?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this study, the effects of extraction temperature, extraction time and liquid–solid ratio on the extraction yield of polysaccharide (HSSEP) from Umbilicaria esculenta cultivated in Huangshan Mountain were investigated using Box–Behnken response surface design. A second-order polynomial equation was employed to optimize the heat water extraction of HSSEP. The optimal extraction conditions of HSSEP were: 100.37 °C extraction temperature, 0.98 h extraction time and 11.02 mL/g liquid–solid ratio. Under these conditions, the maximum extraction yield of HSSEP was 27.68%. Additionally, two purified polysaccharide fractions, designated as HSSEP1 and HSSEP2, were prepared from HSSEP. The results indicated that HSSEP1 and HSSEP2 were acidic polysaccharides. HSSEP1 was mainly composed of mannose, glucose and galactose, in a ratio of 12%, 76% and 12%, respectively, while HSSEP2 was mainly composed of glucose. This study demonstrated the optimal extraction conditions and structure characterizations of HSSEP for the first time.
Introduction
The extraction is a fundamental step before further research and application of natural products. Therefore, it is essential to design an appropriate extraction technology. Response surface methodology (RSM) is an efficient strategy for process optimization and is usually used to optimize technical parameters.[Citation1–3] The main advantages of RSM are that it is a less laborious and less time-consuming strategy.[Citation4] There are several types of response surface designs in process optimizing, such as central composite design, central composite rotatable design and Box–Behnken design.[Citation5,Citation6] In particular, Box–Behnken design is more efficient and easier for the optimization of complex processes, in comparison with the others.[Citation7] It is widely applied for the optimization of polysaccharides’ extraction conditions.[Citation8,Citation9]
Umbilicaria esculenta (Chinese name Shi Er) is a widely distributed lichen in China and in other Asian countries. Several components, extracted from U. esculenta, have shown pharmacological activities.[Citation10,Citation11] Polysaccharides are the main bioactive compounds of U. esculenta. In the past several years, many researches have focused on the biological activities of polysaccharides from U. esculenta.[Citation12–15]. Huangshanshier (HSSE), a kind of U. esculenta, cultivated in Yellow Mountain, is a local specialty.[Citation16] Today it helps local people meet their basic needs and improve their quality of life. HSSE is regarded as both food and medicine source in China. It contains proteins, carbohydrates, dietary fibers, minerals and vitamins and can be a key to a healthy diet. HSSE has been used as a traditional Chinese medicine, because it possesses haemostatic and antifebrile effects, and it can moisten the lungs to remove phlegm.[Citation17] However, up to now, there has not been any scientific researches regarding the extraction and chemical structure of the polysaccharides from HSSE. Therefore, a basic understanding of the extraction and chemical structure of the polysaccharides from HSSE is a significant prerequisite for its further utilization in food industry.
In the present study, we investigated the optimal conditions for heat water extraction of polysaccharide isolated from HSSE (HSSEP). Box–Behnken design (with three factors and three levels) was first employed to study the effects of extraction temperature, extraction time and liquid–solid ratio on the extraction yields of the polysaccharide from HSSE. In addition, we found that the polysaccharide from HSSE was easily separated into two phases by a freeze–thawing step. Hence, two polysaccharide fractions (HSSEP1 and HSSEP2) were obtained from HSSE using this step. Further, the preliminary structure characterizations of the two polysaccharide fractions were investigated. This study will provide a new guide to deep processing and utilization of HSSE.
Materials and methods
Materials
HSSE was obtained from Huangshan Mountain, Anhui province of China. Bovine serum albumin, glucose, galactose, mannose, rhamnose, arabinose and xylose were obtained from Sigma Chemicals Co. All other chemical reagents were of analytical grade.
