Abstract
An extremophilic Chlorella strain R-06/2, isolated from a geothermal spring (+42 °C) in the region of Rupite village (SW Bulgaria), was investigated for species identification. This was done by observation of the cell morphology, reproduction and ultrastructure by light microscopy, scanning electron microscopy and transmission electron microscopy, and by investigation of the cell-wall chemistry. The pyrenoid ultrastructure with a double-layered thylakoid traversing the matrix, the shape of the starch envelope, as well as the cell wall, composed of glucosamine and developed around young autospores, were the features that allowed us to classify the thermophilic strain Chlorella R-06/2 as Chlorella vulgaris Beijerinck 1890.
Introduction
In 2006 a strain of Chlorella was isolated from a geothermal spring (+42 °C) in the region of Rupite village (SW Bulgaria). It was labelled as R-06/2 and cultivated in the Institute of Plant Physiology and Genetics-BAS (Sofia). Physiological studies showed its high light, temperature and salt resistance and, together with the relative stability of its chemical composition (proteins 39%–53%, carbohydrates 20%–26%, lipids 15%–30% and pigments 1.5%–4%), proved that it is of a particular interest for further experimental work and mass outdoor cultivation.[Citation1, Citation2] Further investigations of the strain outlined the antibacterial and antifungal activity of its ethanol fatty acid extracts and culture media. The studied strain inhibited the growth of human cervical carcinoma cells (HeLa) with its crude hot water extract. Its fatty acids also had a potential activity against these cells.[Citation1, Citation3, Citation4] These interesting results raised the question about the strain identification, additional to the general knowledge that it belongs to the unicellular green autosporic genus Chlorella Beijerinck. The simple morphology of this genus causes taxonomical difficulties, discussed by many authors for a very long time.[Citation5–Citation7] Recently, the reliable identification of Chlorella species require documentation of its cell ultrastructure and cell-wall composition, in addition to light microscopic (LM) data. Therefore, the isolated strain was additionally cultivated on agar with Bold's Basal Medium (BBM) and studied in the Institutes of Botany in Innsbruck University and Faculty of Biology of Sofia University, where it is recently kept in the Algal Collection of the University of Sofia (ACUS).[Citation8]
In the present study, we investigated strain R-06/2's ultrastructure of the pyrenoid and the starch envelope, as well as the cell wall's ultrastructure, chemical composition and development. We compared the obtained results with results from the literature, which led to the taxonomical designation of the investigated strain to Chlorella vulgaris Beijerinck.
Materials and methods
The isolated Chlorella strain R-06/2 from a water body in the thermal region of Rupite village was cultivated on BBM liquid and agarized medium [Citation9] and kept under standard conditions, which were light intensity 30–35 μmol photons m−2 s−1 under a light/dark (L / D) cycle of 12 / 12 hours (the light sources were OSRAM Daylight Lumilux Cool White lamps, 36 W), in the collection of ACUS [Citation8] and the Algal Collection of the Botanical Institute at Innsbruck (ASIB).[Citation10] For the transmission electron microscopy (TEM) investigation, the studied cells were fixed in 3% glutaraldehyde in 0.1 mol/L cacodylate buffer and also in 1% aqueous OsO4 in 0.1 mol/L cacodylate buffer, dehydrated in acetone and embedded in Spurr resin. Ultrathin sections were stained with uranyl acetate and lead citrate.[Citation11] Electron micrographs were taken with a Tecnai 12 (FEI) microscope, equipped with a Gatan CCD camera. For the scanning electron microscopy (SEM), the investigated algal cells were dehydrated in gradually increasing ethanol concentrations (up to 96% ethanol), transferred in formaldehyde dimethyl acetal for 24 and 2 hours, critical point dried with CO2, sputter coated with palladium/gold and examined with a Philips XL20 SEM microscope.
For the investigation of the cell wall's chemical composition and for detecting the presence or absence of glucosamine, in particular, acid hydrolysis, thin layer chromatography (TLC) and lysozyme treatment were applied. For cell-wall isolation, Chlorella cells, collected from solid growth medium, were grinded in liquid nitrogen and resuspended in dH2O. The resulting homogenate was subjected to centrifugation for 15 min at 15 000 × g at 4 °C. An overnight extraction with 2 mol/L NaCl for the complete removal of proteins was performed after washing in dH2O three times. The cell-wall pellet was subjected to acid hydrolysis with concentrated HCl (12.08 mol/L), following the method of Varum,[Citation12] proposed for chitosan polymers. After hydrolysis for 3 hours at 30 °C, the reaction was stopped by cooling down to 0 °C, followed by neutralization process with equal volume of 12.08 mol/L NaOH. Then, the resulting hydrolysate was freeze-dried and resuspended in dH2O.
