Abstract
The phytohormone auxin is transported by two distinct pathways in plants. Indole-3-acetic acid is mainly transported throughout the plant by an unregulated bulk flow in the mature phloem. The major auxin distribution is regulated via direct transport from cell to cell, known as polar auxin transport (PAT). PAT is maintained by the coordinated action of efflux (PIN) and auxin influx (AUX/LAX) carrier proteins. In this study, we examine, compare and localize the expression of a gene encoding an auxin influx carrier (MtLAX3) from Medicago truncatula in the model plants M. truncatula, Lotus japonicus and Arabidopsis thaliana. Transgenic plants with overexpression and down-regulation of MtLAX3, as well as with expressed promMtLAX3 transcriptional reporters, were constructed for the three model species, using Agrobacterium-mediated transformation. Histochemical and transcriptional analyses revealed the expression of MtLAX3 during various stages of somatic embryogenesis and plant development, as well as during formation of symbiotic nodules. The alteration of the MtLAX3 expression, as well as its overexpression in the analysed model species, results in various abnormal phenotypes and disturbance of leaf and root development. The reported results show that MtLAX3 plays an important role in proper plant growth and development, modelling of the root system and the number of formed nodules and seeds.
Introduction
The phytohormone auxin is a critical signal molecule, regulating several processes in plant growth and development.[Citation1,Citation2] Auxin is crucial for the regulation of zygotic embryo development,[Citation3,Citation4] root development,[Citation2,Citation5,Citation6] including initiation and emergence of lateral roots [Citation7–9] and the development of leaves and flowers.[Citation10,Citation11] It is involved in the response of plants to light and gravity,[Citation5,Citation12] general shoot and root architecture,[Citation13,Citation14] organ patterning and vascular development [Citation15–17] and apical dominancy regulation.[Citation18] Auxin is vital for the regulation of cell division, initiation and radial position of plant lateral organs [Citation19] and is likely to be an important regulator of nodulation.[Citation20,Citation21] De Billy et al. [Citation22] suggest that auxin is required in two general stages in secondary rooting and nodulation, as primordium development and nodulation.
Auxin is mainly synthesized in the young leaves and apical meristem of the shoot and roots.[Citation23–25] Indole-3-acetic acid (IAA) is the most abundant natural auxin.[Citation26] IAA biosynthesis occurs via two pathways in plants: the tryptophan (Trp) dependent and Trp-independent pathway.[Citation14,Citation24] Weak organic acid IAA is derived from the shoot; it is taken up by cells by a combination of carrier-mediated uptake and diffusion of the dissociated lipophilic acid and is transported acropetally from the shoot to the root.[Citation24,Citation27,Citation28] At a long distance, most IAA is transported throughout the plant from young leaves and flowers by an unregulated flow in the mature phloem.[Citation29,Citation30] At a short distance, auxin moves from cell to cell by forming local maxima and creating a gradient. This movement mechanism is controlled by the coordinated action of influx and efflux carriers and is known as polar auxin transport (PAT).[Citation2,Citation31–35] It has been proved that PAT is unique in the plant tissue and is not detected for any other signalling molecule.[Citation36] There have been identified three main classes of auxin transporters in Arabidopsis thaliana: LIKE-AUX1 (from the AUX/LAX family), PIN-formed proteins and ABC transporter family P-glycoproteins (PGP). LAX proteins are auxin influx carriers.[Citation29,Citation37,Citation38] The first putative efflux carrier to be characterized was AtPIN1.[Citation39,Citation40] AtPIN is a family of polar auxin efflux transporters localized in the plasma membrane with a central role in many plant processes.[Citation32,Citation41] PGPs are involved in both influx and efflux,[Citation42] but with a minor role.[Citation43] Influx and efflux auxin transporters are with asymmetric cellular localization, which appears to be a dynamic process, and their action is required for maintaining PAT.[Citation44,Citation45] It has been shown that in protophloem cells, AUX1 and PIN1 show localization at opposite sides of the same cell. This suggests that AUX1 and PIN1 are targeted by divergent vesicle trafficking pathways and are established at opposite sides of the completed cell wall.[Citation46]
The auxin influx carrier protein AUXIN-RESISTANT1 (AUX1) is a member of the amino acid permease family of proton-driven transporters and plays a role in the uptake of the Trp-like auxin molecule IAA.[Citation47] In the genome of A. thaliana, there are four genes: AUX1 and three LIKE-AUX1 (LAX) genes (LAX1, LAX2 and LAX3).[Citation48] AtAUX1 and AtLAX3 are localized at the plasma membrane and actively transport auxin inside the cell.[Citation7,Citation49] It has been shown that LAX3 promotes lateral root emergence [Citation2,Citation7] and is involved in the regulation of phyllotaxis, the arrangement of leaves on the stem.[Citation10] Medicago truncatula contains a family of five genes related to AUX1 of A. thaliana (MtLAX).[Citation50] MtLAX genes are involved in local auxin transport, development of lateral roots and root nodules.[Citation22] It has been shown that during lateral root and nodule development, MtLAX genes are expressed in the primordia and in the regions of the developing organs where the vasculature arises at later stages.[Citation22]
Here, we describe a study of the expression pattern and biological function of the MtLAX3 gene from M. truncatula in three model plants, M. truncatula, Lotus japonicus and A. thaliana. The histochemical and fluorescent analyses of transcriptional reporter plants demonstrated expression of MtLAX3 during different stages of somatic embryogenesis, symbiotic nodulation and in plant organs and tissues. The phenotype analyses of transgenic lines with overexpressed (OE) or down-regulated MtLAX3 showed a crucial role of MtLAX3 during the growth and development of the model plants.
Materials and methods
Cloning and plasmid construction
Recombinant plasmids were obtained based on the GATEWAY system [Citation51] (Invitrogen Life Technologies, Inc., http://www.lifetechnologies.com). To create plants overexpressing MtLAX3, the open reading frame (ORF) of MtLAX3 (MT3G072870, Plaza 2.5) was incorporated into the pDONR221 donor vector. The obtained entry clone was transferred into pK7WG2 or pK7FWG2 destination vectors [a C-terminal translational green fluorescent protein (GFP) fusion] under the control of CaMV 35S promoter. Both destination vectors contain the neomycin phosphotransferase (nptII) gene for plant selection.[Citation52] Silenced constructs were created by using the RNA interference method [Citation53] and a pK7GWIWG2D(II) hairpin RNA expression vector was used. The Xwin Razor software program predicted in silico a fragment of 130 bp from the ORF of LAX3, optimal for silencing the gene. In M. truncatula, these 130 bp correspond to nucleotide positions from 935 to 1064 bp of the ORF of the MtLAX3 gene and from 935 to 1065 bp of the ORF of LJ0G013110 in L. japonicus. In A. thaliana, the AT1G77690 gene was silenced from position 950 to 1079 bp in the ORF.
