?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Terpene esters of short-chain fatty acids are essential oils that have big importance in food, cosmetic and pharmaceutical industries as flavours and fragrances. Geraniol and citronellol are the most important substances. Considering the ever-increasing demand for such products, their enzymatic production from natural raw materials by using environmentally friendly and economically attractive processes may prove advantageous. In this contribution, we would like to present an alternative option for the production of geranyl propionate using nanobioconjugates consisting of Candida rugosa lipase adsorbed onto multi-walled carbon nanotubes (CRL-MWCNTs). We investigated the effects of incubation time, temperature, solvent log P and substrate molar ratio, and determined the optimum conditions. The yield of geranyl propionate catalysed by CRL-MWCNTs nanobioconjugates was significantly influenced by two factors, namely, temperature and time of the reaction. Under the optimum reaction conditions of 55 °C, solvent n-heptane (log P = 4.0), geraniol to propionic acid molar ratio of 5:1 and reaction time of 6 h, the use of CRL-MWCNTs resulted in 51.3% production of geranyl propionate. Therefore, the investigation revealed that geranyl propionate was successfully synthesized under mild conditions with reasonably high yield within a short period of time. The CRL-MWCNTs nanobioconjugates demonstrated a potential as economical and environmentally smarter biocatalysts for the production of geranyl propionate.
Introduction
Geraniol and citronellol are one of the main components that form terpene esters of short-chain fatty acids, commonly used as flavours and fragrances in food, cosmetic and pharmaceutical industries.[Citation1] Conventionally, these esters are produced via various methods, viz. chemical synthesis, extraction from natural products, as well as fermentation.[Citation2] The current chemical route that is being used for the production of these esters is usually associated with numerous shortcomings, such as utilization of strong acid catalyst and hazardous chemicals, which entail the use of high energy processes and a considerably long reaction time.[Citation3] There is also a downstream processing that incurs extreme contact with toxicants, as well as production of superfluous harmful by-products.[Citation3] Alternative enzyme-based methods that would overcome such disadvantages and improve the reaction yields and productivity would be of a significant advantage.[Citation4]
In addition, the approval of REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation by the European Parliament and of the Council and the environmental restrictions imposed by US, Japan and EU has opened up new challenges in utilizing Green Chemistry philosophy for industrial processes.[Citation5] The philosophy emphasizes the industrial use of chemicals acquired from biomass, the use of green solvents and more sustainable industrial processes,[Citation6] incorporating catalytic technologies (chemical or enzymatic) into a general organic synthesis scheme. In this sense, the pro-Green Chemistry enzyme-based methods offer many attractive features that embrace gentle reaction conditions (temperature and physiological pH) and an efficient environmentally friendly catalysts (a cell or an enzyme).[Citation6]
Lipases (triacylglycerol ester hydrolysis EC 3.1.1.3) are commercially important biomolecules that have acquired popularity as biocatalysts [Citation7] for reasons, such as high specificity, efficient reaction rate, non-toxicity and biodegradability,[Citation8] as well as ability to convert a large number of substrates with high stereospecificity.[Citation9] In this context, Candida rugosa lipase (CRL) has been commonly used due to its high activity and broad specificity.[Citation10] In view of the poor stability of the free form CRL in organic solvents and the easy deactivation in prolonged conditions of high temperature and extreme pH,[Citation8] immobilization of CRL onto multi-walled carbon nanotubes (MWCNTs) may be one of the feasible solutions [Citation10] for extending the reaction life. Such extended reaction life of the MWCNTs immobilized CRL (CRL-MWCNTs) nanobioconjugates can be attributed to the excellent binding capability due to the large surface area to volume ratio, physicochemical properties, as well as biological compatibility of the MWCNTs.[Citation11] The enzyme immobilization offers a multitude of advantages, such as structural stability, improved activity, specificity and selectivity, and reduced inhibition,[Citation12] as well as easy separation of the enzyme from the product.[Citation13] In this sense, the enzyme immobilization technique permits the establishment of an essential, economically viable enzyme catalyzed process,[Citation14] which processes are shorter, produces less waste and is economically smarter.[Citation15]
