 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Wild emmer wheat (Triticum turgidum ssp. dicoccoides) is the progenitor of cultivated emmer wheat (ssp. dicoccum) and durum wheat (ssp. turgidum conv. durum). Because of its full interfertility with domesticated emmer wheat, this wild species can serve as one of the most important genetic resources to improve durum as well as bread wheat. To elucidate the magnitude of genetic variation within a population of wild emmer wheat, variation of chloroplast DNA was investigated using 91 plants, in total, collected from two natural habitats in southern Turkey. Allelic variation at 24 microsatellite loci in the chloroplast genome was investigated using these samples. Allelic variations were observed at 15 microsatellite loci. The number of alleles per locus was the same in the two populations, ranging from 1 to 4 with an average of 2.17. The estimated diversity indices (H) were also very close ranging from 0.00 to 0.70 with an average of 0.28 and 0.29 for the two populations. Based on the observed allelic variation at all chloroplast microsatellite loci, a total of 23 chloroplast haplotypes (plastotypes) were identified. Only two plastotypes were shared in common between the two natural populations, indicating that the two populations are highly differentiated. Furthermore, uneven micro-geographic distribution of plastotypes was found within each population, suggesting limited rate of migration (seeds dispersal rate) in this species. Our study demonstrated the presence of a high level of genetic diversity between and within highly structured populations of wild emmer wheat in southern Turkey.
Introduction
Genus Triticum consists of a series of polyploid species and is classified into four groups: einkorn (2n = 14, genome constitution: AA), emmer (2n = 28, AABB), timopheevi (2n = 28, AAGG) and common wheat (2n = 42, AABBDD). Genetic and morphological studies have revealed that wild emmer wheat (Triticum turgidum ssp. dicoccoides) is the ancestral species of cultivated tetraploid wheat with AABB genomes (i.e. emmer wheat: T. turgidum ssp. dicoccum and durum wheat: T. turgidum ssp. turgidum conv. durum). Wild emmer wheat is an annual, predominantly self-pollinated species.[Citation1] It is fully interfertile with domesticated emmer wheat and therefore can serve as one of the most important genetic resources for wheat improvement.
The geographical distribution area of wild emmer wheat covers the Fertile Crescent in South-west Asia from Israel, Jordan, Lebanon, Syria, southern Turkey, northern Iraq and western Iran.[Citation1–3] Wild emmer wheat is found in the open park forest of Quercus species and also abundant in habitats with rocky basalt soils. A number of studies have reported the genetic diversity at the macro- and micro-geographic levels and its correlations with allelic diversity at allozyme or DNA marker loci such as microsatellites in the nuclear genome.[Citation4–9]
The purpose of this study was to document the genetic diversity of chloroplast DNA between and within two natural populations of wild emmer wheat. Chloroplast DNA is inherited maternally in Triticum and its closely related genus Aegilops [Citation10] and therefore it has been effectively and critically studied for elucidating the genetic diversity of cytoplasm as well as the maternal lineage in Triticum and Aegilops (see [Citation11] for review). Herein we report the presence of marked inter- and intra-population diversity of chloroplast DNA in two natural populations in southern Turkey, both located within the core area of diversity and domestication of wild cereal species. The allelic diversity at 24 microsatellite loci found in T. aestivum [Citation12] was used for evaluating plant samples collected from these natural habitats.
Materials and methods
Plant materials
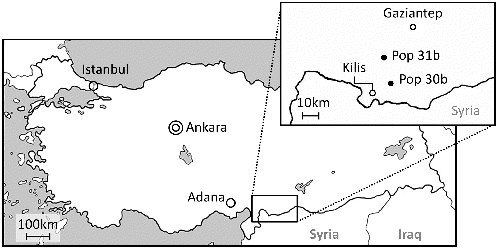
Wild emmer wheat (T. turgidum ssp. dicoccoides) was collected in two natural habitats (population no. 30b and 31b, hereafter designated 30b and 31b, respectively) in southern Turkey (, Table S1 in Supplemental data). Population 30b is located at a rocky gentle slope facing north, while 31b is located at a steep rocky slope facing south-west. The altitude is 647 and 729 m above sea level for 30b and 31b, respectively. These two populations are about 13 km apart from each other.
Figure 1. Two natural populations of the wild emmer wheat (T. turgidum ssp. dicoccoides) investigated in this study.