To create transcriptional reporter plants, a full-length promoter sequence (∼2.0 kb upstream of the start codon) was inserted into donor vector pDONRP4P1R. The obtained entry clone was recombined into the pEX-K7SNFm14GW destination vector (promoter-NLS-GUS/GFP) to create an expression clone. The destination vector possesses an nptII gene as a selection marker for transgenic plants.
Verified plasmids were then transferred to Agrobacterium tumefaciens strain C58C1, maintained on solid YEB medium (nutrient broth; 1.5% agar) supplemented with 100 mg/L rifampicin, 100 mg/L spectinomycin and 50 mg/L gentamicin.
All gene-specific primers for cloning were designed using the Primer 3 software and are given in Table S1 in the Supplemental data.
Plant materials, growth conditions and genetic transformation
M. truncatula cv. ‘Jemalong 2HA’ [Citation54] seeds were scarified, surface-sterilized with 70% (v/v) ethanol and 0.1% (v/v) mercury chloride (HgCl2), and washed several times with sterile distilled water. L. japonicus ecotype B-129 seeds, a kind gift from Dr Hiroshi Kouchi (International Christian University, Tokyo, Japan), were surface sterilized by using the same protocol. Seeds from both model plants were germinated on Murashige and Skoog (MS) basal medium [Citation55] and seedlings were then propagated by cuttings in Magenta boxes (60 × 60 × 96 mm, Sigma) and grown in a growth chamber at 24 °C, with a 16 h photoperiod and light intensity of 350 µmol/(m−2 s−1). Genetic transformation of legumes plants through the A. tumefaciens system was performed using a combined protocol described by Erfurth et al. [Citation56], Chabaud et al. [Citation57] and Iantcheva et al. [Citation58]. Leaves and petioles collected from 35-day-old in vitro plants were used as explants for genetic transformation. There are four phases of in vitro regeneration that leguminous plants undergo: formation of callus tissue, embryo induction, development and conversion.
Leaf and petiole explants were wounded by a scalpel blade and pre-cultivated for 48 h on callus induction medium under dark conditions. For M. truncatula, SHab medium solidified with 3.5% (w/v) gelrite [SH-basal medium (Schenk and Hildebrandt) including vitamins supplemented with 5 mg/L of 2.4-dichlorophenoxyacetic acid (2.4D) and 1 mg/L benzylaminopurine (BAP)] was used. The medium used for L. japonicus was B54/08 (B5-basal medium including vitamins supplemented with 4 mg/L 2.4-D and 0.8 mg/L kinetin) solidified with 7% (w/v) phyto agar. Pretreated explants were inoculated with bacterial suspension with an optical density of OD600 = 0.5 for 1 h on a shaker (100 r/min) and placed for co-cultivation for another 48 h in the same medium. Afterwards, the transformed explants were transferred to selective solid media supplemented with 50 mg/L kanamycin (Km) and 400 mg/L carbenicillin (Cb). For M. truncatula, SHab medium was used and for L. japonicus, B54/08 selective solid medium. Then, 20–40 days after callus-tissue induction in dark, M. truncatula explants were transferred to selective solid callus-inducing medium (CIM) for embryo induction (MS-basal medium including vitamins, supplemented with 2 mg/L zeatin, 1 mg/L 2.4D, 50 mg/L Km and 200 mg/L Cb) and the formed callus tissue from L. japonicus was transferred to solid embryo induction medium 09-03 (MS-basal medium including vitamins supplemented with 0.9 mg/L BAP and 0.3 mg/L α-naphthaleneacetic acid) free of selective pressure, both in light. Callus-tissue from M. truncatula was transferred to 09-03 solid medium in order to develop somatic embryo structures, which were then transferred to solid selective MS1 medium (MS-basal medium including vitamins, 0.05 mg/L BAP and 250 mg/L casein hydrolysate) for another 20–40 days. In the L. japonicus transformation procedure, 20–30 days after pretreatment on 09-03 embryo induction medium, clusters of closely packed thick globular embryos were formed on the top of transformed calli. By using embryo-development medium B5 lotus (B5-basal medium including vitamins and supplemented with 0.2 mg/L BAP and solidified with 3.5% sucrose (w/v) gelrite), dark green globular embryos developed and slowly formed cotyledonary leaves in a period of 40–50 days. Embryo development and embryo conversion were maintained under selective pressure of Km (50 mg/L). As a last phase of plant transformation, when cotyledonary-stage embryo structures were initiated, the material from both model legumes was transferred to solid MS medium containing 20 mg/L sucrose supplemented with 50 mg/L Km for generating transgenic plantlets. Plants with a well-developed root system were screened by polymerase chain reaction (PCR) for the presence of the nptII gene for Km resistance and those that appeared to be transgenic, were moved to a greenhouse for seeds production. To amplify a 550 bp fragment of the nptII gene, the following gene-specific primers were used: FW 5′-GAACAAGATGGATTGCACGC-3′ and REV 5′- GAAGAACTCGTCAAGAAGGC-3′.
In addition to growing legume plants in in vitro and greenhouse conditions, 35-day-old transgenic and control in vitro plants were transferred to hydroponic containers containing well aerated Fahraeus nutrient (1 g/L CaCl2⋅2H2O, 1.2 g/L MgSO4⋅7H2O, 1 g/L K2HPO4 and 1.5 g/L Na2HPO4⋅12H2O). Twenty-one milligrams per litre of Ca(NO3)2⋅4H2O as a staring dose of nitrogen, Gibson microelements and 0.005 g/L ferric citrate was added to the solution kept under constant aeration and pH between 6.5 and 6.8. Plants were grown under light intensity of 200 µmol/(m−2 s−1), with a 16 h photoperiod and day/night temperature about 25 °С/20 °С for 20–40 days. The roots of the plants were inoculated with bacterial suspension from Sinorhizobium meliloti 1021 and Mesorhizobium loti MAFF 319090 (2 × 108 cells/mL) to hydroponic pots.
According to the floral dip protocol,[Citation59] T0 plants from A. thaliana ecotype Col 0, grown in a greenhouse on soil under specific conditions [23 °С ± 2 °С, 1 m3/s ventilation, 300 µmol/(m−2 s−1) light intensity, 60% humidity and a 16/8 h day/night photoperiod], were used for genetic transformation with A.tumefaciens strain C58C1. The seeds collected from transformed plants were sterilized for 2 min with 70% (w/v) ethanol and 12 min in commercial bleach and were washed with sterile distilled water. Then, the seeds were plated on agar-solidified 0.8% MS selective culture medium supplemented with 50 mg/L Km and placed in dark and cold (4 °C). After 48 h, the plates were moved into light at 21 °C–23 °C for 10 days and Km-resistant seedlings were transferred to soil to obtain homozygous T3 plants.
