Abstract
A total of eight cultivable purified Egyptian yeast phenotypes were isolated from different sources, including fruits, juices and paste, and were compared to a baker's yeast factory reference strain. Genotypic characterization of the most promising new isolate (RO1) confirmed its identification as a Saccharomyces cerevisiae strain. In a shake-flask experiment, the Plackett–Burman multi-factorial design was applied to identify factors that considerably affect the RO1 growth rate. Together with the components of the factory molasses-based medium, six other culture factors, hypothesized to affect yeast biomass production, were examined as independent variables. The calculated main effect results and P-values suggested that by increasing the level of molasses, diammonium phosphate and inoculum size, compared to the factory settings, and by supplementing the medium with yeast extract, calcium pantothenate (vitamin B5) and trace elements, the RO1 biomass production was improved. Application of the predicted near-optimum fermentation conditions with scaling up the culture medium to 22 L in a 40 L airlift bioreactor resulted in 93 g L−1 biomass production, which represented approximately a 1.5-fold increase, when compared to the reference culture condition. Moreover, the dough raising test indicated that the newly isolated yeast strain RO1 caused a 1.75-fold increase in the fermentative power, when compared to the factory reference strain.
Introduction
Many yeast strains, which belong to the genera Saccharomyces, Kluyveromyces, Candida and Rhodotorula, are characterized by their ability to utilize cheap raw materials. They are also known for their rapid growth rates, lack of pathogenicity, high protein content, genetic stability and easy digestion to give high nutritional values.[Citation1,Citation2] These useful physiological properties allowed a wide variety of biotechnological applications of yeasts, such as in many food and beverage industries, in bread making, in single cell protein production and in eukaryotic gene expression systems.[Citation3,Citation4] The most commercially used yeast is baker's yeast, which is almost exclusively composed of cells of one or more selected strains of Saccharomyces cerevisiae.[Citation5,Citation6] The yeast S. cerevisiae is extremely well suited for the development of efficient cell factories that can produce fuels, chemicals, pharmaceutical drugs and food ingredients, in addition to its use as a feed additive for cattle, in order to develop dairy productivity.[Citation7,Citation8] One could not deny that yeasts have a great significance in food technology. Today, the whole world, with its rapidly growing population, is facing low agricultural production, which led to an economical targeting towards the production of food-grade yeasts.[Citation9–11] Moreover, the lower content of nucleic acids, when compared to that in bacteria, makes yeasts more acceptable for being used as a food supplement.[Citation1,Citation12]
For the economic production of yeasts, many cheap agricultural and industrial wastes, mainly molasses, which are abundantly available, are being used for successive submerged fermentations.[Citation10,Citation12] By using molasses as a main substrate, the manufacturing process for the production of yeast biomass has been simplified and its cost has been reduced, when compared with the use of other raw materials and grains.[Citation12–14] After fermentation, the yeast biomass is easily harvested due to the bigger cell sizes and flocculation abilities, and may be subjected to downstream processing steps like washing, cell disruption, protein extraction and purification. Food-grade yeast biomass can also be produced as a by-product of industrial ethanol production on molasses.[Citation10]
Rapid growth rate and high biomass yield, accompanied by good dough-leavening ability, are the most important demands for efficient commercial production of baker's yeast.[Citation15–17] These goals are metabolically contradictory, as respiratory catabolism favours a high biomass yield, whereas fermentative catabolism is responsible for a high dough-leavening capacity.[Citation18,Citation19] Therefore, the main objective of this work was to isolate and characterize new baker's yeast strains, to evaluate them versus a factory reference strain and to optimize the biomass production, based on an economic medium, with a special consideration to the dough raising parameter.