Experimental conditions are of key concern in many enzyme-assisted synthetic reactions, since enzymes can be affected by the above-mentioned conditions. Making predictions of the effects of independent variables on the product and rate of a reaction, in order to increase the efficiency of its bioprocesses, can be almost infeasible due to the nonlinearity and complicated structure of the biotechnological practices.[Citation9] In fact, the complexity of the enzyme-catalysed reactions can be enhanced by the sensitivity of the enzyme structure to variables, such as temperature, reaction time, substrate molar ratio and activator or inhibitor concentrations.[Citation9,Citation16] This is the reason why research studies often resort to utilizing a statistical tool known as response surface methodology (RSM).[Citation17] This method uses multiple variables to establish optimum conditions under predetermined reaction preferences, such as high product yield at the lowest cost and/or with the least number of experiments.[Citation18] To facilitate optimization of processes or products, RSM exploits quantitative data in experimental design to conclude and simultaneously resolve multivariate equations.[Citation19] The technique has been successfully applied to optimize enzyme-catalysed synthesis of various esters.[Citation9,Citation20,Citation21] The establishment of optimum conditions with the usage of RSM is advantageous and it is more rapid and less expensive than the conventional one-variable-at-a-time or full factorial experiment.[Citation22,Citation23]
Hence, the objective of the present study was to model the newly developed CRL-MWCNTs catalysed reaction between geraniol and propionic acid and to produce geranyl propionate – an essential oil used in food, cosmetic and pharmaceutical industries as flavours and fragrances. The mutual effects of four reaction parameters (temperature, incubation time, solvent log P and substrate to molar ratio) on the enzymatic production of geranyl propionate were evaluated. The quadratic polynomial model equation for the conversion yield of geranyl propionate was established using the method of RSM and the optimal reaction conditions suggested.
Materials and methods
Materials
Lipase from C. rugosa (EC 3.1.1.3), Type VII, (activity 1410 U mg−1) was procured from Sigma Aldrich Co. (St. Louis, USA). MWCNTs were prepared by chemical vapour deposition and functionalized in a mixture of H2SO4:HNO3 (3:1 v/v) procured from Sigma Chemical Co. (St. Louis, USA). For the esterification reactions, the substrates geraniol (98%) and propionic acid (99%) were acquired from Sigma Chemical Co. Sodium hydroxide and phosphate buffer pH 7 (Merck, Darmstadt, Germany) were used without further purification. Solvents, diethylether, toluene, n-heptane, benzaldehyde and decane were purchased from Merck. Distilled water was produced in the lab and used in all experiments. All chemicals were of analytical grade unless specified otherwise.
Experimental design and optimization for CRL-MWCNTs catalysed esterification
A four-factor, five-level central composite design (CCD) that required 30 experiments was used in this study to evaluate the reaction parameters.[Citation24] The fractional factorial design consisted of 16 factorial points, 8 axial points and 6 centre points. The variable and the levels selected for the CRL-MWCNTs catalysed esterification production of geranyl propionate were – time (2−10 h); temperature (40−60 °C); solvent log P (0.8−6.0) and substrate molar ratio acid to alcohol (1:1−1:5) (). The experiments were randomized and the measurements of esterification percentage for each experiment were run in triplicate.
Table 1. Independent test variables and their levels used for central composite design.
A software package by Design Expert Version 7.1.6 (State-Ease Inc., Statistics Made Easy, Minneapolis, MN, USA) was used to fit the second-order model to the independent variables using(1)
(1) where y is the dependent variable (% yield) to be modelled; xi and xj are the independent variables (factors), b0, bi, bii and bij are the regression coefficients of the model and e is the error of the model. Analysis of variance (ANOVA) was used to determine the adequacy of the constructed model to illustrate the observed data. The percentage of variability of the optimization parameters explained by the model is indicated using the R2 statistic. The three-dimensional surface plots were drawn to illustrate the main and interactive effects of the independent variables on the dependent ones.
Results and discussion
Model fitting and analysis of variance
RSM consists of an empirical modelization technique, which helps to evaluate the relation between experimental and observed results.[Citation9,Citation24,Citation25] In this study the widely accepted CCD was the chosen design for the surface optimization of geranyl propionate synthesis catalysed by the CRL-MWCNTs biocatalysts.[Citation9,Citation26] The selected model consisted of four factors: time (A), temperature (B), solvent (C) and molar ratio (D) and each factor was studied at five different levels (−2, −1, 0, +1, +2) ().
The experimental conditions’ design and their employed responses are presented in . The predicted values were obtained with a model fitting technique by using the software design expert version 7.1.6 and were seen to be sufficiently correlated with the observed values. The best fitting model was established by a regression analysis. Fitting of the data to various models (linear, two factorial, quadratic and cubic) and their subsequent ANOVA showed that the reaction of geraniol and propionic acid was most suitably described with a quadratic polynomial model, as the following equation:(2)
(2) where A is the time, B is the temperature, C is the solvent and D is the substrate molar ratio.