Extraction of crude polysaccharide
The HSSE powder was extracted with distilled water in a designed extraction conditions of extraction temperature (70, 80, 90, 100 and 110 °C), extraction time (0.5, 1.0, 1.5, 2.0 and 2.5 h) and liquid–solid ratio (5, 10, 15, 20 and 25 mL/g). After the extraction, the supernatant was collected and the insoluble residue was treated again as mentioned above. The supernatant was concentrated using a rotary evaporator and then mixed with 95% ethanol at a 1/4 ratio and kept overnight at 4 °C. Subsequently, the precipitate was obtained by centrifugation (10,000 rpm, 10 min). The content of total sugar was determined according to the phenol–sulfuric acid method. The extraction yield of HSSEP was calculated by the following equation [Citation18]:(1)
(1)
Experimental design and statistical analysis
Based on the single-factor experiment, a Box–Bohnken design with three independent variables (extraction temperature, extraction time and liquid–solid ratio) at three different levels was employed in this optimization study ().
Table 1. Box–Behnken design and results of the extraction yield of HSSEP isolated from HSSE.
Design Expert software (Version 8.0.5, Stat-Ease Inc., USA) was used to analyse the experimental data. The data were analysed using F-test and Student's t-test. Significance was defined as a p-value of < 0.05. All analyses were performed in triplicate.
Purification of the polysaccharide
The HSSE powder was extracted with hot water at the optimal extraction conditions. The supernatant was subjected to Sevag method to remove the free proteins. The deproteinated product was collected, dialysed and further fractionated through freeze–thawing procedure. Subsequently, the supernatant and the precipitate were separated by filtration. The precipitate was washed twice with ultrapure water at ambient temperature. Then the supernatant fraction and the precipitate fraction were lyophilized to obtain the polysaccharides, coded as HSSEP1 and HSSEP2.
Determination of total sugar, uronic acid and protein contents
Total sugar content was determined by the phenol–sulfuric acid method,[Citation19] using glucose as a standard. The protein content was determined according to the Coomassie brilliant blue method,[Citation20] using bovine serum albumin as a standard. Uronic acid content was measured by the m-hydroxydiphenyl method [Citation21] with galacturonic acid as a standard.
Ultraviolet and Fourier-transform infrared (FT-IR) spectra analysis
The ultraviolet (UV) spectrum was recorded with a UV–visible spectrophotometer (Beijing Purkinje General Instrument Co., Ltd, China). The IR spectrum was determined using Nicolet 5700 spectrometer (Thermo) at the range from 400 to 4000 cm−1.
Monosaccharide composition analysis
The monosaccharide composition analysis was performed as previously described.[Citation22,Citation23] HSSEP1 (5 mg) and HSSEP2 (5 mg) were hydrolysed with trifluoroacetic acid (2 mol/L, 4 mL) at 120 °C for 4 h. The hydrolysed products were concentrated with rotary evaporator and then reduced with NaBH4 at room temperature for 3 h. The mixture was concentrated under a reduced pressure. Subsequently, the reduced monosaccharide was acetylated with acetic anhydride (3 mL) at 100 °C for 1 h. Finally, the monosaccharide compositions were analysed by gas chromatography (Agilent 7890A) after complete conversion into their acetylated derivation.
Results and discussion
Effect of single-factor on the extraction yield of HSSEP
In this study, the effect of the extraction temperature, extraction time and liquid–solid ratio on the extraction yield of HSSEP were investigated. The extraction temperatures were set at 70, 80, 90, 100 and 110 °C. The extraction time and liquid–solid ratio were fixed at 2.0 h and 20 mL/g, respectively. As shown in (A), the extraction yield of HSSEP reached a maximum value of 25.75% when the extraction temperature was 100 °C. When the extraction temperature was higher than 100 °C, the extraction yield of HSSEP decreased. This could be due to degradation of HSSEP.[Citation24] This result indicated that an extraction temperature from 90 to 110 °C was used in the present work.
Figure 1. Effect of extraction temperature (A), extraction time (B) and liquid–solid ratio (C) on the extraction yield of HSSEP.
