Abstract
One of the major problems in modern dentistry is the recovery of bone defects. The aim of this prospective experimental study was to evaluate the effect of light-emitting diode (LED) photobiomodulation therapy on bone healing of rat calvarial defects. Twenty-eight male Sprague Dawley rats were used for the study. Critical size defects with 5 mm diameter were made with a trephine bur used in a low-speed handpiece under continuous sterile saline irrigation on each side of the sagittal suture. All critical size defects on the right side were filled with corticocancellous bone graft material and all the defects on the left side were left empty. The animals were randomly divided into two groups of 14 rats each. Group I received LED therapy and Group II did not receive any therapy. OsseoPulse® LED device (Biolux Research Ltd.) 618 nm wavelength and 20 mW/cm2 output power irradiation was started immediately after the surgery and was applied for 20 minutes at 24-h intervals for 7 and 14 days. In each group, seven rats were sacrificed on the 8th day and the remaining rats were sacrificed on the 15th day. Bone healing of the non-grafted side was statistically significant in Group I on both 8th day and 15th day; on the other hand, in the grafted side, enhanced bone healing was dominantly observed on the 15th day in Group I, compared to Group II, although the difference was not significant. Within the limits of this study, the findings suggested that LED therapy might have a favourable effect in the early phase of bone healing.
Introduction
Low-intensity light therapy, generally referred to as ‘photobiomodulation’ by light in the far-red to the near-infrared region of the spectrum (630–100 nm), modulates numerous cellular functions. Low level lasers (LLLs) and light-emitting diodes (LEDs) are well-accepted therapeutic tools in the treatment of infected, ischemic and hypoxic wounds, along with other soft tissue injuries.[Citation1–5] Photobiomodulation therapy has been shown to stimulate cell proliferation, including that of fibroblasts, to help for the attachment and synthesis of collagen and procollagen, to promote angiogenesis and to stimulate macrophages and lymphocytes, and in this way to accelerate the bone repair process.[Citation6–8]
LEDs differ from LLLs and have been found to be more beneficial in several aspects. LLL is a laser with the characteristics of coherency, whereas the LED light is not coherent and therefore is expected to result in fewer side effects. Unlike lasers, there is virtually no heat generated by the LED array and, therefore, no potential thermal injury is expected. Furthermore, LED radiation can also be produced at a lower cost, compared to the LLL and it can safely be applied to a larger area of the body surface.[Citation9–11]
Modern dentistry is challenged daily by the need to recover bone loss due to several etiological factors, such as trauma, pathological conditions or as a consequence of surgical procedures. This aspect has led to extensive studies on the process of bone repair. Several techniques for the correction of bone defects have been studied, including grafts and photobioengineering.[Citation6,Citation7,Citation12–16] Laser light has been used for improving bone repair in several conditions, such as dental implants,[Citation17] orthodontic miniscrews,[Citation18] autologous bone grafts [Citation8,Citation19] and several types of bone defects.[Citation20–23]
Despite the growing number of successful applications of LED-phototherapy (LPT) in many fields, there are few reports concerning their effect on bone repair.[Citation24] Several studies have suggested that the use of LPT is mostly suitable for improving bone repair due to its penetration depth in the bone tissue, when compared to the visible light.[Citation13] Although several studies have demonstrated positive results in the healing of bone tissue with the use of LPT, there have been few reports on the use of LPT and biomaterial combinations.[Citation6,Citation7,Citation13–16] The effects of LPT, associated with graft biomaterials in bone repair, need further studies, as each material may cause different responses. As light has been proven to speed up bone repair, it would be desirable to develop a technique that may enhance and shorten the time of bone repair in cases of biomaterials usage.[Citation6]
Autogenous bone grafts are the materials of choice and are regarded as the ‘gold standard’ in the treatment of bone defects. They are a source of osteogenic cells and osteoinductive substances without immunogenic properties.[Citation25–27] Considerable interest has been risen for the usage of bone graft substitutes to fill critical bone defects, because of the significant limitations in terms of donor sites that the autogenous bone grafts have.[Citation27–29] Freeze dried bone allografts (FDBA) have been widely used in the field of implantology and periodontology for the treatment of bone defects with predictable outcomes.[Citation30,Citation31] They are providing osteoconductive scaffold and volume enhancement, as well as an effective site development.