Materials and methods
Chemicals and media
Molasses were purchased from Hawamdeia City, Giza, Egypt. All other chemicals, used in the study, were of analytical grade. The used media included maltose dextrose agar (MDA) used for yeast isolation (1 g L−1 ammonium phosphate, 1 g L−1 potassium dihydrogen phosphate, 2 g L−1 yeast extract, 5 g L−1 dextrose, 5 g L−1 maltose, 20 g L−1 agar) and potato dextrose agar (PDA) for isolation and maintenance (200 g L−1 potatoes, 20 g L−1 dextrose, 20 g L−1 agar) and, finally, nutrient agar (NA) for the production of cells for DNA isolation. For yeast production, the formulae recommended by the Egyptian Company for Starch, Yeast and Detergents, Alexandria, Egypt, were used. These included malt extract medium supplemented with calcium pantothenate (0.03%, w v−1) [Citation20] for the pre-seed cultures, molasses medium I for the seed culture flasks and molasses medium II for the fermentor cultures. Molasses medium I contained 900 mL molasses with 180 g L−1 sugar content and 100 mL malt with 180 g L−1 sugar content and 2 g L−1 magnesium sulphate. Molasses medium II contained molasses with final sugar content of 200 g L−1, 2.3 g L−1 diammonium phosphate, 0.8 g L−1 ammonium sulphate and 0.5 mL antifoam (Struktol). Trace elements, added to some of the cultures, were prepared as described by Velagapudi et al.[Citation21] All media were sterilized by autoclaving and the initial pH was adjusted before sterilization using 0.1 mol L−1 NaOH and 0.1 mol L−1 HCl.
Yeast strains
The reference S. cerevisiae factory strain, used in this study, was provided by the Egyptian Company for Starch, Yeast and Detergents, Alexandria, Egypt. Experimental yeasts were isolated from different sources, including decomposed orange, spoiled mango fruit, fermented orange juice, fermented sugar cane juice and cachaca paste.[Citation22] Serial dilutions were immediately prepared and cultured on MDA and PDA agar plates. The plates were incubated at 30 °C for two to three days and 15 morphologically different microbial isolates were picked up for purification. Gram staining was applied to examine the yeast cells microscopically. The isolated yeasts were preserved for a short period of time (three to six months) on yeast peptone dextrose agar (YPD) slants and the cultures were stored at −4 °C for subsequent studies.
Shake-flask cultures
Batch cultures in 250 mL Erlenmeyer flasks were applied with 100 mL malt or molasses-based medium, inoculated with 2 mL pre-seed culture. The initial pH was adjusted to 4.5 and the flasks were incubated at 30 °C with shaking at 200 rpm. Growth was monitored by measuring the absorbance at 600 nm through time intervals of 2 h using a spectrophotometer (Optima, Japan). A600 records were converted to biomass, expressed as g L−1 fresh weight, based on a standard curve.
Fermentor cultures
Most of baker's yeast production plants use opened reactors,[Citation23] since pH of the medium is manually adjusted using 0.1 mol L−1 NaOH or 0.1 mol L−1 HCl. The fermentor used in this work was a 40 L airlift bioreactor with a 2 mm thick stainless steel vessel, present in the commercial production of baker's yeast at the Egyptian Company for Starch, Yeast and Detergents. The vessel is normally sterilized by steaming for 20–30 min. Air was introduced into the cultivation vessel via a sparger fixed at the bottom of the bioreactor with a flow rate ranging between 0.13 v v−1 min−1 and 0.15 v v−1 min−1. The temperature was measured using a glass thermometer fitted in the lower third portion of the vessel. Cooling of the vessel was carried out by sprinkling of cooled water via a ring tube fitted at the top of the vessel and water flow over walls.
The medium (22 L), which had an increased up concentration of molasses to a sugar content of 260 g L−, was sterilized separately by autoclaving and then transferred aseptically into the vessel. The temperature was maintained at 30 °C ± 2 °C and the pH was 4.5. Aeration was not limited to allow maximum oxygen exchange efficiency, agitation and mixing of the culture components.[Citation24] Growth was allowed for 16 h and fresh weights were determined. After an incubation period, the cells were separated by centrifugation and the residual sugar concentration in the liquids was determined using a saccharometer (Dujardin-Salleron, France). Dissolved oxygen was determined ex situ using a benchtop dissolved oxygen meter (VWR SympHony meter, USA).