Direct somatic embryogenesis of A. thaliana
Immature zygotic embryos collected from siliques of 8- to 10-week-old plants from pAUX1::GUS-GFP transcriptional reporter plants and wild type (WT) Col 0 of A. thaliana were used as initial explants for direct somatic embryogenesis.[Citation60] Siliques were sterilized with 10% (w/v) commercial bleach, rinsed several times with distilled sterile water and incubated at 4 °С for 12–16 h.[Citation61] Afterwards, the immature zygotic embryos were excised from the siliques under a microscope. To induce formation of embryogenic callus tissue formation, approximately 20 immature zygotic embryos were cultured on B5 solid medium supplemented with 0.9 mg/L 2.4D, and placed in dark for 14 days. Formed callus was plated on ½ MS solid medium with vitamins to obtain small plantlets.
Histochemical localization of GUS activity
Histochemical localization of GUS (β-glucuronidase) activity was done as described elsewhere.[Citation62,Citation63] Samples from the three model plants were placed in 90% acetone for 30 min at 4 °С, washed in phosphate buffer and incubated in GUS solution at 37 °С for 16–18 h until a blue colour developed.
Phenotypic analysis and leaf clearing, light and confocal microscopy
Phenotypic analyses of the studied model plants cultivated under in vitro, in vivo and hydroponic conditions were performed. Roots, hypocotyls, siliques and leaves from A. thaliana model plant were collected from 20 selected lines with MtLAX3 overexpression and knockdown, for morphometric measurements. Evaluation of the dynamics of primary root growth was conducted 7 days after seed germination for 5 consecutive days.
For phenotype assessment, leaves from 7- to 8-week-old transgenic and control plants were collected, cleared in absolute ethanol (∼24–48 h) and transferred into a NaOH:EtOH solution (1.25 mmol/L NaOH and absolute EtOH at a 1:1 volume ratio) at 60 °C for 2 h. The leaves were transferred into Hoyer's solution (8 g chloral hydrate, 1 mL glycerol and 3 mL MQ H2O) for 2 h to clear residual starch from the cells and subsequently cleared for 24 h in lactic acid (Acros Organics).
Samples from the three model plants were examined and photographed using an Olympus microscope with high contrast differential interference contrast coupled to a XC50 digital microscope camera (maximal resolution 2576 × 1932 pixels). Using ImageJ 1.4.3 software, 10 individual leaves from A. thaliana lines were measured to determine leaf size, epidermal cell size and number of the abaxial leaf surfaces. Fluorescence imaging of roots was performed using an Axiovert 100M confocal laser scanning microscope with software package LSM510 version 3.2 (Zeiss) and 488 nm line of an argon laser for excitation of GFP. For excitation of GFP, the 488 nm line of an argon laser was used. Images of nodules expressing GFP were collected using a SZX7 fluorescence stereomicroscope with a DP73 digital camera (Olympus) fitted with MGFPA filters (460–490 nm excitation and 510–550 nm emission).
Quantitative real-time PCR
Total RNA was isolated from lines with MtLAX3 overexpression and knockdown of the gene and its orthologous (LJ0G013110 and AT1G77690) (RNAi) and wild-type plants, using the EurEx RNeasy Plant Mini Kit. cDNA synthesis was performed with the First Strand cDNA Synthesis kit (Fermentas) and transcript levels were determined with the 7300 Real-Time PCR System (Applied Biosystems). Two housekeeping genes, actin (ACT) and ubiquitin (UBQ10), were used for data normalization as reference genes. The sequences of used primers for quantitative (qRT) PCR analyses are provided in Table S2.
Statistical analysis
Triplicate assays were performed for each experimental data-set. The data were analysed using OriginPro 8.5.1 software and qBase 1.3.5, differences were assessed by t-test and the results are expressed as means ± standard deviation. Differences were considered statistically significant at P < 0.05.
Results and discussion
In this work, we investigated the role of Auxin influx carrier transmembrane transporter from M. truncatula (MT3G072870, Plaza 2.5, MtLAX3) in plant growth and development of the model species M. truncatula, L. japonicus and A. thaliana. The MtLAX3 gene belongs to a gene family HOMO000823, which includes 132 genes in 20 species. In M. truncatula, MtLAX3 is part of an ORTHO000128 sub-family, which contains two more members, MT5G082220 (auxin influx protein, Plaza 2.5) and MT7G067450 (auxin transporter-like protein 1, Plaza 2.5). The function of MtLAX3 was studied only in ‘composite’ transgenic plants of M. truncatula obtained by A. rhizogenes hairy roots transformation,[Citation22] but never in stable transformants. The orthologous genes of MtLAX3 in L. japonicus (LJ0G013110 and LJ3G034540; Plaza 2.5) are still unknown. In A. thaliana, MtLAX3 gene has three orthologs. The closest ortholog encodes an auxin influx carrier LAX3 (LIKE-AUX1, AT1G77690, Plaza 2.5, AtLAX3).[Citation50] Based on the sequence homology, it is presumed that MtLAX3 is involved in PAT, as it has been proved for AtAUX1 and AtLAX3.[Citation7,Citation29,Citation49]
GUS/GFP activity in transcriptional reporter plants of M. truncatula, L. japonicus and A. thaliana
To follow the expression pattern of MtLAX3 during somatic embryogenesis and plant development, we obtained transcriptional reporter plants by introducing the construct of MtLAX3 promoter fused to GUS–GFP reporters into wild-type M. truncatula, L. japonicus and A. thaliana (pMtLAX3::GUS-GFP). The T1 progeny of GUS-positive transgenic plants of M. truncatula and L. japonicus were used for histochemical analyses after performing a PCR screen for the presence of the nptII gene. Applying Km selection of T1 and T2 seeds, we obtained homozygous transgenic plants (T3 generation) of A. thaliana.
Localization of gene expression was traced in the process of somatic embryogenesis in the three model species. GUS activity was observed in the initial explant, in the process of globular embryo induction, torpedo and subsequent early and late cotyledonary stages (). It is known that the combined action of auxin efflux carriers PIN1, PIN4 and PIN7 plays an essential role in auxin transport, cell division and auxin distribution during all stages of zygotic embryogenesis.[Citation36,Citation61,Citation64,Citation65] Since it is well known that auxin cell-to-cell transport is mediated by auxin influx (LAX proteins) and efflux (PIN proteins) carriers [Citation40] and the plant hormone auxin plays an important role in every aspect of plant growth and development, including embryogenesis,[Citation1,Citation66] we confirmed the involvement of MtLAX3 in somatic embryo development.