To evaluate the within habitat diversity, we further divided 30b into eight plots, and 31b into six plots (see below). In each plot, spikelets from three to eight individual plants were sampled separately. Accordingly, a total of 55 and 36 plants were collected in 30b and 31b, respectively (Table S1 in Supplemental data). In addition, an accession of Aegilops tauschii (KU20-1) was used as an out-group and a common wheat cultivar, Chinese Spring (T. aestivum ssp. aestivum cv. Chinese Spring, hereafter referred to as CS), as a size standard in the analysis of chloroplast microsatellite repeat number.
DNA extraction and analysis of chloroplast microsatellite loci
Plants were grown from a single seed per spikelet in Cukrova University, Adana (Turkey) in 2012. Total DNA was isolated from leaf tissue according to the cetyltrimethylammonium bromide (CTAB) method.[Citation13] Twenty-four microsatellite loci (designated WCt1–WCt24, Table S2 in Supplemental data) were analysed according to Ishii et al. [Citation12]. Polymerase chain reaction (PCR) amplification was performed in 15 μL reaction mixture containing 20 to 40 ng of total DNA, 200 μmol/L of each deoxyribonucleoside triphosphate, 200 μmol/L each of primer, 10 mmol/L Tris-Cl (pH 8.3), 50 mmol/L of KCl, 1.5 mmol/L MgCl2 and 1.0 U of rTaq polymerase (Toyobo, Japan). PCR was carried out in a PCR Thermal Cycler Dice (Takara, Japan), using the following programme: 94 °C for 5 min, followed by 35 cycles of 30 s at 94 °C, 30 s at 55 °C, 30 s at 72 °C and a final extension for 7 min at 72 °C. Amplified PCR products were separated using a 6% denaturing polyacrylamide gel with an SQ3 sequencer (Hoefer, USA) and visualized by the sliver staining method described by Panaud et al. [Citation14]. Two of the loci, WCt20 and WCt21, are located only eight base pairs apart, and thus were amplified in a single DNA fragment containing both loci (Table S2 in Supplemental data).
Data analysis
Allelic diversity at the chloroplast microsatellite loci was estimated based on the gene diversity (H) described by Nei [Citation15] as follows:where n is the number of samples, xij is the frequency of the jth allele for locus i and summation extends over m alleles.
Phylogenetic relationships among the plastotypes were evaluated based on the comparisons of the chloroplast microsatellite alleles. The mean number of common alleles was used to calculate the genetic distance (dij) according to the following formula:where Aij and Bij are the numbers of total and common fragments observed between the ith and jth plastotypes.
Phylogenetic trees showing the genetic relationships among the chloroplast genomes were estimated using both the unweighted pair-group method with arithmetic mean (UPGMA) [Citation16] and the neighbour-joining method (NJ) [Citation17] based on the genetic distances. The reliability of the groupings in the trees was tested using the bootstrapping method of Felsenstein [Citation18] with 1000 times of resampling. Principal coordinate analysis (PCoA) was also applied to evaluate the phylogenetic relationships among the plastotypes, using GenAleEx 6.5.[Citation19] For PCoA, the generalized stepwise mutation model [Citation20] was assumed to estimate the genetic distance among the plastotypes.
Results and discussion
Allelic diversity of chloroplast microsatellites
Good amplification was obtained for all 24 chloroplast microsatellite loci from 91 wild emmer wheat samples collected in two natural populations of 30b and 31b in southern Turkey. Allele types at each locus were determined based on the nucleotide length difference from the corresponding alleles in the standard common wheat cultivar CS. Polymorphic banding patterns were observed for 14 and 15 loci for population 30b and 31b, respectively (). To characterize the polymorphism at each microsatellite locus, the number of alleles and the gene diversity (H) of Nei [Citation15] were examined for the 91 samples collected in two populations (). The number of alleles per polymorphic microsatellite locus ranged from 2 to 5 and 2 to 4 in population 30b and 31b, respectively. Both populations showed the same average number of alleles, 2.17. The gene diversity (H) values per locus ranged from 0.00 to 0.65 (average 0.28) in population 30b and 0.00 to 0.70 (average 0.29) in population 31b (). Hirosawa et al. [Citation21] reported that the average H value was 0.04 in the worldwide collection of common wheat (T. aestivum). In comparison, our results indicate that the genetic diversity of wild emmer wheat, even in small natural populations, is much greater than that in common wheat. A high level of genetic diversity in a population has been reported for allozymes [Citation4] as well as for nuclear simple sequence repeats (SSRs) [Citation6–9] in Israel. If we could assume that the effective population size of the two natural populations has not drastically changed, we could compare the divergence time of each population based on the observed within population variation. Since the average H values were similar between population 30b and 31b, it is suggested that the diversification of these two populations has started at almost the same time in the past. To estimate the divergence time using the chloroplast microsatellite variation, it is necessary to measure the rate of evolution of chloroplast microsatellites. Further studies are needed to obtain the estimated rate of evolution at these microsatellite loci in the chloroplast genome of wild emmer wheat.