On the other hand, LPT may offer advantages as an attractive treatment option for previously grafted bone defect sites, allowing a successful implant placement and osseointegration. Accordingly, the aim of the present study was to evaluate the effect of LPT on the early phase of healing of surgically created calvarial defects with or without FDBA.
Materials and methods
Animal care
The protocol for the study was approved by the Institutional Review Board of Laboratory Animals Ethical Committee (No:257a). Twenty eight 13 weeks old Sprague Dawley male rats (mean weight 280 g) were kept under natural conditions of light and temperature. The animals were fed with standard laboratory diet and had water ad libitum. Controlled day/night light cycle and temperature were performed during the experimental period. The animals were randomly divided into two groups (Group I: Rats receiving LED and Group II: Rats not receiving LED) and then subdivided into two subgroups, according to the sacrification days ().
Table 1. Distribution of the rats in the study.
Study protocol
All procedures were performed under sterile conditions in a surgical operating room. All Sprague Dawley rats were sedated with an intramuscular injection of ketamine (Ketalar®, Pfizer, Turkey, 50 mg/mL) and 2% xylazine (Rompun®, Bayer, Canada, 20 mg/mL, 2 mL/kg). A semilunar incision was made at the scalp in the anterior region of the calvarium allowing the reflection of a full-thickness flap towards the posterior region of the skull. Critical size defects with 5 mm diameter were made with a trephine (3i Implant Innovations Inc., FL, USA) used in a low-speed handpiece under continuous sterile saline irrigation at parietal bones by the reference of bregma point and sagittal suture. The defect located on the right side was filled with FDBA (MinerOss®, Biohorizons/Osteotech, Inc, Eatontown, NJ) material and the defects on the left side were left empty. Group I received LPT and Group II did not receive any therapy. For the ongoing daily LPT sessions, the animals were sedated only with Rompun® 10 mg/mL in order to provide their stability and their survival.
LPT protocol
LPT was carried out using a LED device with 618 nm wavelength and with 20 mW output power (OsseoPulse® AR 300; Biolux, Vancouver, Canada). LPT was started for Group I immediately after the surgery and transcutaneously applied for 20 minutes at 24-h intervals for 7 d and 14 d. The animals were sacrificed on the 8th and 15th days. Sacrifications were made 24-h after the final irradiation days (7th and 14th days) to observe the effect of the last day irradiations. Surgical defects with the surrounding tissues nearby were removed in block. A silk suture was fixed through a hole made by a bur at the neighbourhood of the empty defect in order to differentiate the defects during the histopathological examination.
Histopathological and histomorphometrical analysis
The parietal bones were dissected and were fixed in 10% neutral formalin solution and sent for histological and histomorphometrical analysis. After fixation of the blocks, all materials were decalcified in the solution prepared with 0.5% formic acid (v/v) and 0.2% sodium citrate (w/v). Bone samples were embedded in suitable containers with melted paraffin wax and stored at −4 ºC. Paraffin blocks were sectioned at 5–7 µm thickness and stained with hematoxylin eosin. Bone healing scores were adopted according to the Allen's grading system ().[Citation32] According to Allen's scoring system, score 0 shows non-union, whereas the score 6 shows complete bony union. These bone healing scores were used in and . Histomorphometrical analysis was performed by two pathologists (blinded to the LPT group) under a light microscope (Olympus BX51, Optical Co. Ltd, Tokyo, Japan). Digital images with 400× magnification were examined. The scores for necrosis, inflammation and fibrosis were determined by counting the associated cells and their ratio to the total cell count. Regarding to the staining percentage, which they comprise in the digital image, these findings were scored as–(0%), 1 (+) (5%–30%), 2 (++) (30%–60%) or 3 (+++) (> 60%) by analySIS FIVE image analysis software. (Olympus, Tokyo, Japan). Three different parts of one and the same block section (0.14 mm2 at 400× magnification) were determined. The most central stained section that represented the maximum diameter of the defect was selected in each block. It was not possible to capture the entire defect in one image at the level of magnification that was used, therefore it was obtained from three different parts of the block.[Citation33]
Table 2. Allen's fracture of healing scoring system.
Table 3. Comparison of bone healing, inflammation, necrosis and fibrosis values of 8th day sacrification groups.
Table 4. Comparison of bone healing, inflammation, necrosis and fibrosis values of 15th day sacrification groups.