Figure 1. Expression pattern of рMtLAX3::GUS in all stages of somatic embryogenesis in transcriptional reporter plants: Medicago truncatula (a); Lotus japonicus (b) and Arabidopsis thaliana (c).

In M. truncatula plants in vitro, GUS activity was reported in whole seedlings, in the petiole and vascular system of the leaflets ( and ). GUS expression was detected in the root meristem of in vitro plants and vasculature, and the growth area of root nodules in hydroponically cultivated plants ( and ). Light signal was observed in the hooks of the seed pod in greenhouse plants (). It was recently shown that in A. thaliana, LAX2 is involved in vascular development and regulates vascular patterning in cotyledons and young leaves, by showing the expression in procambial and vascular tissues, and in developing leaves at the sites of initiating veins.[Citation17,Citation67] This suggests that the MtLAX3 gene may also play a role in the regulation of vascular patterning.
Figure 2. Expression pattern of рMtLAX3::GUS in Medicago truncatula (a)–(e), Lotus japonicus (f)–(j) and Arabidopsis thaliana (k)–(p): (a) seedling stage; (b) vascular system of the leaf; (c) in vitro root system; (d) growing area of root nodule; (e) hooks of the pod; (f) stem and leaf petiole; (g) vascular system of leaves and stem; (h) adventitious bud; (i) area of stem branching; (j) roots vascular system; (k) seedling stage; (l) vascular system of the leaf; (m) in vitro primary root and lateral root primordia and vasculature; (n) flower buds; (o) flower sepals, petals, style and stigma of pistil and (p) growth area of siliques and episperm.
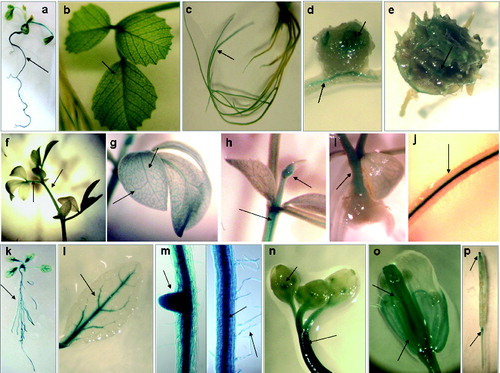
In young L. japonicus plants, GUS activity was detected in the stem and leaf petiole (), the vascular system of the leaves and the stem (), in the adventitious bud (), in the stem branching () and in the vascular system of the roots ().
In A. thaliana, a strong GUS signal was detected in the whole seedling (), the vascular system of the leaves and roots, root meristem, lateral roots and root hair ( and ). GUS activity was observed in the flower buds and stems, in sepals, in the style and stigma of the pistil and in the petal of the flower ( and ). Gene expression was detected in the growth area of siliques, in the episperm, as well as at the base of the silique pedicel ().
A GFP screening was performed for L. japonicus and A. thaliana plants. In L. japonicus, MtLAX3::GFP expression was detected in the root nodules (). In A. thaliana, a GFP signal was detected in the vascular tissue of primary and secondary roots ( and ) and the early stage of emerging lateral root primordia ().
Figure 3. Expression pattern of pMtLAX3::GUS::GFP in Lotus japonicus (a) and Arabidopsis thaliana (b)–(d): nodules (a); vascular system in primary root (b); vascular system (c) and vascular system in emerging lateral root (d).
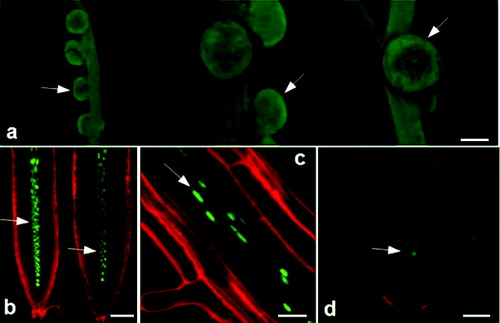
The performed GUS and GFP analyses confirm the role of the gene in lateral root emergency. Péret et al. [Citation8] reported for the role of auxin in lateral root initiation and development in A. thaliana. Swarup et al. [Citation7] provided evidence that the auxin influx carrier AtLAX3 also regulates the emergency of lateral roots. These authors proposed that auxin from the lateral root primordia enters the cortical cells and induces LAX3 expression. At the plasma membrane, LAX3 facilitates auxin uptake in the same cell and reinforces LAX3 expression. More auxin accumulates in the cortical cells, which leads to induction of cell-wall remodelling enzymes and smooth passage of the primordium through the cortex.
Relative expression level of MtLAX3 and its orthologous genes in M. truncatula, L. japonicus and A. thaliana
The general expression level of MtLAX3, LJ0G013110 and AT1G77690 transcripts was analysed by qRT-PCR in OE and knockdown (RNAi) lines of the examined model plants. In M. truncatula and L. japonicus, the transcript level of the LAX3 gene was reported in in vitro plants (designated as T0) and in their progeny (T1 plants). In A. thaliana, the expression level of the gene was evaluated in homozygous T3 plants. Two to four individual plants from each line were randomly selected and assayed for the expression level.
It was shown that the MtLAX3 transcript level in OE in vitro plants and T1 plants of M. truncatula increased 2 to 3.2 times compared with the control (P < 0.001) (). The transcript level of MtLAX3 in in vitro and T1 knockdown plants was up to threefold lower than in WT (P < 0.01) (). The expression profile for L. japonicus OE plants showed a similar trend to that in M. truncatula (). The transcript level increased up to seven times in OE lines (P < 0.001) and decreased up to fivefold in RNAi lines compared to the control (P < 0.001) (). The gene expression level in OE-lines of A. thaliana plants was increased compared to WT plants (P < 0.001) () and slightly decreased in knockdown lines (P < 0.01) ().
Figure 4. Relative expression level of MtLAX3 and its orthologous genes: (a) MtLAX3 transcript levels in T0 and T1 OE lines and WT plants of Medicago truncatula; (b) MtLAX3 transcript level in T0 RNAi lines and WT of Medicago truncatula; (c) MtLAX3 transcript level in T0 and T1 OE lines and WT of Lotus japonicus; (d) LJ0G013110 transcript level in T0 RNAi lines of Lotus japonicus; (e) MtLAX3 transcript level in homozygous OE lines and WT plants of Arabidopsis thaliana; (f) AT1G77690 transcript level in homozygous RNAi lines and WT control plants of Arabidopsis thaliana; (g) MtLAX3 transcript level in T0 OE and RNAi lines of Medicago truncatula; (h) MtLAX3 transcript level in T0 OE lines, LJ0G013110 transcript level in T0 RNAi lines of Lotus japonicus and (i) GH3 transcript levels in OE and RNAi lines of Medicago truncatula, Lotus japonicus and Arabidopsis thaliana.