Table 1. Number of alleles and diversity indices of chloroplast microsatellite loci in the two natural populations 30b and 31b.
Phylogenetic relationship among plastotypes
Based on the allelic variation at the 24 microsatellite loci, 23 chloroplast haplotypes (plastotypes) were identified in 91 wild emmer wheat samples (). Among them, the plastotypes E58 (found in 23 of 91 samples, i.e. 25.3%) and E18 (11 samples, 12.1%) were predominant compared to the other plastotypes (). In addition, only these two plastotypes were found in common between the two populations, 30b and 31b. On the other hand, most of the plastotypes found in each population were unique to the population. These results indicate clear genetic differentiation between the two natural populations, although the two habitats are only about 13 km apart ().
Table 2. Size variation of amplified fragments at 24 chloroplast microsatellite loci found among 23 plastotypes.
Table 3. Frequency of plastotypes found in population 30b and 31b.
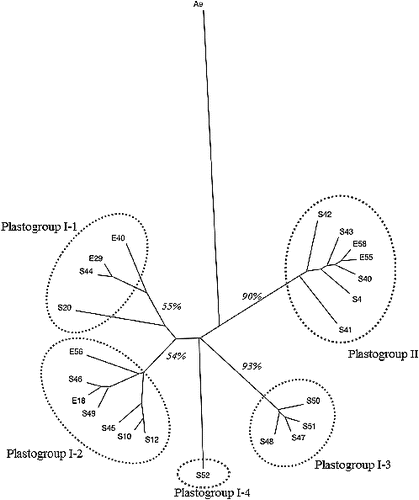
shows a UPGMA tree of 23 plastotypes identified in this study. A NJ tree also showed a very similar topology as that of the UPGMA tree (data not shown). The 23 plastotypes were classified into two groups, plastogroup I and plastogroup II. These two plastogroups were the same groups as previously identified and designated by Mori et al. [Citation22]. The two clades corresponding to plastogroup I and II were well supported (90%) by a bootstrap test [Citation18] with 1000 times of resampling. Plastogroup I was further divided into four subgroups: I-1, I-2, I-3 and I-4 ().
Figure 2. UPGMA tree showing genetic relationship among 23 plastotypes identified in this study. The number in italic shows the bootstrap values for each clade, calculated by 1000 times of resampling. The code ‘Ae’ represents an Aegilops tauschii accession used as an out-group.
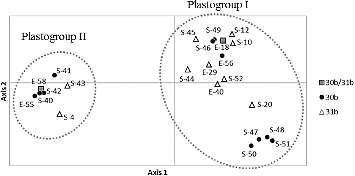
PCoA also showed clear differentiation between plastogroups I and II (). These results indicate clear differentiation between the two plastogroups existing in the wild emmer wheat populations examined, and also suggest that the two plastogroups might have diverged early in the evolutionary history of emmer wheat. Among the 91 samples, plastogroup I was found in 51 samples (56%) and the remaining 40 (44%) belonged to plastogroup II. Interestingly, both plastogroups I and II were found in both populations, 30b and 31b ( and ). The following two scenarios could explain these results: (1) plastotgroups I and II might have existed before the differentiation of the two populations, or (2) either one of the two plastogroups may have been introduced independently into the other after the divergence of the two populations. To test these scenarios, we are now conducting a large-scale survey on the natural populations covering a whole range of the distribution area of wild emmer wheat in Turkey.