Statistical analysis
The statistical analysis was carried out using the Statistical Package for Social Sciences (SPSS, version 17.0). Study and control groups were compared statistically with Mann–Whitney U test, and mean differences in each group were analysed by Wilcoxon signed-rank test with a confidence interval of 95 % (P < 0.05).
Results and discussion
Many therapeutic approaches are being studied to promote cell biostimulation and to improve the regenerative capacity of bone and soft tissues.[Citation34–37] Thus, photobiomodulation therapy, applied by LLL or LED, is considered as a way to accelerate and improve bone tissue regeneration in the treatment of clinical conditions that require tissue regeneration.[Citation18] The present study was conducted to observe the influence of LPT on bone healing in the surgically created rat calvarial defects filled with FDBA or without FDBA. To our knowledge, this is the first histological report on the use of the association of LED light and FDBA.
In the present study 28 rats were used. No postoperative complications were observed, yielding a total of 56 bone defects for final analysis. Calvarial soft tissue healing progressed without any sign of infection. No weight loss and no side effects, such as behavioural changes, or features of pain, were observed.
– show the characteristic histological sections for all of the groups at the end of the first week. Bone healing of the non-grafted site in Group I was significantly higher than the non-grafted site in Group II (P < 0.05). The inflammation of the non-grafted site in Group I was significantly lower than the inflammation of the non-grafted site in Group II (P < 0.05). We did not observe the same significance, concerning the inflammation rate in the grafted side of Group I compared to grafted side of Group II. We supposed that graft itself increased the inflammation rate. Fibrosis in the grafted site in Group I was significantly lower than the grafted site in Group II (P < 0.05) (). We supposed that the presence of bone grafts prevented fibrosis in the early stage and LED did not have any effect on it, because the non-grafted side of Group I and II showed no differences on fibrosis. Necrosis was present on the 8th day in both groups–grafted or non-grafted, but it was not notable.
Figure 1. Seventh day grafted defect with LPT in Group I.
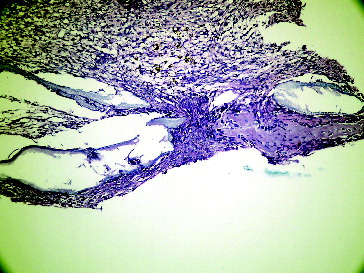
Figure 2. Seventh day empty defect with LPT in Group I.
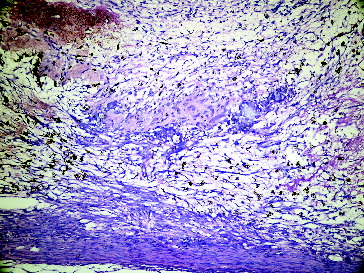
Figure 3. Seventh day grafted defect in Group II.
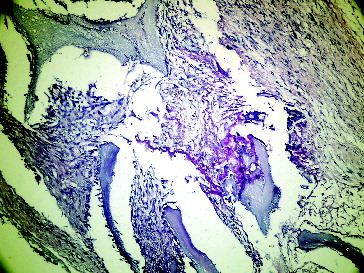
Figure 4. Seventh day empty defect in Group II.
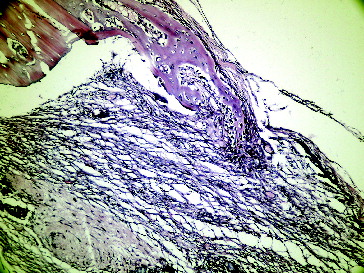
– show the characteristic histological sections for all of the groups at the end of the second week. Bone healing on the non-grafted site in Group I was significantly higher than the non-grafted site in Group II (P < 0.05). Bone healing of the grafted side in Group I was higher than that in Group II, although the difference was not significant. The inflammation of the grafted side in Group I was significantly higher than that in the grafted side in Group II (P < 0.05), suggesting that the inflammation is still proceeding. Fibrosis in the grafted side of Group I was higher when compared with the grafted side of Group II, but no significant differences were detected. There were not significant differences regarding necrosis (). Undistinguishable rates of necrosis provided a healthy healing period in both groups.
Figure 5. Fourteenth day grafted defect with LPT in Group I.
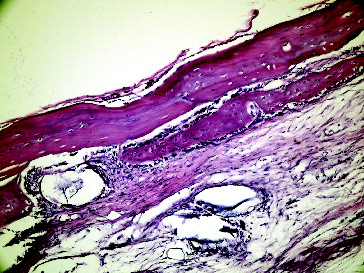
Figure 6. Fourteenth day empty defect with LPT in Group I.