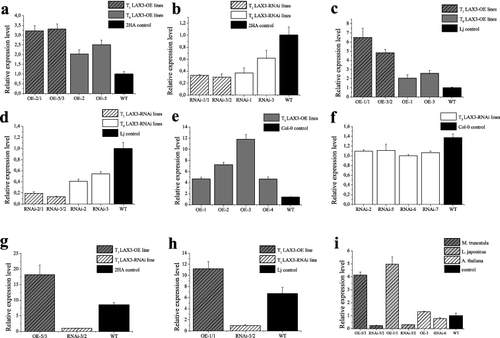
The MtLAX3 transcript level was evaluated in the nodules of T1 plants of M. truncatula and L. japonicus OE and RNAi lines ( and ). In both model plants, the expression level in OE lines was significantly higher (P < 0.001 and P < 0.0001, respectively) and decreased in RNAi lines compared to the control (P < 0.001).
The expression level of the auxin-inducible gene GH3 was analysed in the progenies of OE and RNAi lines as well (). The expression level of GH3 was observed to increase 4 to 5 times in OE lines of M. truncatula (P < 0.001) and L. japonicus (P < 0.001) and up to 0.5 times in A. thaliana compared to WT (P < 0.01). The GH3 transcript level was decreased in the RNAi lines of the three model plants compared to the control (P < 0.01).
Previous studies in supernodulating mutants of M. truncatula, skl (an ethylene insensitive mutant with root-controlled increases of nodule numbers) and sunn (supernumerary nodule),[Citation68,Citation69] showed increased expression of the auxin response gene GH3 in inoculated roots, compared to the WT.[Citation22,Citation70] The accumulation of GH3 transcript is induced by auxin,[Citation14,Citation71] which corresponds to our results for the expression profile of GH3 transcript in young seedlings which display a high level in OE lines and a very low level in the background of RNAi lines. Pacios-Bras et al. [Citation72] observed an up-regulation of auxin levels at the basis of nodule primordial and similar changes in GH3 expression in determinate nodules of L. japonicus.
Phenotypic and morphometric analysis of M. truncatula, L. japonicus and A. thaliana plants with MtLAX3 overexpression and knockdown of the gene and its orthologs
To identify the function of the MtLAX3 gene and its role in plant development, transgenic and control plants were grown under in vitro, in vivo and hydroponic conditions. Primary phenotypic characterization was performed for four to six transgenic lines compared to wild type. Figures illustrating the morphology of transgenic plants compared to the wild type of the studied model species are shown in the Supplemental data.
Overexpressed M. truncatula in vitro plants possessed a long primary root with short multiple secondary branches (Figure S1(a)) compared to 2HA control plants (Figure S1(c)). In RNAi plants, we observed slower growth and shorter root system with thinner secondary branches (Figure S1(b)). These plants displayed negative geotropism, very typical for the early stages of rhizogenesis. De Billy et al. [Citation22] have shown that the AUX1 gene of A. thaliana [Citation47] and MtLAX1-5 of M. truncatula are closely related. It has been proved before that the AUX1 gene plays a role in root gravitropism and maintains high auxin levels in the differentiation zone, root hair cell polarity and facilitates root hair growth response.[Citation17] It is not known for MtLAX3 to be involved in gravity signal response apart from some MtLAX3 expression in columella cells and detection of MtLAX genes in root tips and particularly in provascular bundles and root caps in the primary root.[Citation17,Citation22] All of this suggested the involvement of MtLAX3 in primary root growth and elongation and root hair development, especially considering our detection of MtLAX3 expression in the root tip and root hair of A. thaliana.
When grown in a greenhouse and under hydroponic conditions, M. truncatula plants that overexpressed MtLAX3 showed a faster grow rate than the WT plants and displayed a well-developed aerial part with many branches compared to WT (Figure S1(a) and S1(c)). Hydroponically cultivated plants possessed a long, branched root system with multiple dispersed scattered effective nodules /Nod++/ (Figure S1(c)). It has been reported before that MtLAX genes are involved in the development of the primordial and initiation and differentiation of the vasculature in root indeterminate-type nodules by facilitating auxin transport.[Citation21,Citation22]
Supplementary Table S3 shows the number of seeds and root nodules in T0 and T1 OE and RNAi plants of the model legumes compared to the WT. We observed that the number of root nodules in OE lines increased in T1 progeny compared to T0 and WT plants. The number of T2 seeds in OE plants was higher than the number of seeds in T1 plants and the control.
Knockdown mutants grown in a greenhouse and under hydroponic conditions were characterized with an underdeveloped aerial part (Figure S1(b)). In one of the investigated lines, there were observed leaves with four and five leaflets (Figure S1(b)). This could be considered as evidence for changes in the morphogenesis of the leaves as a result from disrupted auxin transport and decreased expression of MtLAX3. Moreover, the auxin influx carrier has an effect on organ formation and is essential for correct leaf positioning.[Citation73]
Knockdown plants displayed a shorter root system and most of them were not able to form nodules, but those that were capable of nodulating formed few very small nodules which were mostly not effective (Nod+/−). The number of root nodules in RNAi lines of M. truncatula remained persistent in the progeny, but decreased nearly six times compared to WT. The seed number was also lower in the progeny compared to WT (Table S3).
Microscopic observation of epidermal cells showed that the OE lines of M. truncatula had larger leaves and leaf surface covered with multiple well-developed, thick trichomes, whose number was drastically reduced in knockdown lines (Figure S2). These results are in agreement with the report of Mattsson et al. [Citation74], who established that the overexpression of MtLAX3 in A. thaliana leads to increased accumulation of auxin in the cells, which causes more cell divisions and bigger leaf surface.
L. japonicus OE in vitro plants had a shorter and thicker primary root (Figure S3(a)) with many secondary branches compared to control plants (Figure S3(c)). The aerial part featured a strong upright growth habit in greenhouse grown plants and a well-developed, branched stem in hydroponic plants (Figure S3(a)). These plants had large leaves, earlier flowering time than the control, many flowers and pods (Figure S3(a)). We observed increased seed number in two consecutive progenies of the transgenic plants, but no significant difference in comparison to the WT plants, probably due to the heterologous expression of MtLAX3 in L. japonicus (Table S3). Hydroponically cultivated plants displayed a well-developed, long and branched root system with multiple nodules all over the roots (Nod++), capable of nitrogen fixation from the atmosphere (Figure S3(a)). The number of root nodules increased in the progeny over three fold compared to WT (Table S3).
