?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Regenerating islet-derived 3-alpha (REG3α) is a secreted intestinal antimicrobial protein, which shows antibacterial, anti-inflammatory and anti-apoptotic activities. It could significantly promote internal tissue regeneration and wound repair. In the present study, we expressed the codon-optimized murine REG3α in the yeast Pichia pastoris system. The secreted murine REG3α was captured using ProteinIsoTM Ni-NTA resin and further purified using a strong anion exchange resin Poros® 50 HQ. The final protein yield was 20 mg/L. The antibacterial activity and the anticancer efficacy of the recombinant REG3α were assessed using liquid growth inhibition assay, killing kinetics and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. The results showed that the recombinant murine REG3α possessed antimicrobial activity against Escherichia coli, Salmonella paratyphi A, Staphylococcus aureus, Staphylococcus epidermidis, Bacillus subtilis, Bacillus pumilus and Micrococcus luteus. Moreover, 100% killing against E. coli and S. aureus was observed after 30 min. The recombinant murine REG3α had a potent antitumour activity in a time-dependent and dose-dependent manner and had a negligible haemolysis activity against human erythrocytes. Taken together, P. pastoris is an efficient expression system for producing large quantities of antibacterial active REG3α for further research studies and clinical applications.
Introduction
Regenerating islet-derived protein (REG), which belongs to the family of C-type lectins, is a protein, first isolated from pancreatic calculi of men, suffering from chronic calcifying pancreatitis in 1979.[Citation1] Based on the primary structure of the proteins, the members of the family have been classified into four subgroups: REGI, REGII, REGIII and REGIV.[Citation2] The REGIII subclass family comprises REG3α, REG3β, REG3γ and REG3δ. REGIII has been reported to possess anti-inflammatory and anti-apoptotic activity and the ability to promote wound repair.[Citation3] REGIII can influence tissue regeneration after several tissue injuries, such as mucosal damage,[Citation4] liver failure,[Citation5] intestinal epithelial and Paneth cells impairment,[Citation6] as well as pancreatic β-cells damage.[Citation7] Furthermore, human REG3α (REG3A) and mouse REG3γ exhibit antibacterial activity by destroying the bacterial cell wall.[Citation6, Citation8] In addition, REG3A was found to be down-regulated in primary human gastric cancers, but to be up-regulated in primary HCCs.[Citation9–12] REGIII may have a significant application value in clinical studies.
The high cost of chemical synthesis and extraction is one of the limiting factors that hamper the use of REG3α as a therapeutic agent. In recent years, the expression of recombinant proteins by using micro-organisms as host cells has become a more efficient alternative method. Recombinant human REG3A protein was expressed in Escherichia coli expression system,[Citation13] which exhibited a fast and robust growth in bioreactors using a simple media. However, the recombinant protein existed in the form of inclusion bodies and the post-purification process was complicated. The eukaryotic expression system of Pichia pastoris has numerous advantages. Some of them include high-level and large-scale expression, cheap and easy purification, and post-translational modifications.[Citation14, Citation15] Based on the above, P. pastoris may be the best choice for the efficient expression of the murine REG3α.
In this study, in order to achieve an efficient expression of REG3α, pwPICZalpha expression vector was used to produce murine REG3α in a P. pastoris expression system. The study will lay the foundations for further development of a protein drug that might be used as a treatment for bacterial infections and cancer.
Materials and methods
Construction of plasmids encoding REG3α
The codon-optimized gene was designed based on the gene sequence of REG3α (Genbank accession NM_011259.1) according to the codon bias of P. pastoris.[Citation16] To facilitate the downstream purification, six histidines (6 × His tag) were added to the C-terminus. The entire REG3α gene, carrying XhoI and EcoRI restriction sites at each end, was synthesized by Invitrogen. The synthesized REG3α was cut out using XhoI and EcoRI restriction enzymes and cloned into a digested pwPICZalpha vector. Next, double digestion and DNA sequencing were used to confirm the final construction of REG3α in the pwPICZalpha vector. The resulting plasmid was designated as pwPICZalpha-REG3α.