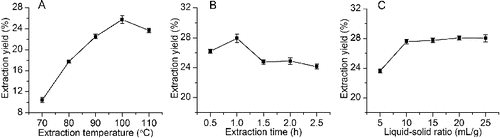
The effect of the extraction time (0.5, 1.0, 1.5, 2.0 and 2.5 h) on the yield of HSSEP is shown in (B). Different extraction times were carried out while the other extraction parameters were set as follows: 100 °C extraction temperature and 20 mL/g liquid–solid ratio. Obviously, the extraction yield of HSSEP increased with the increase of the extraction time and reached a maximum value of 27.93%. A maximum value of the extraction yield was observed when the extraction time was 1.0 h. When the extraction time exceeded 1.0 h, the extraction yield of HSSEP decreased. Therefore, an extraction time from 0.5 to 1.5 h was adopted in the RSM experiments.
As shown in (C), the effect of the liquid–solid ratio (5, 10, 15, 20 and 25 mL/g) on the yield of HSSEP was investigated when the extraction temperature and extraction time were fixed at 100 °C and 1.0 h, respectively. When the liquid–solid ratio was higher than 10 mL/g, the extraction yield changed insignificantly. Therefore, a liquid–solid ratio from 5 to 15 mL/g was investigated in the present study.
Hence, the obtained optimal ranges (extraction temperature from 90 to 110 °C; extraction time from 0.5 to 1.5 h; liquid–solid ratio from 5 to 15 mL/g) of the three variables were selected for further optimization.
Optimization of HSSEP extraction
Model fitting
Heat water extraction parameters were optimized using Box–Bohnken design. There were 17 runs for optimizing the extraction parameters in the current design. The experimental conditions and data are shown in . The extraction yield of HSSEP ranged from 21.47% to 27.70%. The maximum yield of HSSEP was recorded under the following experimental conditions: extraction temperature 100 °C, extraction time 1 h and a liquid–solid ratio of 10 mL/g. The extraction yield of HSSEP was expressed by the equation,(2)
(2) where Y is the extraction yield of HSSEP. X1, X2 and X3 are the coded values for the extraction temperature, extraction time and liquid–solid ratio, respectively.
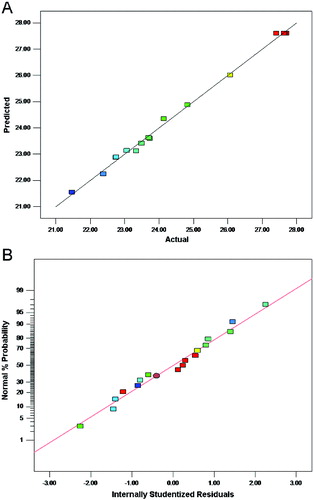
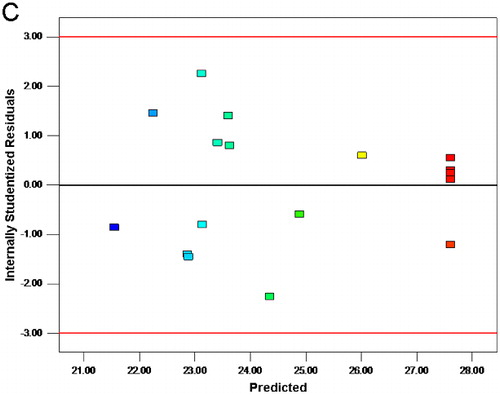
The predicted values of the extraction yield of HSSEP were quite close to the actual response values ((A)). The deviation of variance was not observed ((B)).[Citation5] (C) suggested that the variance of original observation was constant for all values of the extraction yield. Hence, it is indicated that the model is suited for the predicting yield of HSSEP.[Citation25]
Figure 2. Diagnostic plots for the fitted model adequacy.
Diagnostic plots for the fitted model adequacy.
Note: Predicted versus actual (A); normal % probability versus internally studentized residuals (B); internally studentized residuals versus predicted (C).