RNAi lines grown in vitro showed very short roots (Figure S3(b)). The knockdown in vitro plants possessed a short stem and displayed slower growth than the control ones. The plants grown in a greenhouse and under hydroponic conditions were characterized with an underdeveloped aerial part, a short and less branched root system with less and dispersedly located nodules (Nod+), some of them efficiently fixing atmospheric nitrogen (Figure S3(b)). The number of root nodules and seeds in RNAi lines of L. japonicus decreased in the progeny compared to WT (Table S3). It was established that epidermal cells were larger in OE lines than in the control plants, but were reduced in size in knockdown mutants (Figure S4).
Overexpression of MtLAX3 in both model legumes caused elongation of the primary root and multiple secondary branches. On the other hand, knockdown plants showed reduced growth and had a shorter root system with less and thinner secondary branches and nodules as we expected. Our results confirmed previous studies for the function of the investigated gene in root development. Ugartechea-Chirino et al. [Citation75] proved the role of auxin influx carriers in embryonic root patterning by showing that the quadruple aux/lax mutants had severely disorganized radicle apex and had significant increase in the average cell size, root-capcell number or both. Péret et al. [Citation8] and Swarup et al. [Citation7] reported for the role of auxin in lateral root initiation and development.
In order to better understand the role of auxin influx carrier in the processes of plant growth, we used A. thaliana as a referent plant. To investigate the function of MtLAX3 in Arabidopsis development, we selected OE and RNAi T3 homozygous lines. At least 20 plants from each line were scored for morphometric and morphological changes in in vitro and in vivo conditions. The following parameters were evaluated: dynamics of root growth, dynamics of hypocotyl growth, leaf size, biometric evaluation of epidermal cells (size and number) and length of siliques.
In A. thaliana OE and RNAi lines, the dynamics of root growth were observed five days after germination for five consecutive days. Statistical analysis of root growth for the OE line showed significantly higher (P < 0.0001) root growth rate compared to the wild type. Slower root growth in the RNAi line was observed compared to WT Col 0 (P < 0.05) (Figure S5(a)). The analysed data showed significantly higher root growth rate for the OE line compared to WT at 24 h (P < 0.0001), 72 h (P < 0.05), 120 h (P < 0.05) and no significant difference at 48 h and 96 h. The root growth rate was slower for the RNAi line as compared to WT at 24 h (P < 0.0001), 48 h (P < 0.0001), 120 h (P < 0.001) and the differences for 72 h and 96 h were not statistically significant.
The dynamics of hypocotyl growth in A. thaliana seedlings grown in darkness were measured for 10 days (Figure S5(b)). The hypocotyl growth in the OE line was similar to the growth in WT Col-0 plants and significant differences were not observed, but the hypocotyl growth in the OE line was more than two fold faster compared to the RNAi line (P < 0.0001). The RNAi line showed slower hypocotyl growth than Col-0 plants (P < 0.0001). The dynamics of root growth confirmed the role of MtLAX3 in primary root growth, showing that the OE line of A. thaliana grew faster than the RNAi line. We established that the dynamics of hypocotyl growth does not depend on the MtLAX3 auxin transporter.
Next, we further investigated how overexpression and silencing of the gene reflected on leaf size and morphology of epidermal cells. The leaf size did not show significant differences between the OE lines and control plants (Figure S5(c)), but was considerably reduced in the RNAi lines compared to OE and WT leaves (P < 0.0001). When the epidermal cells of OE and RNAi lines were morphologically evaluated for size and number of cells, giant pavement cells were found in the upper and lower part of the leaf both in the OE and RNAi lines (Figure S6).
The epidermal cells in the OE lines of Arabidopsis were observed to be of a smaller size, but not statistically significant, and more in number (P < 0.0001) than the cells in the knockdown lines (Figure S5(d) and S5(e)). The epidermal cells in WT plants were significantly smaller in size than the cells in OE (P < 0.05) and RNAi lines (P < 0.05), but more in number (P < 0.0001). No significant difference was observed between the siliques length in the OE lines, RNAi lines and WT Col-0 plants (Figure S5(f)). It is very likely that both overexpression and down-regulation caused abnormal division patterns in the leaf cells, resulting in changed expression of a subset of target genes controlling leaf cell division.
As a next step in our study, we screened 25-day-old OE and RNAi Arabidopsis plants for morphological changes in soil conditions. The OE lines of A. thaliana (Figure S7(a)) were bigger in size, with larger leaves and flowering time later than the control (Figure S7(c)). Some of the observed plants displayed many trichomes on the leaf surface. The knockdown mutants showed slower growth, smaller leaf size, shorter stem and late flowering time (Figure S7(b)).
Taken together our results throw more light on identification of the function of MtLAX3 in model legumes. Further studies will focus on optimizing the growth and development of important leguminous crop plants.
Conclusions
The characterization of transgenic plants of the model legume species M. truncatula, L. japonicus and A. thaliana demonstrated that the MtLAX3 gene was expressed in different plant tissues and organs, and its biological functions are related to plant growth and development. Tracing the expression of marker genes GUS and GFP, we proved that MtLAX3 is transcriptionally active during the processes of indirect somatic embryogenesis and symbiotic nodulation. Our study provides further support to the important role of this gene in PAT and lateral root emergency. The proper expression of MtLAX3 is required for root growth and development, lateral root emergence and normal morphogenesis of the leaves. Overexpression of the gene in both model legumes, M. truncatula and L. japonicus, led to a bigger leaf size, multiple secondary root branches, increased number of root nodules and seed pods, as well as increased seed number in M. truncatula. Up-regulation and, respectively, down-regulation of MtLAX3 and its orthologs caused abnormal phenotypes in transgenic lines from all three investigated model plants. The obtained results significantly augment the information about the function of the MtLAX3 gene in the model legumes and the opportunity to translate this knowledge to crop plants.
Supplemental data
Supplemental data for this article can be accessed at 10.1080/13102818.2015.1031698.
TBEQ-2015-0059-Online_Supplementary_Appendix.pdf
Download PDF (1.1 MB)Acknowledgements
The authors are grateful to Kety Krastanova (AgroBioInstitute) and Bistra Yuperlieva-Mateeva (Institute of Plant Physiology and Genetics) for valuable technical assistance. Cloning and construction of expression vector for transformation was performed at the Department of Plant Systems Biology, VIB/Ghent University in the frame of the above project.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Davies PJ. Plant hormones: their nature, occurrence, and functions. In: Davies PJ, editor. Plant hormones. Dordrecht: Springer; 2010. p. 1–15.
- Péret B, Middleton AM, French AP, Larrieu A, Bishopp A, Njo M, Péret B, Middleton AM, French AP, Larrieu A, Bishopp A, Njo M, Wells DM, Porco S, Mellor N, Band LR, Casimiro I, Kleine-Vehn J, Vanneste S, Sairanen I, Mallet R, Sandberg G, Ljung K, Beeckman T, Benkova E, Friml J, Kramer E, King JR, De Smet I, Pridmore T, Owen M, Bennett MJ. Sequential induction of auxin efflux and influx carriers regulates lateral root emergence. Mol Syst Biol. 2013;9:699. doi:10.1038/msb.2013.43.