For conducting the TLC, both hydrolysates from the Chlorella cell walls and chitosan were applied to silica TLC plates (Merck), along with glucosamine as a positive control. The TLC plates were run and developed as described by Liu et al.[Citation13]
The inhibitory effect of lysozyme on growth was followed by a change in the absorbance of Chlorella cultures at 750 nm, normalized to OD750 = 0.6.[Citation14] One unit of commercially available lysozyme (Serva) was added and the cultures were left on a rotary shaker, under an L/D of 12/12 hours photoperiod at 25 °C for 24 hours.
Results and discussion
The vegetative cells of strain R-06/2 were globular to slightly ellipsoidal with a relatively thick, slightly rough, but unsculptured outer wall ((a), 1(b) and 1(c)). The diameter of the adult cells was (4)–5–6–(7) μm. The cell wall of the vegetative cells was visible under TEM as multi-layered and the same structure was also seen in the remnants of the autosporangial walls ((e), 1(f) and (b), 2(d)). Each cell contained a parietal cup-shaped chloroplast, one nucleus and a pyrenoid with starch sheath, consisting of two large concave–convex cup-shaped starch plates ((a) and 2(b)). The pyrenoid matrix was penetrated by a double thylakoid, which divided the pyrenoid into two almost similar halves ((b), 2(c) and 2(d)). The pyrenoid architecture of the autospores was the same ((f)).
Figure 1. Vegetative cells and autospores of strain R-06/2.
Note: vegetative cells under the light microscope (40 ×) (a); young vegetative cells of strain Rupite R-06/2 under SEM (b, c); liberation of two autospores from the autosporangium through rupture of the autosporangila wall seen under SEM (d); autosporangium with two autospores and clearly visible electrodense layer of the cell wall around them (arrows), seen under TEM (e); autospores with remnants of multi-layered autosporangial wall; in one autospore the thick starch sheath of two biconvex plates surrounding the pyrenoid is visible (arrow), seen under TEM (f). Scale bar is included in the figures.
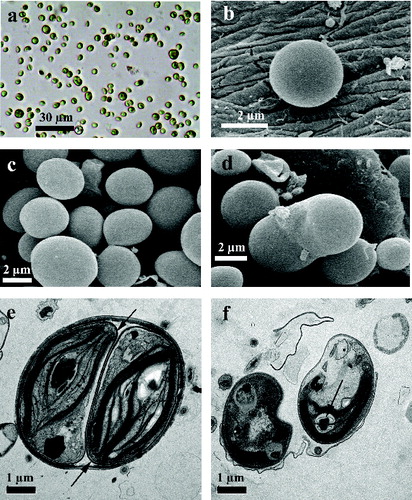
Figure 2. Vegetative cells of strain R-06/2 under LM, TEM and results of cell wall biochemical analysis.
Note: vegetative cells under LM (100 × objective with oil immersion; the scale bar is shown in the figure); the pyrenoid starch sheath is stained with Lugol's solution and is arrowed (a); vegetative cells under TEM (arrow shows the thylakoid penetrating the pyrenoid matrix) (b); pyrenoid and starch sheath of the vegetative cell with a multi-layered cell wall from strain R-06/2 at different magnifications under TEM: one double layered thylakoid penetrates the pyrenoid matrix (the arrow on d), starch sheath of two biconvex starch plates and additional starch grains in the chloroplast (the arrowheads) (c, d); TLC analysis of chitosan and Chlorella cell-wall acid hydrolysates. Commercial glucosamine (1); Chitosan hydrolysate (2); Chlorella cell-wall hydrolysate (3) (e); control (left) and lysozyme treated (right) Chlorella cultures with sedimented dead Chlorella cells on the bottom of the culture (f).
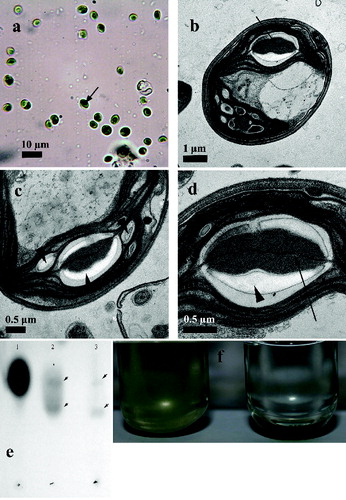
The observations under LM, SEM and TEM proved that the reproduction was done by autosporulation, in which mainly two, but also four autospores per sporangium were developed ((d), 1(e) and 1(f)). The young autospores within the sporangium were covered by a clearly visible electron dense layer of the developing cell wall ((e)). The glucosamine content in the Chlorella cell walls was determined by a TLC analysis ((e)). The acid hydrolysed profile was identical to that of the acid hydrolysed chitosan and shows the similarity of Chlorella cell-wall polysaccharides to chitosan polymers. Additionally, lysozyme treatment of Chlorella culture suspension showed growth inhibition and sedimentation of cells, unlike the control culture ((f)). The ability of lysozyme to hydrolyse the β (1-4) linkages between N-acetylglucosamine and glucosamine in chitosan and chitin [Citation15] would lead to growth inhibition and/or cell death, as cell sedimentation in cultures, treated with lysozyme, showed. Both analyses demonstrated that the investigated Chlorella strain possessed glucosamine-containing cell wall.