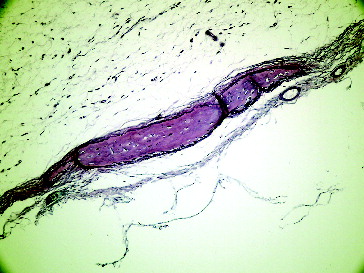
Figure 7. Fourteenth day grafted defect in Group II.
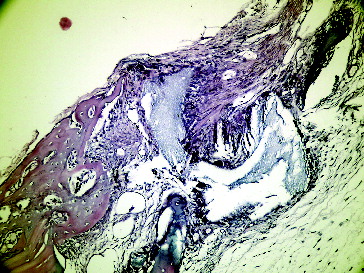
Figure 8. Fourteenth day empty defect in Group II.
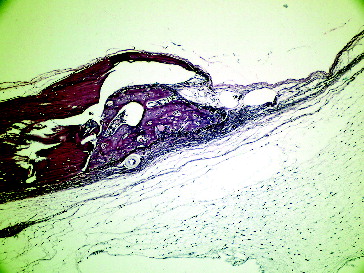
If we compare the 8th day and the 15th day sacrification periods, in Group I, the grafted site revealed a significant bone formation from the 8th day to the 15th day (P < 0.05). The bone healing in non-grafted site in Group I was higher on the 15th day compared to the 8th day (), but the difference was not statistically significant. In Group II, bone healing in both grafted and non-grafted sites was significantly increased from the 8th day until the 15th day (P < 0.05). In Group II, inflammation in both grafted and non-grafted sites significantly decreased from the 8th day until the 15th day (P < 0.05). In the present study, fibrosis was significantly increased from 8th day to 15th day in the grafted LPT group. Furthermore, it was determined that the bone healing was also significantly increased in the same period and in the same group. This immature bone shows low levels of formation of bone matrix, which incorporates with FDBA, characterizing bone maturation.
These findings suggest that the rate of bone regeneration was significantly higher in the non-grafted-LPT group (Group I) than in non-grafted and not received LPT group (Group II). Furthermore, enhanced bone healing was observed in the grafted-LPT group when compared with grafted-non-irradiated group, but no significant differences were detected. These findings may indicate an increased activity of irradiated osteoblasts in bone tissue. This is because in the early stages of bone repair, the cellular component (fibroblasts and osteoblasts) is more prominent and more prone to be affected by the light.[Citation16,Citation38,Citation39] The deposition of bone matrix starts later and becomes the main component of the repairing tissue.[Citation7]
Studies on the effects of laser light on bone regeneration have indicated that its effect on bone healing depends not only on the total dose of irradiation, but also on the irradiation time and the irradiation mode.[Citation7,Citation14,Citation17,Citation19–23,Citation40–46] In the literature, there are different LPT protocols concerning time intervals and durations. Soares et al.,[Citation6] Pinheiro et al. [Citation24] and Aciole et al. [Citation47] reported that they applied LPT at 48-h intervals for two weeks. On the other hand, Uysal et al. [Citation18] applied LPT at 24-h intervals for 10 consecutive days and Casalechi et al. [Citation48] preferred to apply LPT every 24-h for 7, 14 and 21 days periods, according to their study groups. All of these referred authors suggested that LPT significantly increased bone regeneration. In the present study LPT was applied at 24-h intervals for 20 minutes per session for 7 and 14 consecutive days. Consistent with the previous studies, high bone regeneration values were obtained in the present study.
The wavelength delivered by the OsseoPulse® LED device in the present study was 618 nm with 20 mW output power, which was close to that used in LLL (600–1000 nm) with similar energy. It has been shown that both LED and LLL radiations result in photobiomodulation effects.[Citation9,Citation49] The energy density used in this study was 5 J/cm2, which is aligned with several previous reports, suggesting that 1–5 J/cm2 is effective to induce positive effects on both bone and soft tissues.[Citation6,Citation20] Our results showed an increase in the formation of bone matrix stimulated by LPT.