- Steinmann T, Geldner N, Grebe M, Mangold S, Jackson CL, Paris S, Gälweiler L, Palme K, Jürgens G. Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science. 1999; 286(5438):318–388.
- Wolters H, Anders N, Geldner N, Gavidia R, Jürgens G. Coordination of apical and basal embryo development revealed by tissue-specific GNOM functions. Development. 2011;138(1):117–126.
- Swarup R, Kramer EM, Perry P, Knox K, Leyser HM, Haseloff J, Beemster GT, Bhalerao R, Bennett MJ. Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nat Cell Biol. 2005;7(11):1057–1065.
- Overvoorde P, Fukaki H, Beeckman T. Auxin control of root development. Cold Spring Harbor Pespect Biol. 2010;2(6):a001537.
- Swarup K, Benková E, Swarup R, Casimiro I, Péret B, Yang Y, Parry G, Nielsen E, De Smet I, Vanneste S, Levesque MP, Carrier D, James N, Calvo V, Ljung K, Kramer E, Roberts R, Graham N, Marillonnet S, Patel K, Jones JD, Taylor CG, Schachtman DP, May S, Sandberg G, Benfey P, Friml J, Kerr I, Beeckman T, Laplaze L, Bennett MJ. The auxin influx carrier LAX3 promotes lateral root emergence. Nat Cell Biol. 2008;10:946–954.
- Péret B, De Rybel B, Casimiro I, Benkova´ E, Swarup R, Laplaze L, Beeckman T, Bennett MJ. Arabidopsis lateral root development: an emerging story. Trends Plant Sci. 2009;14:399–408.
- Lewis DR, Negi S, Sukumar P, Muday GK. Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin efflux carriers. Development. 2011;138:3485–3495.
- Bainbridge K, Guyomarc'h S, Bayer E, Swarup R, Bennett M, Mandel T, Kuhlemeier C. Auxin influx carriers stabilize phyllotactic patterning. Genes Dev. 2008;22:810–823.
- Guenot B, Bayer E, Kierzkowski D, Smith RS, Mandel T, Žádníková P, Benková E, Kuhlemeier C. Pin1-independent leaf initiation in Arabidopsis. Plant Physiol. 2012;159(4):1501–1510.
- Rossen E, Chen R, Masson PH. Root gravitropism: a complex response to a simple stimulus? Trends Plant Sci. 1999;4:407–412.
- Davies PJ. Plant hormones: physiology, biochemistry and molecular biology. 2nd ed. London: Kluwer; 1995.
- Woodward AW, Bartel B. Auxin: regulation, action, and interaction. Ann Bot. 2005; 95(5): 707–735.
- Berleth T, Mattsson J, Hardtke CS. Vascular continuity and auxin signals. Trends Plant Sci. 2000;5:387–393.
- Reinhardt D. Vascular patterning: more than just auxin? Curr Biol. 2003;13:R485–R487.
- Péret B, Swarup K, Ferguson A, Seth M, Yang Y, Dhondt S, James N, Casimiro I, Perry P, Syed A, Yang H, Reemmer J, Venison E, Howells C, Perez-Amador MA, Yun J, Alonso J, Beemster GT, Laplaze L, Murphy A, Bennett MJ, Nielsen E, Swarup R. AUX/LAX genes encode a family of auxin influx transporters that perform distinct functions during Arabidopsis development. Plant Cell. 2012;24:1–12.
- Prusinkiewicz P, Crawford S, Smith RS, Ljung K, Bennett T, Ongaro V, Leyser O. Control of bud activation by an auxin transport switch. Proc Natl Acad Sci USA. 2009;106(41):17431–17436.
- Reinhardt D, Mandel T, Kuhlemeier C. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell. 2000;12(4):507–518.
- Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol. 2005;15:1899–1911.
- Noorden GE, Kerim T, Goffard N, Wiblin R, Pellerone FI, Rolfe BG, Ulrike Mathesius U. Overlap of proteome changes in Medicago truncatula in response to auxin and Sinorhizobium meliloti. Plant Physiol. 2007;144:1115–1131.
- De Billy F, Grosjean C, May S, Bennett M, Cullimore JV. Expression studies on AUX1-like genes in Medicago truncatula suggest that auxin is required at two steps in early nodule development. Mol Plant-Microbe Interactions. 2001;14:267–277.
- Lomax TL, Mehlhorn RJ, Briggs WR. Active auxin uptake by zucchini membrane vesi cles: quantitation using ESR volume and delta pH determinations. Proc Natl Acad Sci USA. 1995;82:6541–6545.
- Ljung K, Hull AK, Celenza J, Yamada M, Estelle M, Nonmanly J, Sandberg G. Sites and regulation of auxin biosynthesis in Arabidopsis roots. Plant Cell. 2005;17:1090–1104.
- Chen Q, Dai X, De-Paoli H, Cheng Y, Takebayashi Y, Kasahara H, Kamiya Y, Zhao Y. Auxin overproduction in shoots cannot rescue auxin deficiencies in Arabidopsis roots. Plant Cell Phisyol. 2014;55(6):1072–1079.
- Perrot-Rechenmann C, Napier RM. Auxins. Vitamins Horm. 2005;72:203–233.
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G. Efflux-dependent auxin gradient establish the apical basal axis of Arabidopsis. Nature. 2003;426:147–153.
- Teale WD, Paponov IA, Palme K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol. 2006;7:847–859.
- Swarup R, Friml J, Marchant A, Ljung K, Sandberg G, Palme K, Bennett M. Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev. 2001;15:2648–2653.
- Marchant A, Bhalerao R, Casimiro I, Eklöf J, Casero PJ, Bennett M, Sandberg G. AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell. 2002;14:589–597.
- Goldsmith MH. The polar transport of auxin. Ann Rev Plant Physiol. 1977;28:439–478.
- Friml J, Wiґsniewska J, Benkov E, Mendgen K, Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002;415:806–809.
- Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J. Local efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602.
- Vieten A, Sauer M, Brewer PB, Friml J. Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci. 2007;12:160–168.
- Vanneste S, Friml J. Auxin: a trigger for change in plant development. Cell. 2009;136:1005–1016.
- Petrásek J, Friml J. Auxin transport routes in plant development. Development. 2009;136:2675–2688.
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA. Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science. 1996;273:948–950.
- Palme K, Gaelweiler L. PIN-pointing the molecular basis of auxin transport. Curr Opin Plant Biol. 1999;2:375–381.