The analysis of variance of the fitted model is given in . The coefficient of determination (R2) and the adjusted determination coefficient (adj-R2) values were 0.9969 and 0.9929, respectively. The model p-value was 0.0001, which indicated that the model was significant. The lack-of-fit p-value for the model was 0.0852, which indicated that the lack-of-fit was not significantly relative to the pure error.[Citation25]
Table 2. Analysis of variance of the fitted model.
Optimization for the extraction of HSSEP
In order to investigate the effect of process variables on the extraction yield of HSSEP, experiments were performed using Box–Bohnken design. Three-dimensional (3D) response surface and two-dimensional (2D) contour plots were obtained by Design expert software (version 8.0.5). The results of the extraction yield of HSSEP, affected by the extraction temperature, extraction time and liquid–solid ratio are shown in .
Figure 3. The 3D plots (A), (C) and (E) and 2D contour plots (B), (D) and (F) showing the effects of extraction temperature, extraction time and liquid–solid ratio on the extraction yield of HSSEP.
Note: The effect of extraction temperature and extraction time on the extraction yield of HSSEP at a fixed liquid–solid ratio of 10 mL/g (A) and (B); the effect of extraction temperature and liquid–solid ratio on the extraction yield of HSSEP at a fixed extraction time of 1.0 h (C) and (D); the effect of extraction time and liquid–solid ratio on the extraction yield of HSSEP at a fixed extraction temperature of 100 °C (E) and (F).
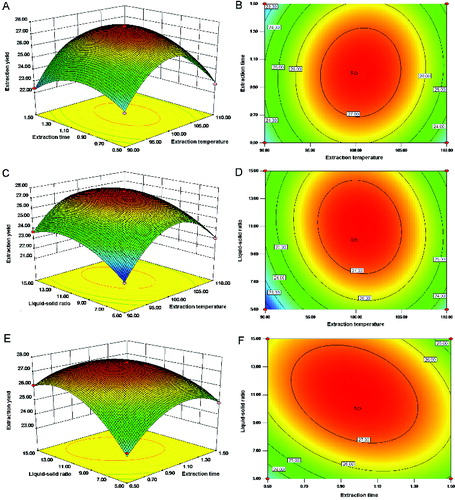
(A) and (B) showed the effect of extraction temperature and extraction time on the extraction yield of HSSEP at a fixed liquid–solid ratio of 10 mL/g. The extraction yield of HSSEP increased with the increase of the extraction temperature from 90 to 110 °C and began to decrease when the extraction temperature continued to rise. The extraction yield also increased with the increase of the extraction time from 0.5 to 1.0 h.
The 3D response surface plot and 2D contour plot based on the independent variables extraction temperature and liquid–solid ratio are shown in (C) and (D), while the extraction time was set at 1.0 h. The results indicated that the maximum extraction yield of HSSEP was achieved when the extraction temperature and liquid–solid ratio was 100 °C and 10 mL/g, respectively.
(E) and (F) illustrated the effect of the extraction time and the liquid–solid ratio on the extraction yield of HSSEP at a fixed extraction temperature of 100 °C. The extraction yield of HSSEP increased with the increase of the extraction time from 0.5 to 1.0 h and decreased when the extraction time continued to increase from 1.5 to 2.0 h. The extraction yield also increased with the increase of the liquid–solid ratio.
The above results showed that the optimal extraction conditions of HSSEP isolated from HSSE were as follows: extraction temperature 100.37 °C, extraction time 0.98 h and a liquid–solid ratio of 11.02 mL/g. Under these conditions, the predicted extraction yield was 27.68%. It has been reported that the optimal extraction yield of polysaccharides from U. esculenta using the combined-enzyme method was 12.52%.[Citation26] Therefore, the optimal extraction yield of HSSEP using water extraction was higher in this study.