The mechanism by which LPT produces its biological effects remains to be elucidated. Several in vitro and in vivo studies have demonstrated that light stimulates the cytochrome C oxidase, a major photo acceptor molecule, causing increased energy metabolism and production and stimulation of mitochondrial oxidative metabolism. Better cell function especially in cells with a suboptimal metabolic condition which produces ATP is provided by cytochrome C oxidase. Acceleration of mitoses, improvement of tissue repair, stimulation of bone repair, balancing of the production of fibroblasts with normalization of collagen and elastic fibres deposited in the repairing tissue and increment of the peripheral blood circulation, improving anti-inflammatory action and tissue healing are all supplied by ATP.[Citation18,Citation37]
FDBA has been used extensively in oral surgery.[Citation50–52] This material is composed of crushed cortical and cancellous bone. Its ‘dual’ nature seems to contribute to the attainment of an excellent biological response after grafting. The mineralized cancellous particles may provide a rapidly resorbable, osteoconductive scaffold for the ingrowth of bone cells and angiogenesis, leading to optimal bone remodelling. On the other hand, mineralized cortical chips may offer an adequate structure to maintain the space due to its size and slower resorption rate.[Citation51] In the present study, histological analyses revealed that allograft residual particles were integrated into the tissue of the host with no signs of inflammation or foreign body reaction. Most allograft particles were in direct contact with well-organized lamellar bone, which is an evidence of the biocompatibility and osteoconductivity of this material. Furthermore, the rate of bone regeneration in the grafted-Group I was higher than the grafted-Group II, but the difference was not statistically significant.
In the present study, the authors questioned the effect of LPT on bone regeneration rate during the early stages of healing. Based on histological observations, fracture healing (secondary healing) in human occurs in four overlapping phases, including the hematoma formation phase, early inflammatory phase (2–4 weeks), repair phase (proliferation and differentiation, which is within 1–2 months) and a late remodelling phase, which lasts for months or years.[Citation53,Citation54] In the present study, we evaluated the effect of LPT on the early inflammatory phase, in which the osteoblastic activity had just started with the formation of bone matrix. These osteoblasts were less in number and meaningless to be counted. Therefore a seven-point scoring (modified Allen's scoring) system was used to assess bone healing. Furthermore, the authors propose additional latent period of time to get more predictable results.
The interpretation of differences between reported studies for LED arrays may be related to the difference in parameters, graft types and animal models used, creating difficulties in hindering the comparison of treatment outcomes and extrapolation to the clinical practice in humans.[Citation35] The question here shall be identifying the optimal light and experimental parameters to obtain the optimal biological outcomes.
As a major limitation of this study, additional data from in vivo human trials are required to validate this assumption. Additionally, long term viability and mechanical properties of the LPT treated and FDBA grafted bone defect sites should be further evaluated to contribute to a better understanding of the long-term clinical performance of this treatment modality.
Conclusions
As a conclusion, the present preliminary data suggested that LPT with a wavelength of 618 nm might have a positive effect on the early phase of wound healing of bone defects with or without the use of FDBA. The use of photobiomodulation therapy, applied by LED, was suggested as a way of accelerating and improving the bone tissue healing process in order to reduce the healing time.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Desmet KD, Paz DA, Corry JJ, Eells JT, Wong-Riley MT, Henry MM, Buchmann EV, Connelly MP, Dovi JV, Liang HL, Henshel DS, Yeager RL, Millsap DS, Lim J, Gould LJ, Das R, Jett M, Hodgson BD, Margolis D, Whelan HT. Clinical and experimental applications of NIR-LED photobiomodulation. Photomed Laser Surg. 2006;24:121–128.
- Conlan MJ, Rapley JW, Cobb CM. Biostimulation of wound healing by low-energy laser irradiation. A review. J Clin Periodontol. 1996;23:492–496.
- Sommer AP, Pinheiro AL, Mester AR, Franke RP, Whelan HT. Biostimulatory windows in low-intensity laser activation: lasers, scanners, and NASA's light-emitting diode array system. J Clin Laser Med Surg. 2001;19:29–33.
- Whelan HT, Smits RL, Buchman EV, Whelan NT, Turner SG, Margolis DA, Cevenini V, Stinson H, Ignatius R, Martin T, Cwiklinski J, Philippi AF, Graf WR, Hodgson B, Gould L, Kane M, Chen G, Caviness J. Effect of NASA light-emitting diode irradiation on wound healing. J Clin Laser Med Surg. 2001;19:305–314.