Table 2. Composition of the various runs of the central composite design, actual and predicted responses.
The ANOVA for the RSM is shown in . The computed value of the model (183.84) is very high compared to the tabular F0.05 (14,14) = 2.48, implying that the degree of freedom relative to the residual, obtained for the model, is significant at the 5% confidence level. The very small P-value (0.0001) and a suitable coefficient of determination (R2 = 0.9946) also confirmed that the quadratic polynomial model is highly significant and sufficient to explain the actual relationship between the response and the significant variables. P-values less than 0.05 indicate that the model terms are significant. In this case, the terms AB (P-value = 0.4467) and BD (P-value = 0.5130) are not significant and can be removed. However, all coefficients were considered in the design in order to minimize the possible error.
Table 3. Analysis of variance and model coefficients.
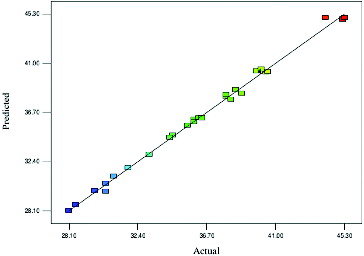
According to the ANOVA of factors, the F-value for the lack of fit was 1.15 which was lower than the tabulated value of F0.05 (10,4) = 5.964, which implied that the lack of fit was not significantly relative to the pure error (). Therefore, the relationships of the reactions represented by the model were well within the chosen range. In order to assess the optimization technique, the actual and predicted values were compared and the results were depicted in . The predicted values of the response from the model agreed with the observed values and could be properly applied to navigate the design space.[Citation27,Citation28]
Mutual effects of factors оn the production of geranyl propionate
Effect of solvent log P and time
shows the effect of the reaction time and solvent and their mutual interaction on the CRL-MWCNTs catalysed production of geranyl propionate at constant conditions of geraniol:propionic acid molar ratio of 3:1 and 50 °C. The F-value for time versus solvent (log P) showed that the effect of time on the percentage conversion of geranyl propionate was more significant than the used solvent (log P) (). However, the interaction between both of the studied parameters was significant because of the very small P-value (<0.0003).
Figure 2. Response surface plot showing the effect of time (A) and solvent (C) and their interaction in the enzymatic production of geranyl propionate.
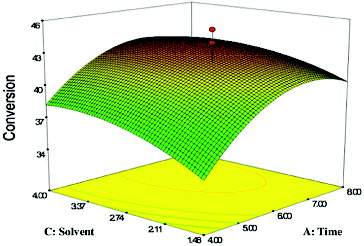
A high conversion of geranyl propionate (42.02%) at time 6.5 h and solvent (log P) 2.3 was observed. This was due to the production of molecules, which had increased in an adequate value to accommodate the hydrolysis process of ester, apart from the reaction, which had achieved the equilibrium state.[Citation29] As the reaction proceeded, the substrate concentration decreased, which consequently reduced the degree of saturation of the enzyme with the substrate.[Citation30] It can be seen that the percentage yield increased slightly with the increasing of the solvent hydrophobicity (log P) up to 6 h. The observed increase in yield was attributed to the activity of the enzyme, which was affected by the polarity of the employed organic solvent.[Citation31] Corroborating previous reports has shown that solvent with a higher log P had a better rate of esterification.[Citation32]
Effect of molar ratio and time
The effect of varying molar ratio (geraniol:propionic acid) and reaction time on the production of geranyl propionate at constant conditions of 50 °C and a solvent log P of 2.5 is depicted in . Again, it is clear that the effect of time on the yield of geranyl propionate was more significant than the molar ratio of the substrates. The F-value indicated that the effect of time (577.57) was more significant, compared to the molar ratio of geraniol:propionic acid (2.26) (). The interaction between both parameters was significant because of the very small P-value (0.0008). A high percentage conversion was achieved with moderate molar ratio and solvent log P.
Figure 3. Response surface plot showing the effect of reaction time (A) and molar ratio (D) and their interaction in the enzymatic production of geranyl propionate.