Figure 3. Two-dimensional plot of a principal coordinate analysis (PCoA) of 23 plastotypes identified in wild emmer wheat. Black circle, empty triangle and grey square represent plastotypes found in 30b, 31b and in both 30b and 31b, respectively. Axis 1 and axis 2 explained 39.3% and 25.3% of the total variation, respectively.
Distribution of plastotypes in natural habitats at a micro-geographic level
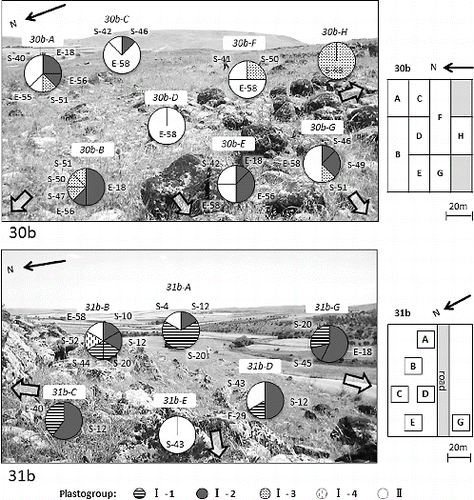
We investigated the micro-geographic distribution of the plastotypes in the two natural populations by dividing the habitats into several plots (see Materials and methods). shows the frequency of plastotypes within each plot. The analysis reveals remarkable differences in the plastotypes and their frequencies between and within the plots. It is particularly notable that there is uneven distribution of plastotypes within the natural population of wild emmer wheat. Non-random allelic distribution was previously reported at nuclear microsatellite loci in wild emmer wheat in Israel.[Citation6–9] These studies suggested the presence of natural selection associated with the micro-environment. To test if there has been a natural selection towards generating the observed uneven distribution of plastotypes, it is needed to further evaluate the macro- as well as micro-environmental factors in the habitats and also to examine if there is correlation between the environmental factors and plastotypes in southern Turkey.
Figure 4. Frequency of plastotypes within the two populations, 30b (upper panel) and 31b (lower panel). Number in italic indicates the sampling plot in the map (right). Circles show the proportion of plastotypes found in each plot.
Since the chloroplast DNA is inherited maternally, seed dispersal can effectively be evaluated by monitoring the spatial distribution of plastotypes in a natural habitat. In this context, it can be inferred based on the variation in chloroplast microsatellite that the wild emmer wheat population in southern Turkey has a limited (very short) seed dispersal distance and accordingly does not migrate long distances. Therefore, in a stable environment, wild emmer wheat might stay in the same place for a time long enough to accumulate a variation within the population. Our results agree with the report by Golenberg [Citation23], who showed that a genetic neighbourhood for wild emmer wheat may cover an area defined by 5 m radius.
Taken together, the results from this study demonstrated the existence of a high level of genetic variation between and within closely located populations and thus indicated a highly structured genetic nature of wild emmer wheat in southern Turkey. It is intriguing to study if there might be some balancing mechanism that acts to maintain such diversified plastotypes, thus a seemingly highly structured population, in natural habitats of wild emmer wheat. Further studies on both cytoplasmic and nuclear DNA variations are necessary to elucidate the genetic mechanism of the maintenance of such a large variation within a natural population in this agronomically important wild species.
Conclusions
Haplotype variation of chloroplast DNA was investigated in two natural populations of the wild emmer wheat in southern Turkey. To our knowledge, this is the first report on the chloroplast DNA diversity in a natural population of the species. The average gene diversity within a natural population was much higher than that of a worldwide collection of common wheat. Furthermore, uneven micro-geographic distribution of plastotypes was found within each of the populations, suggesting a limited migration rate in this species. Our study demonstrated the presence of a high level of genetic diversity within a highly structured population in the wild emmer wheat in southern Turkey.
Supplemental data
Supplemental data for this article can be accessed online http://dx.doi.org/10.1080/13102818.2015.1012648.
T-Shizuka-Online-Supplement.pdf
Download PDF (105.9 KB)Acknowledgements
We thank Dr Chiharu Nakamura, professor Emeritus at Kobe University, for his valuable suggestions in the preparation of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported in part by Japan Society for the Promotion of Science (JSPS) [grant-in-Aid number 15255012], [grant-in-Aid number 25640102].