REG3α expression and purification
The pwPICZalpha-REG3α was linearized by SacI digestion and was transformed into P. pastoris strain X33 using the gene transfection instrument (NingBo Scientz Biotechnology Co., Ltd, Ningbo, China). The transformants were selected on yeast extract–peptone–dextrose (YPD) agar plates (10 g/L yeast extract, 20 g/L peptone, 15 g/L agar and 20 g/L dextrose) containing 100 μg/mL of zeocin. The positive transformants were cultivated on YPD (10 g/L yeast extract, 20 g/L peptone and 20 g/L dextrose) and incubated for 48 h at 25 °C, 225 rpm, as a seed culture. Next, 5% of the seed culture were transferred into 250 mL YPD and were cultured at 30 °C, 250 rpm. After 24 h the YPD was replaced with 250 mL yeast extract–peptone–glycerol (10 g/L yeast extract, 20 g/L peptone and 10 g/L glycerol). We continued to culture the yeasts at 30 °C at 250 rpm for another 24 h. Subsequently, the yeasts were centrifuged at 3000 rpm for 10 min and the pellet was resuspended in 125 mL buffered methanol-complex medium (BMMYC) (10 g/L yeast extract, 20 g/L peptone, 100 mmol/L potassium phosphate, pH 7.0, 13.4 g/L yeast nitrogen base without amino acids, 0.04 g/L biotin, 10 mL/L methanol and 10 g/L bacto casamino acids) and induced at 25 °C, 225 rpm for 48 h. We added methanol every 12 h to sustain a 0.5% level.[Citation17] The supernatant, containing the recombinant murine REG3α, was collected for protein purification after centrifugation at 3000 rpm at 4 °C for 10 min.
The culture supernatant was loaded on a ProteinIsoTM Ni-NTA resin column (TansGen, Beijing, China), pre-equilibrated with 10 column volumes (CV) of 50 mmol/L sodium phosphate, 0.3 mol/L NaCl, 10 mmol/L imidazole and 10 mmol/L Tris-HCl pH 8.0. The supernatant was eluted with 50 mmol/L sodium phosphate, 0.3 mol/L NaCl, 500 mmol/L imidazole and 10 mmol/L Tris-HCl pH 8.0 after an extensive washing with 50 mmol/L sodium phosphate, 0.3 mol/L NaCl, 10 mmol/L imidazole and 10 mmol/L Tris-HCl pH 8.0. The first purification fractions were analysed using sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). The fractions containing the protein of interest were pooled and dialysed using a 3.5 kDa cut-off Spectra/Por® membrane tubing (Spectrum Laboratories, Inc., CA, USA) against 20 mmol/L Tris-HCl pH 8.0, 1 mmol/L ethylenediaminetetraacetic acid (EDTA) pH 8.0, 50 mL/L glycerol at 4 °C. The dialysis buffer was replaced once.
The dialysed protein was loaded on a Strong anion exchange resin Poros® 50 HQ column (Applied Biosystems, CA, USA), pre-equilibrated with 20 mmol/L Tris-HCl pH 8.0, 1 mmol/L EDTA, 50 ml/L glycerol (10 CV), after this followed the washing of the column with 20 mmol/L Tris-HCl pH 8.0, 1 mmol/L EDTA, 5 ml/L glycerol (8 CV). The bound protein was eluted into seven fractions with 100 mmol/L sodium borate and six fractions 200 mmol/L sodium borate in 20 mmol/L Tris-HCl pH 8.0, 1 mmol/L EDTA and 5 mL/L glycerol. The purification fractions were analysed using SDS-PAGE and western blot with mouse anti-His monoclonal antibody. The recombinant murine REG3α was concentrated by ultra centrifugal filter units and dialysed using a 3.5 kDa cut-off Spectra/Por® membrane tubing against phosphate buffered saline (PBS) pH 7.4 at 4 °C. The concentration of the recombinant murine REG3α was determined by bicinchoninic acid protein assay kit (Beyotime, Haimen, China).