- Gälweiler L, Guan C, Müller A, Wisman E, Mendgen K, Yephremov A, Palme K. Regulation of polar auxin transport by AtPIN1in Arabidopsis vascular tissue. Science. 1998;282:2226–2230.
- Saini S, Sharma I, Kaur N, Pati PK. Auxin: a master regulator of plant root development. Plant Cell Rep. 2013;32:741–757.
- Paponov IA, Teale WD, Trebar M, Blilou I, Palme K. The PIN auxin efflux facilitators: evolutionary and functional perspectives. Trends Plant Sci. 2005;10:170–177.
- Zazímalová E, Murphy AS, Yang H, Hoyerová K, Hosek P. Auxin transporters – why so many? Cold Spring Harbor Perspect Biol. 2010;2:a001552.
- Kramer EM, Bennett MJ. Auxin transport: a field in flux. Trends Plant Sci. 2006;11:382–386.
- Estelle M. Transporters on the move. Nature. 2001;413:374–375.
- Muday GK, Peer WA, Murphy AS. Vesicular cycling mechanisms that control auxin transport polarity. Trends Plant Sci. 2003;8:301–304.
- Kleine-Vehn J, Dhonukshe P, Swarup R, Bennett M, Friml J. Subcellular trafficking of the Arabidopsis Auxin influx carrier AUX1 uses a novel pathway distinct from PIN1. The Plant Cell. 2006;18:3172–3181.
- Bennett MJ, Marchant A, May ST, Swarup R. Going the distance with auxin: unravelling the molecular basis of auxin transport. Philosophical Trans B. 1998;353:1511–1515.
- Parry G, Delbarre A, Marchant A, Swarup R, Perrot-Rechenmann C, Bennett M. Physiological characterization of a novel class of auxin influx carrier inhibitors. Plant J. 2001;25:399–406.
- Yang Y, Hammes UZ, Taylor CG, Schachtman DP, Nielsen E. High-affinity auxin transport by the AUX1 influx carrier protein. Curr Biol. 2006;16:1123–1127.
- Schnabel E, Frugoli J. The PIN and LAX families of auxin transport genes in Medicago truncatula. Mol Genet Genomics. 2004;272:420–432.
- Karimi M, Inze D, Depicker A. GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 2002;7:193–195.
- Karimi M, Bleys A, Vanderhaeghen R, Hilson P. Building blocks for plant gene assembly. Plant Physiol. 2007;145:1183–1191.
- Limpens E, Ramos J, Franken C, Raz V, Compaan B, Franssen H, Bisseling T, Geurts R. RNA interference in Agrobacterium rhizogenes-transformed roots of Arabidopsis and Medicago truncatula. J Exp Bot. 2004;55:983–992.
- Nolan KE, Rose RJ, Gorst JR. Regeneration of Medicago truncatula from tissue culture: increased somatic embryogenesis using explants from regenerated plants. Plant Cell Rep. 1989;8:278–281.
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plantarum. 1962;15:473–497.
- d'Erfurth I, Cosson V, Eschstruth A, Lucas H, Kondorosi A, Ratet P. Efficient transposition of the Tnt1 tobacco retrotransposon in the model legume Medicago truncatula. Plant J. 2003;34:95–106.
- Chabaud M, Larsonneau C, Marmouget C, Huguet T. Transformation of barrel medics Medicago truncatula Gaetrn by Agrobacterium tumefaciens and regeneration via somatic embryogenesis of transgenic plants with the MtENOD 12 nodulin promoter fused to gus reporter gene. Plant Cell Rep. 1996;15:305–310.
- Iantcheva A, Chabaud M, Cosson V, Barascud M, Schutz B, Primard-Brisset C, Durand P, Barker DG, Vlahova M, Ratet. Osmotic shock improves Tnt1 transposition frequency in Medicago truncatula cv Jemalong during in vitro regeneration. Plant Cell Rep. 2009;28:1563–1572.
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743.
- Gaj MD. Direct somatic embryogenesis as a rapid and efficient system for in vitro regeneration of Arabidopsis thaliana. PCTOC. 2001;64:39–46.
- Bassuner BM, Lam R, Lukowitz W, Yeung EC. Auxin and root initiation in somatic embryos of Arabidopsis. Plant Cell Rep. 2007;26:1–11.
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: betaglucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6: 3901–3907.
- Willemsen V, Wolkenfelt H, Vrieze G, Weisbeek P, Sheres B. The HOBBIT gene is required for formation of the root meristem in the Arabidopsis embryo. Development. 1998;125:521–531.
- Friml J, Benkova E, Blilou I, Wisniewska J, Hamann T, Ljung K, Woody S, Sandberg G, Scheres B, Jürgens G, Palme K. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell. 2002;108:661–673.
- Jenic PD, Barton MK. Surge and destroy: the role of auxin in plant embryogenesis. Development. 2005;132:3577–3585.
- Quint M, Ito H, Zhang W, Gray WM. Characterization of a novel temperature-sensitive allele of the CUL1/AXR6 subunit of SCF ubiquitin-ligases. Plant J. 2006;43:371–383.
- Farquharson KL. An auxin influx transporter regulates vascular pattering in Arabidopsis. Plant Cell. 2012;24(7):2707.
- Penmetsa RV, Cook DR. A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science. 1997;275:527–530.
- Schnabel E, Journet EP, de Carvalho-Niebel F, Duc G, Frugoli J. The Medicago truncatula SUNN gene encodes CLV1-likr leucine-rich repeat receptor kinase that regulatesnodule number and root length. Plant Mol Biol. 2005;58:809–822.
- Penmetsa RV, Frugoli JA, Smith LS, Long SR, Cook DR. Dual genetic pathways controlling nodule number in Medicago truncatula. Plant Physiol. 2003;131:998–1008.
- Hagen G, Kleinschmidt A, Guilfoyle T. Auxin-regulated gene expression in intact soybean hypocotyl and excised hypocotyl sections. Planta. 1984;162:147–153.
- Pacios-Bras C, Schlaman HR, Boot K, Admiraal P, Langerak JM, Stougaard J, Spaink HP. Auxin distribution in Lotus japonicus during root nodule development. Plant Mol Biol. 2003;52:1169–1180.
- Stieger P, Reinhardt D, Kuhlemeier C. The auxin influx carrier is essential for correct leaf positioning. Plant J. 2002;32:509–517.
- Mattsson J, Ckurshumova W, Berleth T. Auxin signaling in Arabidopsis leaf vascular development. Plant Physiol. 2003;131(3):1327–1339.
- Ugartechea-Chirino Y, Swarup R, Swarup K, Péret B, Whitworth M, Bennett M, Bougourd S. The AUX1 LAX family of auxin influx carriers is required for the establishment of embryonic root cell organization in Arabidopsis thaliana. Ann Bot. 2010;105:277–289.