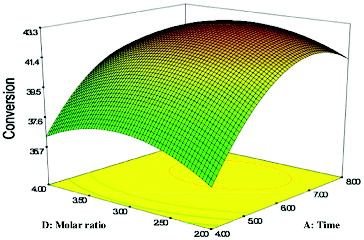
Reaction with low substrate molar ratio and short incubation time showed the lowest conversion, which corresponded to 36.97%. The percentage conversion of geranyl propionate slightly increased to 42.1% with the increasingof the incubation time and molar ratio geraniol:propionic acid up to 3:1. Conversely, at ratio higher than 3:1, the conversion of geranyl propionate decreased. This was attributable to the high alcohol concentration that slowed down the conversion of the esters.[Citation33] It has been suggested that around the critical molar ratio, the alcohol molecules are competing for the same active site, as the acyl group of the acid reduces for the formation of an acyl-enzyme complex and, consequently, are reducing the rate of alcoholysis.[Citation18] It is pertinent to highlight that the presence of excess alcohol molecules in an enzyme-catalysed esterification reaction is predisposed to promote distortion of the essential water layer from the enzyme molecules.[Citation16]
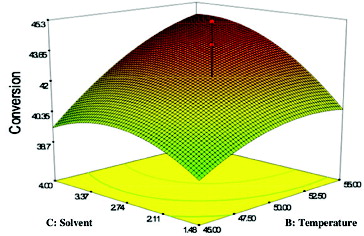
Effect of temperature and solvent log P
illustrates the effect of varying temperature and solvent log P on the esterification production of geranyl propionate at constant conditions of 6 h and a geraniol:propionic acid molar ratio of 3:1. The highest conversion of geranyl propionate of 46.39% was obtained at 52 °C in solvent log P ∼ 3.0. The percentage conversion of geranyl propionate increased slightly with the increasing of log P of the solvent and the temperature of the reaction. According to the F-value, the effect of the temperature (F-value = 346.39) was more significant than the effect of solvent log P (F-value = 152.02). The interaction between temperature and solvent (log P) was significant because of the very small P-value (0.0001). Increasing of the temperature had a significant effect on the reaction yield, which could be attributed to greater unfolding of the enzyme protein at elevated temperatures.[Citation9] With regards to the log P of the solvent, the polarity of solvents, employed in the enzyme-catalysed esterification reactions, has been reported to affect the enzyme activity,[Citation18] whereby the non-polar solvents (log P > 2) are reported to result in more favourable conditions for many lipase-catalysed reactions.[Citation34]
Effect of solvent log P and molar ratio
depicts the response surface plot as a function of solvent (log P) versus molar ratio on the CRL-MWCNTs catalysed production of geranyl propionate at constant conditions of 6 h and 50 °C. The F-value indicated that the effect of solvent log P (F-value = 152.02) was more significant, as compared to the molar ratio of geraniol:propionic acid (F-value = 2.26). According to the ANOVA of factors, the mutual interaction of solvent log P and molar ratio was very significant because of the small P-value (0.0001).
Figure 5. Response surface plot showing the effect of solvent (C) and molar ratio (D) and their interaction in the enzymatic production of geranyl propionate.
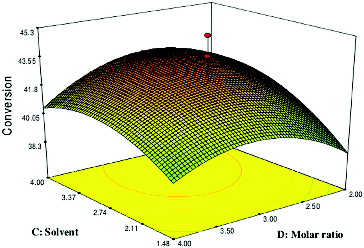
A high percentage conversion was achieved with a medium molar ratio of geraniol:propionic acid and medium values of solvent log P. A high percentage conversion of geranyl propionate was accomplished in a reaction, which consisted of 2.57 solvent log P and a geraniol:propionic acid molar ratio of 2.99:1. On the other hand, the yield of geranyl propionate was particularly low at high geraniol:propionic acid molar ratios, which indicated that the alcohol is a terminal inhibitor of lipases [Citation35] and decreases the enzyme activity. Additionally, hydrophilic substrates, such as alcohol, have been known to be able to strip off the essential water from the enzyme surface, leading to an insufficiently hydrated enzyme molecule.[Citation35] In this context, it is noteworthy to emphasize that solvents of low log P values increase the penetration of the solvent molecules into the lipase protein and accelerate the deformation of the lipase structure[Citation36] and loss of its catalytically competent form.
