proliferation. The initial phase of this process is closely dependent on the physicochemical bonds between the cell and the substrate through ionic forces.[Citation47,Citation48] For example, Lee et al. [Citation36] analysed the impact of graphene of various rigidity and roughness of the surface on the differentiation of hMSCs and preosteoblasts into osteoblasts. Both GO and pure graphene accelerated cell adhesion and differentiation comparing to the polydimethylsiloxane (PDMS) substrates, polyethylene tetraphtalate (PET), glass substrates and silicon oxide. Wang et al. [Citation30] observed that fluorinated graphene induced a higher rate of proliferation and stronger polarization of MSCs, stimulating neuronal differentiation.
Graphene as a substrate can also stimulate induced pluripotent stem cells cultures (iPSCs) by allowing them to spontaneously differentiate.[Citation3] Chen et al. [Citation3] observed that the form of graphene has a significant effect on the process of cell differentiation. In comparison to glass substrates, cells cultured on GO proliferated faster and differentiated in endodermal direction, while cells on pure graphene proliferated at a rate similar to that observed on glass substrates. In the latter case, there was inhibition in endodermal differentiation. These results suggest that cultures on GO may be used to direct the differentiation of pluripotent stem cells towards endoderm-derived cells, such as hepatocytes or pancreatic islet beta-cells producing insulin, while the substrate comprised of pure graphene may be useful for cell proliferation and maintenance of their pluripotentiality.
Nerve cells
Similar to its applications in bone tissue reconstruction, graphene seems to be an ideal material for tissue reconstruction involving cells of the nervous system. A crucial role is played by two factors. The first one is the fact that the nerve cells are characterized by electrical activity, and the activity of the entire nervous system includes the spontaneous and evoked electrical activity of its individual components. Thus, numerous diagnostic and therapeutic activities require electrical stimulation of the cells. The unique electrical properties of graphene seem to be helpful in achieving this goal. Another factor influencing the use of graphene in the study of cells of the nervous system is the fact that the electric properties of the nanostructures of graphene can be adapted for the transport of electric charges necessary for mobile connection.[Citation49,Citation50] The chemical stability of graphene stimulates the integration of the nervous tissue. Li et al. [Citation51] assessed the response of mouse hippocampal cells cultured on graphene and evaluated the potential applicability of this material in the regeneration of nerve tissue damage. The authors observed that the presence of graphene had no effect on the growth of neurons. The morphology and the density of cultured nerve cells were similar to those observed in neurons cultured on tissue culture polystyrene (TCPS). The analysis of scanning electron microscope images revealed that neurons cultured on graphene form a neurite network after 7 days. They also showed proper adhesion. Li et al. [Citation51] found that graphene exhibits excellent biocompatibility with mice primary cultured hippocampal neurons and also has the ability to stimulate neurons outgrowth and their branching. This phenomenon was particularly visible in the early stage of cell development. Tang et al. [Citation52] also observed that hippocampal neurons cultured on graphene have the ability to form correct networks. Additionally, these authors found that graphene as a substrate has a regulatory effect on the excitability of cultured neurons. This indicates that the activity of the network obtained and the effectiveness of neuronal signal can be enhanced by the use of graphene substrates.
Although the effect of graphene and other carbon nano-compounds on different cell cultures is not fully understood, studies performed so far indicate that these materials hold great promise in various fields of tissue engineering. Despite the numerous studies, there is still limited knowledge about the toxicological profile of graphene and its derivatives. Thus, further in vitro and in vivo trials will be needed to throw more light on the safety of these materials and their potential medical applications.
Conclusions
Accumulated results give good grounds for hope for the possibility of graphene use in the regeneration of almost all tissues, including neural tissue implants or neural chips. This may potentially allow for the treatment of degenerative diseases and injuries of the central nervous system in the future. Recent cell studies on various types of carbon nanocompounds show that graphene and its derivatives can also be potentially used in reconstructive surgery as a scaffold material for new types of bone implants stimulating cell growth and differentiation. Therefore, carbon nanomaterials should be further studied to allow for their possible use in clinical applications.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bacakova L, Filova E, Parizek M, Ruml T, Svorcik V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol Adv. 2011;29:739–767.
- Ryoo SR, Kim YK, Kim MH, Min DH. Behaviors of NIH-3T3 fibroblasts on graphene/carbon nanotubes: proliferation, focal adhesion, and gene transfection studies. ACS Nano. 2010;4:6587–6598.
- Chen GY, Pang DWP, Hwang SM, Tuan HY, Hu YC. A graphene-based platform for induced pluripotent stem cells culture and differentiation. Biomaterials. 2012;33:418–427.
- Wang B, Luo PG, Tackett II KN, Ruiz ON, Bunker CE, Cheng SH, Parenzan A, Sun YP. Graphene oxides as substrate for enhanced mammalian cell growth. J Nanomater Mol Nanotechnol. 2012;1:1–4.