- Yu W, Naim JO, Lanzafame RJ. The effect of laser irradiation on the release of bFGF from 3T3 fibroblasts. J Clin Laser Med Surg. 1997;20:55–63.
- Soares LG, Marques AM, Aciole JM, da Guarda MG, Cangussú MC, Silveira L Jr., Pinheiro AL. Do laser/LED phototherapies influence the outcome of the repair of surgical bone defects grafted with biphasic synthetic microgranular HA + β-tricalcium phosphate? A Raman spectroscopy study. Lasers Med Sci. 2014;29:1575–1584.
- Pinheiro AL, Gerbi ME. Photoengineering of bone repair processes. Photomed Laser Surg. 2006;24:169–178.
- Karu TI, Pyatibrat LV, Afanasyeva NI. A novel mitochondrial signaling pathway activated by visible–to-near infrared radiation. Photochem Photobiol. 2004;80:366–372.
- Karu TI, Pyatibrat LV, Kolyakov SF, Afanasyeva NI. Absorption measurements of a cell monolayer relevant to phototherapy: reduction of cytochrome c oxidase under near IR radiation. J Photochem Photobiol. B 2005;81:98–106.
- Wong-Riley MT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, Kane M, Whelan HT. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280:4761–4771.
- El-Bialy T, Alhadlaq A. New therapeutics in promoting and modulating mandibular growth in cases with mandibular hypoplasia. Biomed Res Int. 2003;2003:789679.
- Pinheiro AL, Aciole GT, Cangussú MC, Pacheco MTT, Silveira L. Effects of laser phototherapy on bone defects grafted with mineral trioxide aggregate, bone morphogenetic proteins, and guided bone regeneration: a Raman spectroscopic study. J Biomed Mater Res A. 2010;15:1041–1047.
- Lopes CB, Pacheco MT, Silveira L Jr, Cangussú MC, Pinheiro AL. The effect of the association of near infrared laser therapy, bone morphogenetic proteins, and guided bone regeneration on tibial fractures treated with internal rigid fixation: a Raman spectroscopic study. J Biomed Mater Res. A 2010;94:1257–1263.
- Torres CS, Santos JN, Monteiro JS, Amorim PG, Pinheiro AL. Does the use of laser photobiomodulation, bone morphogenetic proteins, and guided bone regeneration improve the outcome of autologous bone grafts? An in vivo study in a rodent model. Photomed Laser Surg. 2008;26:371–377.
- Soares LG, Magalhães EB, Magalhães CA, Ferreira CF, Marques AM, Pinheiro AL. New bone formation around implants inserted on autologous and xenografts irradiated or not with IR laser light: a histomorphometric study in rabbits. Braz Dent J. 2013;24:218–223.
- Soares LG, Marques AM, Barbosa AF, Santos NR, Aciole JM, Souza CM, Pinheiro AL, Silveira L Jr. Raman study of the repair of surgical bone defects grafted with biphasic synthetic microgranular HA + β-calcium triphosphate and irradiated or not with λ 780 nm laser. Lasers Med Sci. 2014;29:1539–1550.
- Lopes CB, Pinheiro AL, Sathaiah S, Da Silva NS, Salgado MA. Infrared laser photobiomodulation (λ 830 nm) on bone tissue around dental implants: a Raman spectroscopy and scanning electronic microscopy study in rabbits. Photomed Laser Surg. 2007;25:96–101.
- Uysal T, Ekizer A, Akcay H, Etoz O, Guray E. Resonance frequency analysis of orthodontic miniscrews subjected to light-emitting diode photobiomodulation therapy. Eur J Orthod. 2012;34:44–51.
- Weber JBB, Pinheiro AL, de Oliveira MG, Oliveira FA, Ramalho LM. Laser therapy improves healing of bone defects submitted to autogus bone graft. Photomed Laser Surg. 2006;24:38–44.
- Pinheiro AL, Martinez Gerbi ME, de Assis Limeira F Jr, Carneiro Ponzi EA, Marques AM, Carvalho CM, de Carneiro Santos R, Oliveira PC, Nóia M, Ramalho LM. Bone repair following bone grafting hydroxyapatite guided bone regeneration and infra-red laser photobiomodulation: a histological study in a rodent model. Lasers Med Sci. 2009;24:234–240.