Antibacterial activity assays
The antibacterial activity of the recombinant murine REG3α was assessed by liquid growth inhibition assay against a panel of micro-organisms, including E. coli, Salmonella paratyphi A, Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus epidermidis, Bacillus subtilis, Bacillus pumilus and Micrococcus luteus. The determination of the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) was done by a modification of a previously described method by using different micro-organisms.[Citation18]
The bactericidal or bacteriostatic effect was demonstrated using killing kinetics against E. coli or S. aureus, as previously described.[Citation19] We incubated 20.30 μmol/L REG3α with the bacteria for 0, 10, 20, 30, 60, 90 and 120 min. At these time points, 5 μL from the mixture was serially diluted in PBS (pH 7.4) and was plated on nutrition broth agar. The plates were incubated at 37 °C for 24 h and the colonies were counted. The percentage of colony-forming units (CFU) was defined relative to the CFU obtained in the control (100% CFU at 0 min).
Cytotoxicity assay
The human hepatocellular carcinoma (HCC) cell line SMMC-7721 was treated with fresh medium, fresh medium containing the recombinant murine REG3α with different concentrations (0, 0.63, 1.27, 2.54, 5.08, 10.15 and 20.30 μmol/L) or PBS (pH 7.4), as a control, for 24, 48 and 72 h at 37 °C in a 5% CO2 humidified atmosphere. The results from each concentration were obtained in triplicate. After the treatment, the cytotoxic effect of REG3α to SMMC-7721 tumour cells was analysed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The percent inhibitory rate (IR%) of the treated cells was calculated by the formula:
(1)
(1)
The half-maximal inhibitory concentration (IC50) value for SMMC-7721 cell line was evaluated using GraphPad Prism 5 (GraphPad Software, Inc., CA, USA) and it was representing the concentration at which the viability of the tumour cells was reduced to 50%, compared with the untreated tumour cells.[Citation20,Citation21] The results are presented as means ± standard deviation.
Haemolytic activity
Human fresh blood was collected in a heparinized tube and centrifuged at 1500 rpm (800 g) for 10 min. The pellet was gently washed three times with cold PBS (pH 7.4) then the erythrocytes were resuspended in cold PBS (pH 7.4) and adjusted to 8%. About 100 µL of the erythrocyte suspension was added into a 96-well microtitre plate. Different concentrations (0.63, 1.27, 2.54, 5.08, 10.15, 20.30 and 40.60 μmol/L) of REG3α solution were added to each well and were incubated for 60 min at 37 °C. Triton-X 100 (0.2%) and PBS were used as positive and negative controls, respectively. The release of haemoglobin of the supernatant was measured after centrifugation [3000 rpm (1000 g) for 10 min] by microplate reader (EL × 808; Gene Co., Ltd., Hong Kong, China) at 490 nm.[Citation20]
Results and discussion
Expression and purification of the recombinant murine REG3α
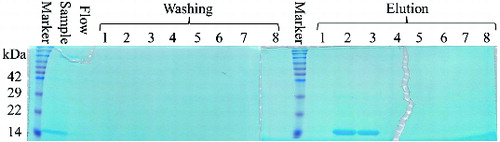
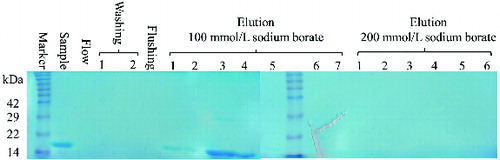
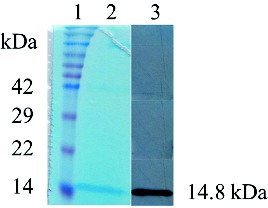
The yeast P. pastoris has been considered as a highly successful expression system for the production of a variety of heterologous proteins, because of its high production levels, easy manipulation, inexpensive growth media and post-translational modifications.[Citation22–25] Previous studies have produced vaccine, candidate therapeutic protein and antimicrobial peptide using the yeast P. pastoris.[Citation26–28] P. pastoris may be the best choice for the efficient expression of the murine REG3α. In our study, the murine REG3α carrying a 6 × His tag in the C-terminus was expressed using a shake-flask system. The supernatant containing the secreted REG3α was collected and captured directly by ProteinIsoTM Ni-NTA resin through its His-tags (). The second and third eluted fractions, containing REG3α, were collected and dialysed to remove the salts. Based on the anticipated isoelectric point (pI = 5.71), the second step purification was completed by a strong anion exchange resin Poros® 50 HQ. Sodium borate was used to separate the murine REG3α from the yeast host's protein and aggregates.[Citation29] The pure REG3α was obtained from the first to the fourth fractions with 100 mmol/L sodium borate (). The Western blot analysis using an anti-His monoclonal antibody confirmed the presence of the recombinant REG3α (). The REG3α band was detected with the expected size (∼14.8 kDa). These results demonstrated that the murine REG3α was successfully expressed and purified in P. pastoris system and we found that the P. pastoris expression system is on a higher level than E. coli's expression system.[Citation13] The final purification yield of murine REG3α was 20 mg/L from the original harvested supernatant in our study. Previously, Human Reg IV was expressed to a maximal expression level (20 mg/L) after 5% methanol induction.[Citation30] Apart from this study, there are no additional reports of the production of a Reg family protein by P. pastoris system.