- Goenka S, Sant V, Sant S. Graphene-based nanomaterials for drug delivery and tissue engineering. J Control Release. 2014;173:75–88.
- Smart SK, Cassady AI, Lu GQ, Martin DJ. The biocompatibility of carbon nanotubes. Carbon. 2006;44:1034–1047.
- Kalbacova M, Broz A, Kong J, Kalbac M. Graphene substrates promote adherence of human osteoblasts and mesenchymal stromal cells. Carbon. 2010;48:4323–4329.
- Zhang Y, Ali SF, Dervishi E, Xu Y, Li Z, Casciano D, Biris AS. Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytomaderived PC12 cells. ACS Nano. 2010;4:3181–3186.
- Rezek B, Michalikova L, Ukraintsev E, Kromka A, Kalbacova M. Micro-pattern guided adhesion of osteoblasts on diamond surfaces. Sensors. 2009;9:3549–3562.
- Kalbacova M, Rezek B, Baresova V, Wolf-Brandstetter C, Kromka A. Nanoscale topography of nanocrystalline diamonds promotes differentiation of osteoblasts. Acta Biomater. 2009;5:3076–3085.
- Kalbacova M, Broz A, Babchenko O, Kromka A. Study on cellular adhesion of human osteoblasts on nano-structured diamond films. Phys Status Solidi B. 2009;246:2774–2777.
- Sniadecki N, Desai RA, Ruiz SA, Chen CS. Nanotechnology for cell-substrate interactions. Ann Biomed Eng. 2006;34:59–74.
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane extracellular matrix-cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805.
- Giancotti FG, Ruoslahti E. Transduction-integrin signaling. Science. 1999;285:1028–1032.
- Armentano I, Dottori M, Fortunati E, Mattioli S, Kenny JM. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym Degrad Stabil. 2010;95:2126–2146.
- Feng L, Liu Z. Graphene in biomedicine: opportunities and challenges. Nanomedicine (Lond). 2011;6(2):317–324.
- Shen H, Zhang L, Liu M, Zhang Z. Biomedical applications of graphene. Theranostics 2012;2(3):283–294.
- Gurunathan S, Han JW, Eppakayala V, Dayem AA, Kwon DN, Kim JH. Biocompatibility effects of biologically synthesized graphene in primary mouse embryonic fibroblast cells. Nanoscale Res Lett. 2013;8(393):1–13.
- Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA. Electric field effect in atomically thin carbon films. Science. 2004;306:666–669.
- Geim AK, Novoselov KS. The rise of graphene. Nat Mat. 2007;6:183–191.
- Berger C, Song Z, Li T, Li X, Ogbazghi AY, Feng R, Dai Z, Marchenkov AN, Conrad EH, First PN, de Heer WA. Ultrathin epitaxial graphite: 2D electron gas properties and a route toward graphene-based nanoelectronics. J Phys Chem B. 2004;108(52):19912–19916.
- Strupinski W, inventor; Instytut Technologii Materialów Elektronicznych, assignee. Method of graphene manufacturing. European patent EP 2392547 A2. 2011 Dec 7.
- Strupiński W, Grodecki K, Wysmolek A, Stepniewski R, Szopek T, Gaskell PE, Grüneis A, Haberer D, Bozek R, Krupka J, Baranowski JM. Graphene epitaxy by chemical vapor deposition on SiC. Nano Lett. 2011;11(4):1786–1791.
- Ciuk T, Pasternak I, Krajewska A, Sobieski J, Caban P, Szmidt J, Strupiński W. Properties of chemical vapor deposition graphene transfer red by high-speed electrochemical delamination. J Phys Chem C. 2013;117(40):20833–20837.
- Hebda M, Łopata A. Graphene – material of the future. Tech Trans Mech. 2012;22:45–53.
- Sanchez VC, Jachak A, Hurt RH, Kane AB. Biological interactions of graphene-family nanomaterials: an interdisciplinary review. Chem Res Toxicol. 2012;25(1):15–34.
- Zhang Y, Nayak TR, Hong H, Cai W. Graphene: a versatile nanoplatform for biomedical applications. Nanoscale. 2012;4(13):3833–3842.
- Chang Y, Yang ST, Liu JH, Dong E, Wang Y, Cao A. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol Lett. 2010;200:201–210.
- Zhao S, Wang Q, Zhao Y. Rui Q, Wang D. Toxicity and translocation of graphene oxide in Arabidopsis thaliana. Environ Toxicol Pharmacol. 2014;39(1):145–156.
- Wang Y, Lee WC, Manga KK, Ang PK, Lu J, Liu YP, Lim CT, Loh KP. Fluorinated graphene for promoting neuro-induction of stem cells. Adv Mater. 2012;24:4285–4290.