Molecular characterization of the selected yeast strain
Genomic DNA of the selected yeast isolate was obtained using the GeneJET™ genomic DNA purification kit (Fermentas). Primer3 software was used to design six universal primers targeting different conserved locations, distributed in sequences of seven different S. cerevisiae 18S rRNA genes, arbitrary chosen from the GenBank database (). These included the primer pair F13 and R1729 for the polymerase chain reaction (PCR) amplification (resulted in a fragment of about 1700 bp of the18S rRNA gene in the selected new isolate). For complete DNA sequencing of the two strands of the amplified fragment, the forward primers F13, F542 and F1302, and the reverse primers R559, R962 and R1729 were separately used. All of the used primers were obtained from Bioneer(Korea).
Table 1. Universal primers used for amplification of the 18S rRNA region.
PCR was performed in a reaction volume of 50 µL containing 1 × Amplitaq Gold buffer (ThermoScientific, USA), 1.25 U of AmpliTaq Gold DNA Polymerase, 2 mmol L−1 dNTPs mixture, 25 mmol L−1 MgCl2 and 2 µL DNA. The reaction parameters were as follows: 95 °C for 5 min, 30 cycles of 95 °C for 1 min, 55 °C for 1 min and 72 °C for 2 min, and final extension at 72 °C for 7 min. Amplification was done using the Perkin Elmer Gene Amp PCR system 2,400. The amplicons were visualized by electrophoresis on 1% agarose gel.[Citation25] The amplicon was purified using GeneAll® Expin™ Combo GP according to the manufacturer's instructions. The primers F13, F542, F1302, R559 and R962 were used for sequencing of the amplified PCR product, on both sense and antisense strands, using a Perkin Elmer ABI 377 sequencer and a Taq FS Dye Terminator Sequencing Kit. Homology search for the obtained sequences was performed using the BLAST program through Biology WorkBench software to search for closely related sequences.
Multi-factorial experimental design
The fermentation factors affecting yeast production (expressed as fresh weight in g L−1) were evaluated by applying the Plackett–Burman experimental design.[Citation26] The design allowed the identification of the significant factors in addition to finding out their near-optimum levels through a relatively limited number of experiments. The examined independent variables, including molasses, (NH4)2SO4, (NH4)2HPO4, urea, calcium pantothenate, yeast extract, pH, inoculum size, medium volume, trace elements and antifoam, were screened by conducting 12 trials (). Each variable was investigated at two different levels listed in . All experiments were conducted in triplicate and the average value of biomass yield was used for statistical analysis.
Table 2. Randomized Plackett–Burman experimental design for evaluating 11 factors influencing the biomass production.
Table 3. Variables used in the optimization experiments and their examined levels.
Determination of consumed nutrients
Sugar content was measured by a saccharometer (Dujardin-Salleron, France), phosphate concentration was measured according to the microdetermination method [Citation27] and the ammonium levels were estimated by QUANTOFIX® Ammonium test strips (Macherey-Nagel, Germany).
Determination of the fermentation capacity
A crucial biotechnological feature of the bakery yeast end product is the fermentative or dough raising power. The most widely used method for measuring this capacity is the Svenska, JästAktiebolaget (SJA) method.[Citation28] The dough raising power of pressed yeast was determined using an SJA-fermentograph.(Svenska, JästAktiebolaget, Sweden).
Results and discussion
Isolation of yeast cells from different sources
The increasing demand of bread as a staple food of human beings has led to the development of the baker's yeast industry.[Citation29] In this study, 15 microbial isolates were picked up from different sources on MDA or PDA solid media. Only eight (M6 and M7 from mango, RO1, RO2 and RO3 from rotten orange, FOJ from fermented orange juice, FSCJ 1 from fermented sugar cane juice and P4 from cachaca paste Citation[22]) were selected after being cultivated on malt medium at 30 °C, where they gave the characteristic odour of yeast fermentation in addition to the formation of foam. These two criteria proved to be primary yeast characterizing features.[Citation30] The eight isolates were further examined morphologically on solid media. Five isolates showed rounded colonies, whereas the other three were star-shaped. Vegetative cells showed positive Gram stain reactions.
Bobye and Dayo-Owoyemi [Citation31] succeeded in isolating five different yeast strains from orange juice, while Ma'aruf et al. [Citation32] could isolate six yeast strains from different local Malaysian fruits.