Attaining optimum conditions and verification of the model
The response surface can indicate the optimal combination of parameters in order to obtain the highest percentage yield. The highest yield accomplished from the various runs was 51.3% using 55 °C temperature, solvent n-heptane (log P = 4.0), geraniol to propionic acid molar ratio of 5:1 and a reaction time of 6 h. Using the Design Expert 7.1.6 optimization functions, several experimental conditions were proposed to find the optimum point and maximize the conversion under a variety of chosen conditions. However, only two sets of conditions were selected, according to the highest conversion in the shortest reaction time. The obtained actual experimental values were 47.4% and 44.6%, which corresponded to the optimized conditions of 4 h, 50 °C, geraniol:propionic acid molar ratio of 3:1, solvent log P = 2.50 and 4.01 h, 53.34 °C, geraniol:propionic acid molar ratio of 3.49:1, solvent log P = 3.29, respectively. The results of the proposed conditions are tabulated in . The experimental values were relatively close to the predicted values by the model and were therefore confirming the adequacy and validity of the predicted model. Hence, the CRL-MWCNTs nanobioconjugates could catalyse the production of geranyl propionate with percentage conversion close to 50% in just 4 h reaction time.
Table 4. The attained optimum conditions for geranyl propionate enzymatic production under the preferred conditions for the shortest reaction time.
Conclusions
A high degree of conversion was possible by simply seeking the optimum point on the response surface. Thus, in the present study, we demonstrated that the RSM can be applied effectively to predict the conditions for a high conversion of geranyl propionate catalysed by the CRL-MWCNTs nanobioconjugates that could give a yield close to 50% in a rather short time period, when the optimized conditions were determined. Considering the cost of 10 g commercial enzymes, i.e. Novozyme (>900 USD) and Lipozyme (400 USD), the prepared nanobioconjugates could provide economical and environmentally smarter biocatalysts for the production of geranyl propionate.
Acknowledgements
The authors would like to express their gratitude to the Central Laboratory, Universiti Teknologi Malaysia for the use of their facilities.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Radzi SM, Mustafa WAF, Othman SS, Noor HM. A pineapple flavour via lipase catalyzed reaction. Acad Sci Eng Technol. 2011;59:677–680.
- Malcata FX, Reyes HR, Garcia HS, Hill CG, Amundson CH. Immobilized lipase reactor for modification of fats and oils: a review. J Am Oil Chem Soc. 1990;67:890–910.
- Charpe TW, Rathod VK. Biodiesel production using waste frying oil.Waste Manage. 2011;31:85–90.
- Paroul N, Grzegozeski LP, Ciaradia V, Treichel H, Cansian RL, Vladimir Oliveira J, de Olivera D. Production of geranyl propionate by enzymatic esterification of geraniol and propionic in solvent free system. J Chem Technol Biotechnol. 2010;12:1636–1641.
- Anastas P, Eghbali N. Green chemistry: principles and practice. Chem Soc Rev. 2010;39:301–312.
- Nestl BM, Nebel BA, Hauer B. Recent progress in industrial biocatalysis. Curr Opinion Chem Biol. 2011;15:187–193.
- Salah RB, Ghamghui H, Miled N, Mejdoub H, Gargouri Y. Production of butyl acetate ester by lipase from novel strain of Rhizopusoryzae. J Biosci Bioeng. 2007;103:368–372.
- Zhou G, Wu C, Jiang X, Ma J, Zhang H, Song H. Active biocatalysts based on Candida rugosa lipase immobilized in vesicular silica. Process Biochem. 2012;47:953–959.
- Wahab RA, Abdul Rahman MB, Zaidan UH, Basri M, Raja Abdul Rahman RNZ, Salleh AB, Leow TC. Enzymatic production of a solvent-free menthyl butyrate via response surface methodology catalyzed by a novel thermostable lipase from Geobacilluszalihae. Biotech Biotech Eq. 2014;28(6):1065–1072.
- Ansorge-Schumacher MB, Thum O. Immobilised lipases in the cosmetics industry. Chem Soc Rev. 2013;42:6475–6490.
- Zhang P, Henthorn DB. Synthesis of PEGylated single wall carbon nanotubes by a photoinitiated graft from polymerization. AIChE J. 2010;56:1610–1615.
- Cesar M, Jose MP, Gloria FL, Jose MG, Roberto FL. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enz Microb Technol. 2007;40:1451–1463.
- Tischer W, Kascher V. Immobilized enzymes: crystals or carriers? Trends Biotechnol. 1999;17:326–335.
- Norouzian D. Enzyme immobilization: the state of art in biotechnology. Iran J Biotechnol. 2003;1:197–206.