The simple morphology of the unicellular autosporic green alga Chlorella always leads to taxonomical difficulties. The first monograph of Fott and Novakova,[Citation6] based on morphological features, established the nomenclatural types, but also showed the phenotypic plasticity of the investigated species. Physiological, biochemical and molecular phylogenetic investigations of Kessler,[Citation16] Kessler and Huss,[Citation17] and Huss et al. [Citation7] made evident that the genus Chlorella consisted of a taxonomically heterogenous complex and its species were dispersed among two classes: Trebouxiophyceae and Chlorophyceae.[Citation18]
The studies of the cell wall's chemical composition by Takeda [Citation19, Citation20] allowed him to group Chlorella species into two clusters: a glucosamine-type group and a glucan-type group. Additionally Ikeda and Takeda [Citation21] showed that the species of the glucosamine-type (C.vulgaris, C.kessleri and C.sorokiniana) are uniform in their pyrenoid morphology, with only one double-layered thylakoid penetrating the pyrenoid matrix. This grouping was on conformity with the same cluster, depicted earlier by Wilcox et al. [Citation22] according to the 18s rRNA coding region sequences. In the investigated strain R-06/2, the cell wall exhibited glucosamine and, therefore, in combination with the cell morphology and dimensions, it doubtless belonged to the cluster mentioned above.
The multi-layered cell wall, observed in the studied strain under TEM, most probably contained an acetolysis resistant biopolymer (algaenan) as stated by Atkinson et al.,[Citation23] Pickett-Heaps [Citation24] and Nĕmcová and Kalina.[Citation25] The development of the cell wall and its structure are both important features for separation of C. kessleri from C. vulgaris and C. sorokiniana.[Citation25] The cell wall structure of C. kessleri is hardly visible in both young autospores, which are not divided by a thin electron dense layer (Figure 6 in [Citation25]) and in adult cells.[Citation25] The young autospores, of the studied strain R-06/2, were surrounded by a thin electron dense layer as a first visible structure of the cell wall ((e)). The same was shown for C.vulgaris ( in [Citation25]) and C.sorokiniana (Figure 4 in [Citation25]).
The next important taxonomic separation feature of Chlorella species and of C. vulgaris, and C. sorokiniana, particularly, was the structure of the starch sheath of the pyrenoid.[Citation21] The starch sheath around the pyrenoid consists of thin starch plates, whereas in C. vulgaris, two thick concavo–convex cup-shaped starch plates surround the pyrenoid matrix, as it was earlier shown by Ikeda and Takeda (Figure 6 and Figure 5 in [Citation21]). The starch sheath around the pyrenoid in the studied strain R-06/2 is doubtless from C.vulgaris type ((b), 2(c) and 2(d)).
Conclusions
The presence of glucosamine in the cell wall, the ultrastructure of the pyrenoid with a double thylakoid penetrating the pyrenoid matrix, the starch envelope of two thick cup-shaped plates and the thin electrodense layer, as a first visible structure of the cell wall around the young autospores, allow us to identify the studied thermophilic strain R-06/2 as C.vulgaris Beijerinck 1890.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Gacheva G. Influence of cultivation conditions on the physiological and biochemical characteristics and biological activities of perspective microalgal strains. [dissertation]. Sofia: Bulgarian Academy of Sciences, Institute of Plant Physiology and Genetics; 2013.
- Gacheva G, Pilarski P. The resistance of a new strain Chlorella sp. R-06/2, isolated from an extreme habitat, to environmental stress factors. Gen Appl Plant Physiol. 2008;34:347–360.
- Gigova GL, Toshkova RA, Gardeva EG, Gacheva GV, Ivanova NJ, Yossifova LS, Petkov GD. Growth inhibitory activity of selected microalgae and cyanobacteria towards human cervical carcinoma cells (HeLa). J Pharm Res. 2011;4:4702–4707.