Selection of a promising yeast isolate
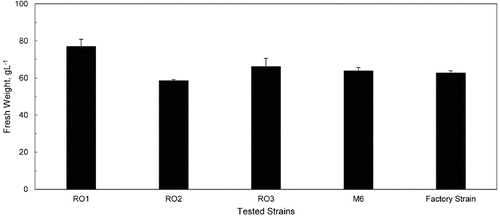
The biomass yield of each isolate was determined on malt liquid medium supplemented with calcium pantothenate in 12 h shake-flask cultures. The factory yeast strain was also included as a reference. The four isolates (RO1, RO2, RO3 and M6) had 1.1–1.3-fold higher biomass formation than the reference factory strain (data not shown). However, biomass determination in bioreactors is a basic parameter in fermentation processes by S. cerevisiae.[Citation33] Accordingly, the four selected isolates were further evaluated via 40 L airlift bioreactor cultures, each containing 22 L of molasses II medium. This resulted in the formation of about 62.66 g L−1 fresh weight yeast biomass by the reference culture. The highest biomass yields (78.24 g L−1 fresh weight and 66.06 g L−1 fresh weight) were recorded by RO1 and RO3, respectively (). According to these results, RO1 was selected as a promising new yeast isolate.
Figure 1. Biomass yield of RO1, RO2, RO3, M6 and factory strain in 40 L airlift bioreactor cultures, each containing 22 L of molasses II medium.
Vicente et al. [Citation22] reported that biomass determination is a basic parameter in fermentation processes. Therefore, simple and reliable online estimation procedures are highly desirable, particularly in fermentation processes using S. cerevisiae, which is widely used in industry as a source of a variety of products. Perepelitsa et al. [Citation34] succeeded in cultivating S. cerevisiae cells in the finesse glass vessel bioreactor with a maximum working volume of 2 L.
Molecular characterization of the isolate RO1
For further characterization of the selected RO1, its 18S rDNA was PCR amplified and subjected for sequencing. Using the primer pair F13 and R1729 (), a fragment of about 1700 bp was amplified. The obtained 18S rRNA partial sequence of the experimental yeast isolate RO1 was submitted to EMBL and received the accession number LK021686. The obtained rDNA nucleotide sequence was aligned with complete or nearly complete 18s rRNA gene sequences, retrieved from the GenBank data libraries by using BLAST homology search. The result showed 100% identity with S. cerevisiae strains YJM993 and YJM789. Because of this sequence identity result and the fact that the strain was isolated from a food material, we concluded that the newly isolated strain RO1 is safe to be used in biotechnology applications.[Citation35]
RO1 pre-seed cultures on malt-based versus molasses-based media
In yeast industry, molasses are used as a main raw material. However, some yeast factories use malt-based media for the preparation of yeast pre-cultures.[Citation20] In the local market, the cost of cane molasses is about $181/ton, whereas malt costs $1400–$1680/ton. Therefore, a comparative study was carried out in which RO1 was allowed to grow on malt medium versus economic molasses-based medium. The yeast biomass results achieved from malt medium and from molasses medium were 68 g L−1 and 65 g L−1, respectively, which were not very different.
It is well known that sugarcane molasses are very rich in nutrients required by most microorganisms.[Citation36] They are rich in fermentable sugars (about 55%, w v−1) and contain appreciable amounts of non-sugar organic substances in addition to nitrogen, phosphorus, sodium, potassium, calcium and other valuable minerals.[Citation37] Accordingly, with this study, we suggested a replacement of the malt-based medium with a molasses-based medium for the preparation of a yeast pre-seed culture. This replacement is cost effective and will reduce the lag phase required in a next molasses-based culture.
Elucidation of cultural factors influencing yeast production on a molasses-based medium
The composition of the medium used for cultivation of microorganisms is directly reflected in their physiological phenotype and their fermentation performance.[Citation38,Citation39] In this work, the Plackett–Burman multi-factorial experimental design [Citation23] was applied to formulate an economic cultivation medium suitable for the production of RO1 biomass. Together with the components of molasses II medium, we evaluated the influence of four other nutrient factors, hypothesized to increase the yeast biomass production. These included yeast extract, calcium pantothenate, urea and trace elements. In addition, initial pH and culture volume of 2 mL were involved. Accordingly, the design included 11 different cultural factors, examined as independent variables, each at two different levels in a total of 12 trials ( and ). Cultures were carried out under aerobic flask-scale conditions.