Antimicrobial activity of the recombinant murine REG3α
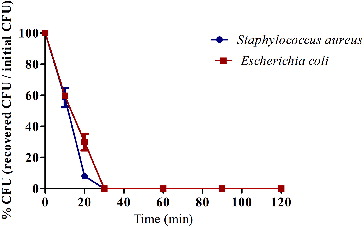
Human REG3A and mouse REG3γ have been reported to inhibit the growth of gram-positive bacteria,[Citation6] but whether other REGs possess antimicrobial activity was not clear. The antibacterial activity of the recombinant murine REG3α was evaluated against a panel of bacteria by using MIC and MBC assays. The recombinant murine REG3α showed antibacterial activity against E. coli, S. paratyphi A, S. aureus, S. epidermidis, B. subtilis, B. pumilus and M. luteus (). The killing kinetics results showed that approximately 40%, 70% and 100% of E. coli were killed after incubation with 20.30 μmol/L concentration of the recombinant murine REG3α for 10, 20 and 30 min, respectively. Approximately 40%, 92% and 100% of S. aureus were killed after incubation for 10, 20 and 30 min, respectively (). Our results showed that the recombinant murine REG3α possesses an antibacterial activity against gram-positive and gram-negative bacteria. Another study showed that RegIIIγ kills gram-positive bacteria by specifically binding with peptidoglycan.[Citation6] Further research is needed to demonstrate the inhibitory mechanism of REG3α.
Table 1. Antimicrobial activity of the recombinant murine REG3α.
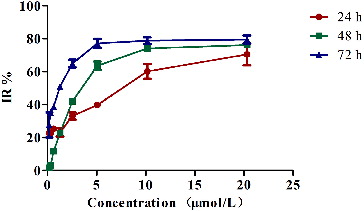
Antitumour efficacy of the recombinant murine REG3α
Human REG3A is overexpressed in liver carcinoma and is a paracrine hepatic growth factor promoting both proliferation and viability of liver cells in vivo.[Citation10] However, the studies for the murine REG3α are very few and the antitumour efficacy of this protein are still not clear. In our study, we assessed the efficacy of the recombinant murine REG3α to SMMC-7721 tumour cells using MTT assay at different time intervals (24, 48 and 72 h) of treatment. As shown in , the IR% of 20.30 μmol/L REG3α treatment was 70%, 76% and 79% at 24, 48 and 72 h, respectively. The results showed that the recombinant murine REG3α significantly inhibited the growth of SMMC-7721 tumour cells in both concentration-dependent and time-dependent manner. The IC50 values at 24, 48 and 72 h were 6.48, 3.75 and 1.01 μmol/L. Our results suggested that the recombinant murine REG3α may inhibit the growth of human HCC cells. The roles of the recombinant murine REG3α against human HCC were different from previous reports, related to the human REG3A. Moreover, the human REG3A is one of the few anti-acute liver failure (ALF) drug candidates and a free-radical scavenger that targets a broad spectrum of death effectors, favours liver regeneration and exhibits significant curative properties against ALF in mice.[Citation31]
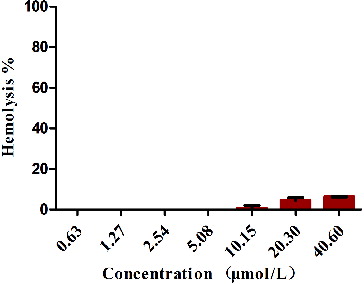
Haemolytic activity of recombinant murine REG3α
In our study, we have analysed the haemolytic activity of the recombinant murine REG3α by the level of human erythrocytes lysis. As shown in , no lysis of the human erythrocytes was observed at 0.63, 1.27, 2.54 and 5.08 μmol/L REG3α solution. Almost no lysis was observed at 10.15 μmol/L REG3α and even at 20.30 μmol/L, and 40.60 μmol/L concentration of REG3α solution, under which the protein exhibited a potent antimicrobial and antitumour activity. Hence, its haemolytic activity could be negligible at the tested concentrations. This haemolytic activity analysis indicated that the recombinant murine REG3α is safe to the host. We speculated that the recombinant murine REG3α would be an ideal anticancer and therapeutic agent for inhibiting microbial infections, without posing any risks of antibiotic resistance and other side effects to the host organisms.