- Gerbi ME, Marques AM, Ramalho LM, Ponzi EA, Carvalho CM, Santos R de C, Oliveira PC, Nóia M, Pinheiro AL. Infrared laser light further improves bone healing when associated with bone morphogenic proteins: an in vivo study in a rodent model. Photomed Laser Surg. 2008;26:55–60.
- Pinheiro AL, Martinez Gerbi ME, Carneiro Ponzi EA, Pedreira Ramalho LM, Marques AM, Carvalho CM, Santos R de C, Oliveira PC, Nóia M. Infrared laser light further improves bone healing when associated with bone morphogenetic proteins and guided bone regeneration: an in vivo study in a rodent model. Photomed Laser Surg. 2008;26:167–174.
- Gerbi ME, Pinheiro AL, Ramalho LM. Effect of IR laser photobiomodulation on the repair of bone defects grafted with organic bovine bone. Lasers Med Sci. 2008;23:313–317.
- Pinheiro AL, Soares LG, Cangussú MC, Santos NR, Barbosa AF, Siveira Júnior L. Effects of LED phototherapy on bone defects grafted with MTA, bone morphogenetic proteins and guided bone regeneration: a Raman spectroscopic study. Lasers Med Sci. 2012;27:903–916.
- Iwama T, Yamada J, Imai S, Shinoda J, Funakoshi T, Sakai N. The use of frozen autogenous bone flaps in delayed cranioplasty revisited. Neurosurgery. 2003;52:591–596.
- Mercer C. Lasers in dentistry: a review part 1. Dent Update. 1996;23:74–80.
- Havlucu U, Bölükbaşı N, Yeniyol S, Cetinel S, Ozdemir T. Effects of LPT and BioOss® as single and combined treatment in an experimental model of bone defect healing in rats. J Oral Implantol. Forthcoming 2015.
- Johansson B, Grepe A, Wannfors K, Hirsch JM. A clinical study of changes in the volume of bone grafts in the atrophic maxilla. Dentomaxillofac Radiol. 2001;30:157–161.
- Raghoebar GM, Meijndert L, Kalk WW, Vissink A. Morbidity of mandibular bone harvesting: a comparative study. Int J Oral Maxillofac Implants. 2007;22:359–365.
- Tabrizi R, Khorshidi H, Shahidi S, Gholami M, Kalbasi S, Khayati A. Use of lincomycin-impregnated demineralized freeze dried bone allograft in the periodontal defect after third molar surgery. J Oral Maxillofac Surg. 2014;72:850–857.
- Viswambaran M, Arora V, Tripathi RC, Dhiman RK. Clinical evaluation of immediate implants using different types of bone augmentation materials. Med J Armed Forces India. 2014;70:154–162.
- Allen HL, Wase A, Bear WT. Indomethacin and asprin: effect of nonsteroidal anti-inflammatory agents on the rate of fracture repair in rat. Acta Orthop. 1980;51:595–600.
- Arslan A, Altundal H, Cevik O, Olgac V. Comparison of the effects of local application of hydroxyapatite graft soaked with alendronate solution and pure hydroxyapatite graft in the mandible of ovariectomised rats. Biotechnol & Biotechnol Equip. 2011;25(3):2513–2518.
- Hübler R, Blando E, Gaião L, Kreisner PE, Post LK, Xavier CB, de Oliveira MG. Effects of low-level laser therapy on bone formed after distraction osteogenesis. Lasers Med Sci. 2010;25:213–219.
- Khadra M, Rønold HJ, Lyngstadaas SP, Ellingsen JE, Haanæs HR. Low-level laser therapy stimulates bone-implant interaction: an experimental study in rabbits. Clin Oral Implants Res. 2004;15:325–332.
- Anders JJ, Geuna S, Rochkind S. Phototherapy promotes regeneration and functional recovery of injured peripheral nerve. Neurol Res. 2004;26:233–239.
- Kawasaki K, Shimizu N. Effects of low-energy laser irradiation on bone remodeling during experimental tooth movement in rats. Lasers Surg Med. 2000;26:282–291.
- Soares LGP, Marques AMC, Guarda MG, Aciole JMS, Pinheiro ALB, Santos JN. Repair of surgical bone defects grafted with hydroxylapatite + β-TCP and irradiated with λ; = 850 nm LED Light. Braz. Dent. J. 2015;26(1):19–25.