References
- Zohary D, Hopf M. Domestication of plants in the old world. 3rd ed. New York (NY): Oxford Univ. Press; 2000. Chapter 2, Cereals; p. 16–91.
- Harlan JR, Zohary D. Distribution of wild wheats and barley. Science. 1966;153:1074–1080.
- Ozkan H, Willcox G, Graner A, Salamini F, Kilian B. Geographic distribution and domestication of wild emmer wheat (Triticum dicoccoides). Genet Resour Crop Evol. 2011;58:11–53.
- Nevo E, Golenberg E, Beiles A. Genetic diversity and environmental associations of wild wheat, Triticum dicoccoides in Israel. Theor Appl Genet. 1982;62:241–254.
- Nevo E, Beiles A, Kaplan D. Genetic diversity and environmental associations of wild emmer wheat, in Turkey. Heredity. 1988;61:31–45.
- Li Y-C, Roder MS, Fahima T, Kirzhner VM, Beiles A, Korol AB, Nevo E. Natural selection causing microsatellite divergence in wild emmer wheat at the ecologically variable microsite at Ammiad, Israel. Theor Appl Genet. 2000;100:985–999.
- Li Y-C, Fahima T, Peng JH, Roder MS, Kirzhner VM, Beiles A, Korol AB, Nevo E. Edaphic microsatellite DNA divergence in wild emmer wheat, Triticum dicoccoides, at a microsite: Tabigha, Israel. Theor Appl Genet. 2000;101:1029–1038.
- Fahima T, Roder MS, Wendehake K, Kirzhner VM, Nevo E. Microsatellite polymorphism in natural populations of wild emmer wheat, Triticum dicoccoides, in Israel. Theor Appl Genet. 2002;104:17–29.
- Peleg Z, Saranga Y, Krugman T, Abbo S, Nevo E, Fahima T. Allelic diversity associated with aridity gradient in wild emmer wheat populations. Plant Cell Environ. 2008;31:39–49.
- Tsunewaki K, Ogihara Y. The molecular basis of genetic diversity among cytoplasms of Triticum and Aegilops species. II. on the origin of polyploid wheat cytoplasms as suggested by chloroplast DNA restriction fragment patterns. Genetics. 1983;104:155–171.
- Tsunewaki K. Plasmon analysis in the Triticum-Aegilops complex. Breeding Sci. 2009;59:455–470.
- Ishii T, Mori N, Ogihara Y. Evaluation of allelic diversity at chloroplast microsatellite loci among common wheat and its ancestral species. Theor Appl Genet. 2001;103:896–904.
- Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acid Res. 1980;8:4321–4325.
- Panaud O, Chen X, McCouch SR. Development of microsatellite markers and characterization of simple sequence length polymorphism (SSLP) in rice (Oryza sativa L.). Mol Gen Genet. 1996;252:597–607.
- Nei M. Molecular evolutionary genetics. New York (NY): Columbia University Press; 1987. Chapter 11, Phylogenetic trees; p. 287–326.
- Sneath PA, Sokal RR. Numerical taxonomy. San Francisco (CA): Freeman; 1973.
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425.
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39(4):783–791.
- Peakall R, Smouse PE. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research – an update. Bioinformatics. 2012;28:2537–2539.
- Kimmel M, Chakraborty R. Measures of variation at DNA repeat loci under a general stepwise mutation model. Theor Popul Biol. 1996;50:345–367.
- Hirosawa S, Takumi S, Ishii T, Kawahara T, Nakamura C, Mori N. Chloroplast and nuclear DNA variation in common wheat: insight into the origin and evolution of common wheat. Genes Genet Syst. 2004;79:271–282.
- Mori N, Ishii T, Ishido T, Hirosawa S, Watatani H, Kawahara T, Nesbitt M, Belay G, Takumi S, Ogihara Y, Nakamura C. Origins of domesticated emmer and common wheat inferred from chloroplast DNA fingerprinting. In: Pogna NE, Romano M, Pogna EA, Galterio G, editors. Tenth International Wheat Genetics Symposium [Proceedings]; 2003 Sep 1–6; Paestum. Campania (Italy): Instituto Sperimentale per la Cerealicoltura; 2003. p. 25–28.
- Golenberg EM. Estimation of gene flow and genetic neighborhood size by indirect methods in a selfing annual, Triticum dicoccoides. Evolution. 1987;41(6):1326–1334.