Verification of the model
In order to validate the reliability of the fitted model, the modified conditions (100 °C extraction temperature, 1.0 h extraction time and 11 mL/g liquid–solid ratio) were tested. Under these conditions, the yield of HSSEP was 27.46%, which was close to the predicted value (27.68%).
Preliminary characterization of HSSEP
In the preliminary experiments, two polysaccharide fractions, coded as HSSEP1 and HSSEP2, were easily separated and purified from U. esculenta using a freeze–thawing method. This behaviour showed that there is a potential for a large-scale commercial production. In addition, it indicated that the physicochemical properties of the two fractions were significantly different. Further, the physicochemical parameters and the monosaccharide composition of HSSEP1 and HSSEP2 were investigated. According to the UV spectra ((A)) and Coomassie brilliant blue method, the protein content in the two polysaccharide fractions was undetectable. The total sugar content in HSSEP1 and HSSEP2 was 95.00% and 98.80%, respectively. The percentages of the uronic acid in HSSEP1 and HSSEP2 were 0.73% and 0.54%, respectively (). The monosaccharide composition indicated that HSSEP1 was mainly composed of mannose, galactose and glucose in a ratio of 12%, 76% and 12%, respectively ((B)), while HSSEP2 was mainly composed of glucose ((C)). Gulzar et al. reported the monosaccharide composition of polysaccharides from two Umbilicaria species: Umbilicaria decussate (Vill.) Zahlbr (UDZ) and Umbilicaria virginis Schaer. (UVS).[Citation27] The results indicated that the UDZ and UVS were all composed of mannose, glucose and galactose in a ratio of 3.90:38.08:2.00 and 16.00:50.96:6.17, respectively.
Figure 4. Preliminary characterization of HSSEP isolated from HSSE.
Note: UV spectra of HSSEP1 and HSSEP2 (A); monosaccharide composition of HSSEP1 (B); Monosaccharide composition of HSSEP2 (C); FT-IR spectra of HSSEP1 (D); FT-IR spectra of HSSEP2 (E).
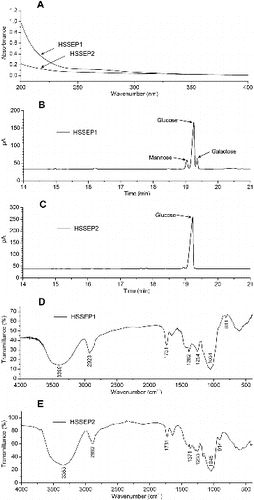
Table 3. Preliminary physicochemical properties of HSSEP1 and HSSEP2.
To investigate the precise characterization of HSSEP1 and HSSEP2, the FT-IR spectra were performed. As shown in (D) and (E), the peaks at 3399 cm−1 (HSSEP1) and 3353 cm−1 (HSSEP2) were due to the hydroxyl stretching vibration.[Citation28,Citation29] The peaks at 2923 cm−1 (HSSEP1) and 2892 cm−1 (HSSEP2) were due to the C–H stretching vibration.[Citation30] The band 1731 cm−1 (HSSEP1) and 1731 cm−1 (HSSEP2) represented the presence of uronic acids.[Citation31] The peaks that appeared at approximately 811 cm−1 of HSSEP1 were attributed to D-galactose or D-mannose.[Citation32] The peaks at 914 cm−1 of HSSEP2 were assigned to glucose.
The above analysis revealed that the structural characterizations of HSSEP1 and HSSEP2 were significantly different. Meanwhile, the glycosidic linkage, molecular mass, degree of branching and conformation are still ambiguous. Therefore, an additional work is required to understand the detailed structure characterizations of HSSEP1 and HSSEP2.