- Pinheiro AZB, Marques AMC, Soares LGP, Barbosa AFS. Bone biomodulation. In: Reitas P, Simoes A, editors. Lasers in dentistry: guide for clinical practice. Oxford: Wiley & Sons, Inc.; 2015. p. 196–206.
- Lopes CB, Pacheco MT, Silveira L Jr, Duarte J, Cangussú MC, Pinheiro AL. The effect of the association of NIR laser therapy BMPs, and guided bone regeneration on tibial fractures treated with wire osteosynthesis: Raman spectroscopy study. J Photochem Photobiol B. 2007;89:125–130.
- Lopes CB, Pinheiro AL, Sathaiah S, Duarte J, Cristinamartins M. Infrared laser light reduces loading time of dental implants: a Raman spectroscopic study. Photomed Laser Surg. 2005;23:27–31.
- Gerbi ME, Pinheiro AL, Ramalho L Marzola C, Limeira FA Jr, Ponzi EA, Soares AO, Carvalho LC, Lima HV, Gonçalves TO. Assessment of bone repair associated with the use of organic bovine bone and membrane irradiated at 830 nm. Photomed Laser Surg. 2005;23:382–388.
- Pinheiro ALB, Limeira FA Jr, Gerbi MEM, Ramalho LMP, Marzola C, Ponzi EAC. Effect of low level laser therapy on the repair of bone defects grafted with inorganic bovine bone. Braz Dent J. 2003;14:177–181.
- Pinheiro ALB, Limeira FA Jr, Gerbi MEM, Ramalho LMP, Marzola C, Ponzi EAC, Soares AO, De Carvalho LCB, Lima HCV, Gonçalves TO. Effect of 830-nm laser light on the repair of bone defects grafted with inorganic bovine bone and decalcified cortical osseous membrane. J Clin Laser Med Surg. 2003;21:383–388.
- Pinheiro AL, Oliveira MA, Martins PP. Biomodulação da cicatrização óssea pós-implantar com o uso da laserterapia não-cirúrgica: estudo por microscopia eletrônica de varredura [Biomodulation of peri-implant bone repair with laser therapy: SEM study]. Rev FOUFBA. 2001;22:12–19. Portuguese.
- Silva N Jr, Pinheiro AL, Oliveira MG, Weismann R, Ramalho LMP, Nicolau RA. Computerized morphometric assessment of the effect of low-level laser therapy on bone repair: an experimental animal study. J Clin Laser Med Surg. 2002;20:83–87.
- Aciole JM, de Castro IC, Soares LG, Barbosa AF, Aciole GT, Silveira L Jr, Pinheiro AL. Assessment of the LED phototherapy on femoral bone defects of ovariectomized rats: a Raman spectral study. Lasers Med Sci. 2014;29:1269–1277.
- Casalechi HL, Nicolau RA, Casalechi VL, Silveira L Jr, De Paula AM, Pacheco MT. The effects of low-level light emitting diode on the repair process of Achilles tendon therapy in rats. Lasers Med Sci. 2009;24:659–665.
- Eells JT, Wong-Riley MT, VerHoeve J, Henry M, Buchman EV, Kane MP, Gould LJ, Das R, Jett M, Hodgson BD, Margolis D, Whelan HT. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion. 2004;4:559–567.
- Saad AY, Abdellatief EM. Healing assessment of osseous defects of periapical lesions associated with failed endodontically treated teeth with use of freeze-dried bone allograft. Oral Surg Oral Med Oral Pathol. 1991;71:612–617.
- Avila G, Neiva R, Misch CE, Galindo-Moreno P, Benavides E, Rudek I, Wang HL. Clinical and histologic outcomes after the use of a novel allograft for maxillary sinus augmentation: a case series. Implant Dent. 2010;19:330–341.
- Gapski R, Misch C, Stapleton D, Mullins S, Cobb C, Vansanthan A, Reissner M. Histological, histomorphometric, and radiographic evaluation of a sinus augmentation with a new bone allograft: a clinical case report. Implant Dent. 2008;17:430–438.
- Estai MA, Soelaiman IN, Shuid AN, Das S, Ali AM, Suhaimi FH. Histological changes in the fracture callus following the administration of water extract of Piper Sarmentosum (Daun Kadok) in estrogen-deficient rats. Iran J Med Sci. 2011;36(4):281–288.
- Harwood PJ, Newman JB, Michael ALR. (ii) An update on fracture healing and non-union. J Orthop Trauma. 2010;24:9–23.