- Sasidharan A, Panchkarla LS, Chandran P, Menon D, Nair S, Rao CNR, Koyakutty M. Differential nano-bio interactions and toxicity effects of pristine versus functionalized graphene. Nanoscale. 2011;3:2461–2464.
- Liao KH, Lin YS, Macosko CW, Haynes CL. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ASC Appl Mater Interfaces. 2011;3:2607–2615.
- Anuszewska E. Badanie aktywności cytotoksycznej produktów leczniczych i wyrobów medycznych [Examination of the cytotoxic activity of medicinal products and medical devices]. Gazeta Farmaceutyczna. 2010;5:36–38. Polish.
- Agarwal S, Zhou X, Ye F, He Q, Chen GCK, Soo J, Boey F, Zhang H, Chen P. Interfacing live cells with nanocarbon substrates. Langmuir. 2010;26(4):2244–2247.
- Biris AR, Mahmood M, Lazar MD, Dervishi E, Watanabe F, Mustafa T, Baciut G, Baciut M, Bran S, Ali S, Biris AS. Novel multicomponent and biocompatible nanocomposite materials based on few-layer graphenes synthesized on a gold/hydroxyapatite catalytic system with applications in bone regeneration. J Phys Chem C. 2011;115(39):18967–18976.
- Lee WC, Lim CH, Shi H, Tang LA, Wang Y, Lim CT, Loh KP. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ASC Nano. 2011;5(9):7334–7341.
- Sanger F, Coulson AR, Barell BJ, Smith AJH, Roe BA. Cloning in single stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980;143:161–178.
- Olive PL, Banáth JP. The comet assay: a method to measure DNA damage in individual cells. Nat Protoc. 2006;1(1):23–29.
- Gedik CM, Ewen SW, Colins AR. Single-cell gel electrophoresis applied to the analysis of UV-C damage and its repair in human cells. Int J Radiat Biol. 1992;62(3):313–320.
- Wang K, Ruan J, Song H, Zhang J, Wo Y, Guo S, Cui D. Biocompatibility of graphene oxide. Nanoscale Res Lett. 2011;6:1–8.
- Wojtoniszak M, Chen X, Kalenczuk RJ, Wajda A, Łapczuk J, Kurzewski M, Drozdzik M, Chu PK, Borowiak-Palen E. Synthesis, dispersion, and cytocompatibility of graphene oxide and reduced graphene oxide. Colloids Surf B Biointerfaces. 2012;1(89):79–85.
- Vietti G, Ibouraadaten S, Palmai-Pallag M, Yakoub Y, Bailly C, Fenoglio I, Marbaix E, Lison D, van den Brule S. Towards predicting the lung fibrogenic activity of nanomaterials: experimental validation of an in vitro fibroblast proliferation assay. Part Fibre Toxicol [Internet]. 2013 [cited 2014 Aug 1];10:52. Available from: http://www.particleandfibretoxicology.com/content/10/1/52
- Coradeghini R, Gioria S, García CP, Nativo P, Franchini F, Gilliland D, Ponti J, Rossi F. Size-dependent toxicity and cell interaction mechanisms of gold nanoparticles on mouse fibroblasts. Toxicol Lett. 2013;217(3):205–216.
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147.
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428.
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495.
- Leenaerts O, Partoens B, Peeters FM. Water on graphene: hydrophobicity and dipole moment using density functional theory. Phys Rev B. 2009;79:235440–235445.
- Akasaka T, Yokoyama A, Matsuoka M, Hashimoto T, Watari F. Thin films of single-walled carbon nanotubes promote human osteoblastic cells (Saos-2) proliferation in low serum concentrations. Mater Sci Eng C – Mater Biol Appl. 2010;30:391–399.
- Navarro X, Krueger TB, Lago N, Micera S, Stieglitz T, Dario P. A critical review of interfaces with the peripheral nervous system for the control of neuroprostheses and hybrid bionic systems. J Peripher Nerv. 2005;10:229–258.
- Kotov NA, Winter JO, Clements IP, Jan E, Timko BP, Campidelli S, Pathak S, Mazzatenta A, Lieber CM, Prato M, Bellamkonda RV, Silva GA, Kam NWS, Patolsky F, Ballerini L. Nanomaterials for neural interfaces. Adv Mater. 2009;21:3970–4004.
- Li N, Zhang X, Song Q, Su R, Zhang Q, Kong T, Liu L, Jin G, Tang M, Cheng G. The promotion of neurite sprouting and outgrowth of mouse hippocampal cells in culture by graphene substrates. Biomaterials. 2011;32:9374–9382.
- Tang M, Song Q, Li N, Jiang Z, Huang R, Cheng G. Enhancement of electrical signaling in neural networks on graphene films. Biomaterials. 2013;34:6402–6411.