Conclusions
In the present study, hot water extraction of polysaccharide from HSSE was performed using Box–Bohnken design. Experimental results showed that the optimal extraction parameters of HSSEP isolated from HSSE were as follows: 100.37 °C extraction temperature, 0.98 h extraction time and 11.02 mL/g liquid–solid ratio. Under these conditions, the extraction yield of HSSEP was 27.68%. Additionally, two polysaccharide fractions, designated as HSSEP1 and HSSEP2 were purified from HSSEP. The total sugar content, protein content, uronic acid content and monosaccharide composition of HSSEP1 and HSSEP2 were investigated. The results showed that HSSEP1 and HSSEP2 are acid polysaccharides with 0.73% and 0.54% of uronic acid content, respectively. The protein content in the two polysaccharide fractions was undetectable. The total sugar content in HSSEP1 and HSSEP2 was 95.00% and 98.80%, respectively. The monosaccharide compositions analysis revealed that the HSSEP1 was composed of galactose, glucose and mannose in a ratio of 12%, 76% and 12%, respectively, while HSSEP2 mainly consisted of glucose. The present study was the first to investigate the optimal extraction conditions and structural characterizations of HSSEP. This work could give a theoretical reference for HSSEP application in food and pharmaceutical industry.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Pierozan MK, Costa RJD, Antunes OAC, Oestreicher EG, Oliveira JV, Cansian RL, Treichel H, Oliveira DD. Optimization of extraction of lipase from wheat seeds (Triticum aestivum) by response surface methodology. J Agric Food Chem. 2009;57:9716–9721.
- Samavati V. Central composite rotatable design for investigation of microwave-assisted extraction of okra pod hydrocolloid. Int J Biol Macromol. 2013;61:142–149.
- Karnachi AA, Khan MA. Box–Behnken design for the optimization of formulation variables of indomethacin coprecipitates with polymer mixtures. Int J Pharm. 1996;131:9–17.
- Wang C, Wang Y, Zhang J, Wang Z. Optimization for the extraction of polysaccharides from Gentiana scabra Bunge and their antioxidant in vitro and anti-tumor activity in vivo. J Taiwan Inst Chem Eng. 2014;45:1126–1132.
- Samavati V. Polysaccharide extraction from Abelmoschus ecsulentus: optimization by response surface methodology. Carbohydr Polym. 2013;95:588–597.
- Liu JL, Zheng SL, Fan QJ, Yuan JC, Yang SM, Kong FL. Optimization of high-pressure ultrasonic-assisted simultaneous extraction of six major constituents from Ligusticum chuanxiong rhizome using response surface methodology. Molecules. 2014;19:1887–1911.
- Zhu C, Liu X. Optimization of extraction process of crude polysaccharides from pomegranate peel by response surface methodology. Carbohydr Polym. 2013;92:1197–1202.
- Ma C, Feng M, Zhai X, Hu M, You L, Luo W, Zhao M. Optimization for the extraction of polysaccharides from Ganoderma lucidum and their antioxidant and antiproliferative activities. J Taiwan Inst Chem Eng. 2013;44:886–894.
- Ji YB, Dong F, Ma DB, Miao J, Jin LN, Liu ZF, Zhang LW. Optimizing the extraction of anti-tumor polysaccharides from the fruit of Capparis spionosa L. by response surface methodology. Molecules. 2012;17:7323–7335.
- Lee KA, Kim MS. Glucosidase inhibitor from Umbilicaria esculenta. Can J Microbiol. 2000;46:1077–1081.
- Kim MS, Lee KA. Antithrombotic activity of methanolic extract of Umbilicaria esculenta. J Ethnopharmacol. 2006;105:342–345.
- Nishikawa Y, Tanaka M, Shibata S, Fukuoka F. Polysaccharides of lichens and fungi. IV. Antitumour active O-acetylated pustulan-type glucans from the lichens of Umbilicaria species. Chem Pharm Bull. 1970;18:1431–1434.
- Hirabayashi K, Iwata S, Ito M, Shigeta S, Narui T, Mori T, Shibata S. Inhibitory effect of a lichen polysaccharide sulfate, GE-3-S, on the replication of human immunodeficiency virus (HIV) in vitro. Chem Pharm Bull. 1989;37:2410–2412.