- Tao J, Kazlaukas RJ. Biotechnology tools for green synthesis: enzymes, metabolic pathways, and their improvement by engineering. In: Tao J, Kazlaukas RJ, editors. Biocatalysis for green chemistry and chemical process development. Hoboken (NJ): John Wiley & Sons Inc.; 2011. p. 3–22.
- Chaibakhsh N, Abdul Rahman MB, Abd-Aziz S, Basri M, Salleh AB, Abdul Rahman RNZ. Optimized lipase-catalysed synthesis of adipate ester in a solvent free system. J Ind Microbiol Biotechnol. 2009;36:1149–1155.
- Baz D, Boyaci IH. Modeling and optimization 1: usability of response surface methodology. J Food Eng. 2007;78:836–845.
- Gunawan ER, Basri M, Rahman MBA, Salleh AB, Rahman RNZA. Study on response surface methodology of lipase-catalyzed synthesis of palm-based wax ester. Enzym Microb Technol. 2005;37:739–744.
- Giovanni M. Response surface methodology and product optimization. Food Technol. 1983;37:41–45.
- Keng PS, Basri M, Abdul Rahman MB, Salleh AB, Rahman RNZ, Ariff A. Optimization of palm-based wax esters production using statistical experimental designs. J Oleo Sci. 2005;54:519–528.
- Macedo GA, Pastore GM, Rodrigues MI. Optimising the synthesis of isoamyl butyrate using Rhizopussp lipase with a central composite rotatable design. Process Biochem. 2004;39:687–892.
- Low TL, Rosfarizan M, Tan CP, Long K, Lo SK, Lai OM. Lipase-catalyzed production of medium-chain triacylglycerols from palm kernel oil distillate: optimization using response surface methodology. Eur J Lipid Sci. 2007;109:107–119.
- Floros JD, Chinnan MS. Optimization of pimiento pepper lye-peeling process using response surface methodology. Trans ASAE. 1987;30:560–565.
- Brereton RG. Central composite or response surface designs. In: Brereton RG, editor. Chemometrics: data analysis for the laboratory and chemical plant. Chichester: John Wiley & Sons Ltd.; 2003. p. 76–84.
- Chowdary GV, Divakar S, Prapulla SG. Modeling on isoamylisovalerate synthesis from Rhizomucormiehie lipase in organic media: optimization studies. World J Microbiol Biotechnol. 2002;18:179–185.
- Jeong GT, Park DH. Response surface methodological approach for optimization of enzymatic synthesis of sorbitan methacrylate. Enzym Microb Technol. 2006;39:381–386.
- Chaibakhsh N, Basri M, Mohamed ASH, Abdul Rahman MB, Rezayee M. Optimization of enzymatic synthesis of eugenol ester using statistical approaches. Biocatal Agri Biotechnol. 2012;1:226–231.
- Zhang D, Bai S, Ren M, Yan S. Optimization of lipase-catalyzed enantioselective esterification of (+)-menthol in ionic liquid. Food Chem. 2008;109:72–80.
- Villeneuve P, Bare B, Sarrazin P, Davrieux F, Boulanger R, Caro Y. Synthesis of pyroglutamic acid fatty esters though lipase-catalysed esterification with chains alcohols. Enzym Microb Technol. 2003;33:79–84.
- Mat Radzi S, Basri M, Salleh AB, Ariff A, Mohamad R, Abdul Rahman MB, Abdul Rahman RNZ. Large scale production of liquid wax ester by immobilized lipase. J Oleo Sci. 2005;54:203–209.
- Gogoi S, Dutta NN. Kinetics and mechanism of esterification of isoamyl alcohol with acetic acid by immobilized lipase. Ind J Chem Technol. 2009;16:209–215.
- Gogoi S, Hazarika S, Rao PG, Dutta NN. Esterification of lauric acid with lauryl alcohol using cross-linked enzyme crystals: solvent effect and kinetic study. Biocatal Biotransform. 2006;24:343–351.
- Kuperkar VV, Lade VG, Prakash A, Rathod VK. Synthesis of isobutyl propionate using immobilized lipase in a solvent free system: optimization and kinetic studies. J Mol Catal B Enzym.2014;99:143–149.
- Klibanov AM. Why enzymes are less active in organic solvents than in water? Trends Biotechnol. 1997;15:97–101.
- Zaks A, Klibanov AM. Enzymatic catalysis in organic media at 100 °C. Science. 1984;224:1949–1951.
- Yang L, Dordick JS, Garde S. Hydration of enzyme in nonaqueous media is consistent with solvent dependence of its activity. Biophys J. 2004;87:812–821.