The calculated main effect results () demonstrated that each of the examined variables exerted a positive or a negative outcome on the response, namely, biomass production. Statistical analyses of the results suggested that the significant independent variables in this experiment were culture volume (P = 0.0053), yeast extract (P = 0.0056) and molasses (P = 0.0075), followed by diammonium phosphate (P = 0.0315).
Figure 2. The main effect of variables on RO1 biomass production under shake-flask conditions based on the Plackett–Burman experimental results.
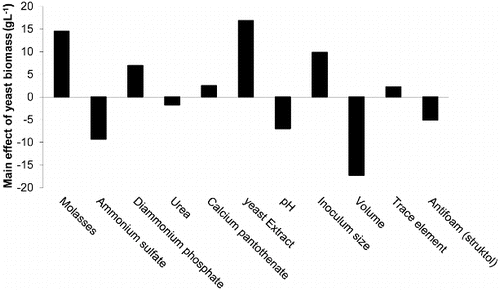
Based on the graphical representation of the calculated main effect results, it was predicted that high levels of molasses, diammonium phosphate and inoculum size are closer to the optimum when compared to their examined low levels. It is also clear that supplementation of the molasses II medium with three of the suggested additives, namely, yeast extract, calcium pantothenate (vitamin B5) and trace elements, improved RO1 biomass production. On the other hand, demonstrates also that the examined low levels of ammonium sulphate, pH and culture volume are nearer to the optimum and that the presence of antifoam and/or urea affects the response negatively. As it can be seen from , an application of maximum levels of molasses, inoculum size and diammonium phosphate in the presence of yeast extract and calcium pantothenate in a relatively small medium volume (represented by trial number 4) resulted in the maximum yield of biomass (71.7 g L−1).
The main effect results, together with the calculated P-values, also suggest that, in the range of the examined levels of variables, culture volume and yeast extract are the most effective key factors in this experiment. Cultures that contained a high volume size resulted in relatively low biomass densities. On the other hand, a lower medium volume in the same bioreactor size allowed more aeration and, consequently, higher yeast growth rates. Similarly, previous investigations [Citation40] proved that the most important parameter, which determines the balance between the fermentative and respiratory activity in many yeasts, is oxygen. It is also known that supplementation of a growth medium with a relatively cheap protein hydrolysate, such as yeast extract, presents a source of amino acids and other biosynthetic precursors that can be channelled directly into anabolic pathways, and hence reducing the need to produce biosynthetic precursors and saving metabolic energy.[Citation38]
Fermentor scale application of RO1 near-optimum conditions
In this experiment, the 40 L airlift bioreactor was used to scale up the RO1 production under the predicted near-optimum conditions. However, it has been reported that the concentrations of sugars and dissolved oxygen are generally the most important factors that interact and control the metabolism of S. cerevisiae.[Citation40] In other words, increased availability of oxygen promotes high rates of yeast growth at the expense of sugar.[Citation41] Therefore, special considerations were given to these parameters in order to reduce the possibility of being limiting factors in this fermentor scale culture. The starting concentration of molasses was arbitrary increased up to a sugar content of 260 g L−1. The near-optimum conditions, predicted through the Plackett–Burman experiment, were applied for all the other cultural factors, with the exception of antifoam, which is essential for aerobic fermentor cultures.