- Najdenski HM, Gigova LG, Iliev II, Pilarski PS, Lukavsky J, Tsvetkova IV, Ninova MS, Kussovski VK. Antibacterial and antifungal activities of selected microalgae and cyanobacteria. Int J Food Sci Technol. 2013;48:1533–1540.
- Bock C, Krienitz L, Pröschold T. Taxonomic reassessment of the genus Chlorella (Trebouxiophyceae) using molecular signatures (barcodes), including description of seven new species. Fottea. 2011;11:293–312.
- Fott B, Nováková M. A monograph on the genus Chlorella. The fresh water species. In: Fott B, editor. Studies in phycology. Prague: Academia; 1969. p. 10–74.
- Huss VAR, Frank C, Hartmann HC, Hirmer M, Kloboucek A, Seidel BM, Wenzeler P, Kessler E. Biochemical taxonomy and molecular phylogeny of the genus Chlorella sensu lato (Chlorophyta). J Phycol. 1999;35:587–598.
- Uzunov B, Stoyneva M, Mancheva A, Gärtner G. ACUS-the new collection of living aeroterrestrial algae of the University of Sofia ‘St Kliment Ohridski’. In: Petrova A, editor. Proceedings of VII National Botanical Conference; 29–30 September 2011; Sofia, Bulgaria.
- Bischoff HW, Bold HC. Phycological studies IV. Some soil algae from Enchanted Rock and related algal species. Univ Tex Publ. 1963;6318:1–95.
- Gärtner G. ASIB - The Culture Collection of Algae at the Botanical Institute, Innsbruck, Austria. Nova Hedwigia. 2004;79:71–76.
- Reynolds ES. The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J Cell Biol. 1963;17:208–212.
- Varum KM, Ottoy MH, Smidsrod O. Acid hydrolysis of chitosans. Carbohydr Polymers. 2001;46:89–98.
- Liu GL, Li Y, Zhou HX, Chi ZM, Madzak C. Overexpression of a bacterial chitosanase gene in Yarrowia lipolytica and chitosan hydrolysis by the recombinant chitosanase. J Mol Catalysis B: Enzym. 2012;83:100–107.
- Gerken HG, Donohoe B, Knoshaug EP. Enzymatic cell wall degradation of Chlorella vulgaris and other microalgae for biofuels production. Planta. 2013;237:239–253.
- Han T, Nwe N, Furuike T, Tokura S, Tamura H. Methods of N-acetylated chitosan scaffolds and its in vitro biodegradation by lysozyme. J Biomed Sci Eng. 2012;5:15–23.
- Kessler E. Chlorella. Biochemische Taxonomie einer fur Forschung und Biotehnologie wichtigen gattung einzelliger Grünalgen. Naturwissenschaften. 1992;79:260–265.
- Kessler E, Huss VAR. Comparative physiology and biochemistry and taxonomic assignment of the Chlorella (Chlorophyceae) strains of the culture collection of the University of Texas at Austin. J Phycol. 1992;28:550–553.
- Friedl T. Inferring taxonomic positions and testing genus level assignmnets in coccoid green lichen algae: a phylogenetic analysis of 18S ribosomal RNA sequences from Dictyochloropsis reticulata and from memebrs of the genus Myrmecia (Chlorophyta, Trebouxiophyceae cl. nova). J Phycol. 1995;31:632–639.
- Takeda H. Sugar composition of the cell wall and the taxonomy of Chlorella (Chlorophyceae). J Phycol. 1991;27:224–232.
- Takeda H. Chemical composition of walls as taxonomic marker. J Plant Res. 1993;106:195–200.
- Ikeda T, Takeda H. Species-specific differences of pyrenoids in Chlorella (Chlorophyta). J Phycol. 1995;31:813–818.
- Wilcox LW, Lewis LA, Fuerst PA, Floyd GL. Assessing the relationships of autosporic and zoosporic chlorococcalean green algae with 18S rDNA sequence data. J Phycol. 1992;28:381–386.
- Atkinson AW, Gunning BE, John PCL. Sporopollenin in the cell wall of Chlorella and other algae: ultrastructure, chemistry, and incorporation of 14C-acetate, studied in synchronous cultures. Planta. 1972;107:1–32.
- Pickett-Heaps J. Green algae: structure, reproduction and evolution in selected genera. Sunderland: Sinauer Associates; 1975.
- Nĕmcová Y, Kalina T. Cell wall development, microfibril and pyrenoid structure in type strains of Chlorella vulgaris, C. kessleri, C. sorokiniana compared with C. luteoviridis (Trebouxiophyceae, Chlorophyta). Algol Stud. 2000;100:95–105.