Conclusions
In summary, we have successfully expressed and purified the recombinant murine REG3α in P. pastoris system. The recombinant murine REG3α possessed antibacterial activity against certain gram-positive and gram-negative bacteria in vitro. Moreover, it showed a strong activity against the human HCC cell lines SMMC-7721 with negligible haemolytic activity. This suggested that this protein may be a potential novel therapeutic agent to use in the treatment of bacterial infections or cancer.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- De Caro A, Lohse J, Sarles H. Characterization of a protein isolated from pancreatic calculi of men suffering from chronic calcifying pancreatitis. Biochem Biophys Res Commun. 1979;87(4):1176–1182.
- Nata K, Liu Y, Xu L, Ikeda T, Akiyama T, Noguchi N, Kawaguchi S, Yamauchi A, Takahashi I, Shervani NJ, Onogawa T, Takasawa S, Okamoto H. Molecular cloning, expression and chromosomal localization of a novel human REG family gene, REG III. Gene. 2004;340(1):161–170.
- Lai Y, Li D, Li C, Muehleisen B, Radek KA, Park HJ, Jiang Z, Li Z, Lei H, Quan Y, Zhang T, Wu Y, Kotol P, Morizane S, Hata TR, Iwatsuki K, Tang C, Gallo RL. The antimicrobial protein REG3A regulates keratinocyte proliferation and differentiation after skin injury. Immunity. 2012;37(1):74–84.
- Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci USA. 2005;102(1):99–104.
- Lieu HT, Batteux F, Simon MT, Cortes A, Nicco C, Zavala F, Pauloin A, Tralhao JG, Soubrane O, Weill B, Brechot C, Christa L. HIP/PAP accelerates liver regeneration and protects against acetaminophen injury in mice. Hepatology. 2005;42(3):618–626.
- Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313(5790):1126–1130.
- Okamoto H. The Reg gene family and Reg proteins: with special attention to the regeneration of pancreatic beta-cells. J Hepatobiliary Pancreat Surg. 1999;6(3):254–262.
- Abe M, Nata K, Akiyama T, Shervani NJ, Kobayashi S, Tomioka-Kumagai T, Ito S, Takasawa S, Okamoto H. Identification of a novel Reg family gene, Reg IIIdelta, and mapping of all three types of Reg family gene in a 75 kilobase mouse genomic region. Gene. 2000;246(1–2):111–122.
- Choi B, Suh Y, Kim WH, Christa L, Park J, Bae CD. Downregulation of regenerating islet-derived 3 alpha (REG3A) in primary human gastric adenocarcinomas. Exp Mol Med. 2007;39(6):796–804.
- Lasserre C, Christa L, Simon MT, Vernier P, Bréchot C. A novel gene (HIP) activated in human primary liver cancer. Cancer Res. 1992;52(18):5089–5095.
- Lasserre C, Simon MT, Ishikawa H, Diriong S, Nguyen VC, Christa L, Vernier P, Brechot C. Structural organization and chromosomal localization of a human gene (REG3A) encoding a C-type lectin overexpressed in primary liver cancer. Eur J Biochem. 1994;224(1):29–38.
- Keim V, Iovanna JL, Rohr G, Usadel KH, Dagorn JC. Characterization of a rat pancreatic secretory protein associated with pancreatitis. Gastroenterology. 1991;100(3):775–782.
- Gao Y, Wen WL, Shi R, Peng YF, Zhou LH, Ma WL. Expression and functional characterization of REG3A protein in Escherichia coli. Microbiol China. 2011;38(8):1249–1255.