- Kim HS, Kim JY, Lee HK, Kim MS, Lee SR, Kang JS, Kim HM, Lee KA, Hong JT, Kim Y, Han SB. Dendritic cell activation by glucan isolated from Umbilicaria esculenta. Immune Netw. 2010;10:188–197.
- Du YQ, Liu Y, Wang JH. Polysaccharides from Umbilicaria esculenta cultivated in Huangshan Mountain and immunomodulatory activity. Int J Biol Macromol. 2015;72:1272–1276.
- Zeng ZX. Huangshanshier. China Food. 1988;7:38.
- Zeng ZX. Huangshanshimuer. Chin Native Produce. 1996;3:22.
- Yang Q, Chen H, Zhou X, Zhang J. Optimum extraction of polysaccharides from Opuntia dillenii and evaluation of its antioxidant activities. Carbohydr Polym. 2013;97:736–742.
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356.
- Lowry OH, Rosebrough NJ, Lewsfarr A, Randall RJ. Protein measurement with the folin reagent. J Biol Chem. 1951;193:265–275.
- Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acid. Anal Biochem. 1973;54:484–489.
- Leung MYK, Liu C, Zhu LF, Hui YZ, Yu B, Fung KP. Chemical and biological characterization of a polysaccharide biological response modifier from Aloe vera L. var. chinensis (Haw.) Berg. Glycobiology. 2004;14:501–510.
- Ana V, Laura MV, Eva G. Structural features and healthy properties of polysaccharides occurring in mushrooms. Agriculture. 2012;2:452–471.
- Li Q, Yu N, Wang Y, Sun Y, Lu K, Guan W. Extraction optimization of Bruguiera gymnorrhiza polysaccharides with radical scavenging activities. Carbohydr Polym. 2013;96:148–155.
- Prakash J, Maran S, Manikandan K, Thirugnanasambandham C, Nivetha V., Dinesh R. Box-Behnken design based statistical modeling for ultrasound-assisted extraction of corn silk polysaccharide. Carbohydr Polym. 2013;92:604–611.
- Yao YF, Zhang HL, Liu Y, Du YQ, Zhang JB, Wang JH. Optimal process of extraction and antioxidant activity of polysaccharide from Umbilicaria esculenta (Miyoshi) in Yellow Mountain. J Hefei Univ Technol. 2014;37:1132–1137.
- Gulzar A, Nurbiya Y, Kerimjan T, Abdulla A. Isolation, purification and monosaccharide composition analysis of polysaccharides from two Umbilicaria species. Food Sci. 2011;32:48–51.
- Chang XL, Chen BY, Feng YM. Water-soluble polysaccharides isolated from skin juice, gel juice and flower of Aloe vera Miller. J Taiwan Inst Chem Eng. 2011;42:197–203.
- Liu Y, Du YQ, Wang JH, Zha XQ, Zhang JB. Structural analysis and antioxidant activities of polysaccharide isolated from Jinqian mushroom. Int J Biol Macromol. 2014;64:63–68.
- Ge Q, Zhang AQ, Sun PL. Structural investigation of a novel water-soluble heteropolysaccharide from the fruiting bodies of Phellinus baumii Pilat. Food Chem. 2009;114:391–395.
- Han XQ, Chan BCL, Dong CX, Yang YH, Ko CH, Yue GGL, Chen D, Wong CK, Lau CBS, Tu PF, Shaw PC, Fung KP, Leung PC, Hsiao WL, Han QB. Isolation, structure characterization, and immunomodulating activity of a hyperbranched polysaccharide from the fruiting bodies of Ganoderma sinense. J Agric Food Chem. 2012;60:4276–4281.
- Chen ZG, Zhang DN, Zhu Q, Yang QH, Han YB. Purification, preliminary characterization and in vitro immunomodulatory activity of tiger lily polysaccharide. Carbohydr Polym. 2014;106:217–222.