- Cregg JM. Introduction: distinctions between Pichia pastoris and other expression systems. Methods Mol Biol. 2007;389:1–10.
- Brondyk WH. Selecting an appropriate method for expressing a recombinant protein. Methods Enzymol. 2009;463:131–147.
- Sreekrishna K. Strategies for optimizing protein expression and secretion in the methylotrophic yeast Pichia pastoris. In: Baltz RH, Hegeman G, Skatrud PL, editors. Industrial microorganisms: basic and applied molecular genetics. Abingdon: Taylor & Francis; 1993. p. 119–126.
- Hermanrud CE, Lucas CL, Sykes M, Huang CA, Wang Z. Expression and purification of soluble murine CD40L monomers and polymers in yeast Pichia pastoris. Protein Expr Purif. 2011;76(1):115–120.
- Peng H, Liu HP, Chen B, Hao H, Wang KJ. Optimized production of scygonadin in Pichia pastoris and analysis of its antimicrobial and antiviral activities. Protein Expr Purif. 2012;82(1):37–44.
- Qu H, Chen B, Peng H, Wang K. Molecular cloning, recombinant expression, and antimicrobial activity of EC-hepcidin3, a new four-cysteine hepcidin isoform from Epinephelus coioides. Biosci Biotechnol Biochem. 2013;77(1):103–110.
- Yan JX, Wang KR, Chen R, Song JJ, Zhang BZ, Dang W, Zhang W, Wang R. Membrane active antitumor activity of NK-18, a mammalian NK-lysin-derived cationic antimicrobial peptide. Biochimie. 2012;94(1):184–191.
- Luo Y, Sun Z, Li Y, Liu L, Cai X, Li Z. Caudatin inhibits human hepatoma cell growth and metastasis through modulation of the Wnt/beta-catenin pathway. Oncol Rep. 2013;30(6):2923–2928.
- Hollenberg CP, Gellissen G. Production of recombinant proteins by methylotrophic yeasts. Curr Opin Biotechnol. 1997;8(5):554–560.
- Cregg JM, Cereghino JL, Shi J, Higgins DR. Recombinant protein expression in Pichia pastoris. Mol Biotechnol. 2000;16(1):23–52.
- Cereghino JL, Cregg JM. Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol Rev. 2000;24(1):45–66.
- Daly R, Hearn MT. Expression of heterologous proteins in Pichia pastoris: a useful experimental tool in protein engineering and production. J Mol Recognit. 2005;18(2):119–138.
- Li K, Gao H, Gao L, Qi X, Gao Y, Qin L, Wang Y, Wang X. Recombinant GP90 protein expressed in Pichia pastoris induces a protective immune response against reticuloendotheliosis virus in chickens. Vaccine. 2012;30(13):2273–2281.
- Peraino JS, Schenk M, Zhang H, Li G, Hermanrud CE, Neville DM Jr, Sachs DH, Huang CA, Duran-Struuck R, Wang Z. A truncated diphtheria toxin based recombinant porcine CTLA-4 fusion toxin. J Immunol Methods. 2013;391(1-2):103–111.
- Fan K, Jiang J, Wang Z, Fan R, Yin W, Sun Y, Li H. Expression and purification of soluble porcine cystatin 11 in Pichia pastoris. Appl Biochem Biotechnol. 2014;174(5):1959–1968.
- Woo JH, Neville DM Jr. Separation of bivalent anti-T cell immunotoxin from Pichia pastoris glycoproteins by borate anion exchange. Biotechniques. 2003;35(2):392–398.
- Li A, Crimmins DL, Luo Q, Hartupee J, Landt Y, Ladenson JH, Wilson D, Anant S, Dieckgraefe BK. Expression of a novel regenerating gene product, Reg IV, by high density fermentation in Pichia pastoris: production, purification, and characterization. Protein Expr Purif. 2003;31(2):197–206.
- Moniaux N, Song H, Darnaud M, Garbin K, Gigou M, Mitchell C, Samuel D, Jamot L, Amouyal P, Amouyal G, Bréchot C, Faivre J. Human hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein cures fas-induced acute liver failure in mice by attenuating free-radical damage in injured livers. Hepatology. 2011;53(2):618–627.