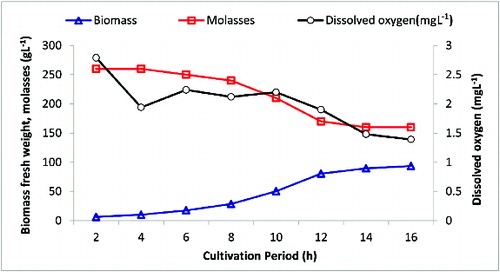
Growth was allowed for 16 h during which RO1 biomass, nutrient consumption and oxygen concentration were monitored at intervals shown in . A relatively short lag phase for about 4 h was observed. This was followed by a gradual transition to an exponential phase that showed a maximum growth rate in the incubation period between 8 h and 12 h. At the end of the cultivation period (16 h), a relatively high yeast biomass (93 g L−1) was achieved, which represented approximately a 1.5-fold increase, when compared to the reference culture's biomass. The rate of molasses consumption as well as the change in dissolved oxygen concentration clearly correlated with the biomass formation. The starting concentration of molasses was reduced from 260 g L−1 to 160 g L−1 and the dissolved oxygen concentration decreased from 2.8 mg L−1 to 1.5 mg L−1, which corresponded to about 22% saturation (v v−1). Phosphate and ammonium analysis results indicated that about 50% of their initial concentrations were consumed within the 16th h (data not shown). At the end of the cultivation period, the cells were collected and analysed for fermentation capacity.
Fermentation capacity of RO1 versus factory reference strain
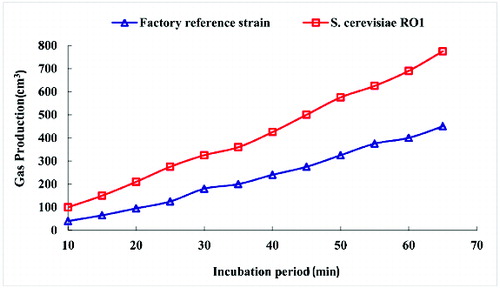
The leavening ability of the yeast is an extremely important parameter in baker's yeast production.[Citation19,Citation42] Therefore, the dough raising test is the main quality parameter for baker's yeast. Hence, the SJA method was applied. The amounts of CO2, produced via fermentation by S. cerevisiae RO1 and a factory reference strain within an hour, were 700 cm3 and 400 cm3, respectively (), which means an increase of 1.75-fold in the fermentative power, when compared to the factory reference strain.
Conclusions
Industrial production of baker's yeast biomass could be easily improved by searching for new isolates from different sources. In the present work, a Saccharomyces cerevisiae strain was isolated from food material, which means that it is safe to be used in biotechnological applications. With this study, it is suggested to replace the malt-based medium with a molasses-based medium for the preparation of a yeast pre-seed culture. Additional optimization of the medium components, using the Plackett–Burman multi-factorial experimental design, was found to significantly increase the biomass productivity.
Acknowledgements
This work was supported by the Egyptian Company for Starch, Yeast and Detergents, Alexandria, Egypt. We acknowledge the company management for advice and facilities, including the laboratory space, reference yeast strain, chemicals, fermentor, analytical tools and other equipment, required for the accomplishment of this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bhattacharjee JK. Microorganisms as potential sources of food. Adv Appl Microbiol. 1970;3:139–161.
- Pederson C. Microbiology of food fermentations. 2nd ed. Westport: Taylor & Francis; 1979.
- Dai Z, Wang B, Liu Y, Shi M, Wang D, Zhang X, Liu T, Huang L, Zhang X. Producing aglycons of ginsenosides in bakers' yeast. Sci Rep. 2014;4:Article number: 3698. doi:10.1038/srep03698.
- Musatti A, Devesa V, Calatayud M, Vélez D, Manzoni M, Rollini M. Glutathione-enriched baker's yeast: production, bioaccessibility and intestinal transport assays. J Appl Microbiol. 2014;116(2):304–313.
- Oliveira HC, Leão CP, Soares FO. An application for control, monitoring and stimulation of baker's yeast fermentation process. Paper presented at: World Congress Computer Science, Engineering and Technology Education; 2006 March 19–22; Sao Paulo, Brazil. p. 152–156.
- Sreekumar PK. Identification of radioprotective activity in the extract of Indian green mussel, Pernaviridis L [dissertation]. Goa: Taylor & Francis; 2007.
- Matsuda F, Ishii J, Kondo T, Ida K, Tezuka H, Kondo A. Increased isobutanol production in Saccharomyces cerevisiae by eliminating competing pathways and resolving cofactor imbalance. Microb Cell Fact. 2013;12:119.
- European Food Safety Authority (EFSA). Scientific opinion on the safety and efficacy of Yea-Sacc® (Saccharomyces cerevisiae) as a feed additive for cattle for fattening, goats for fattening, dairy cows, dairy sheep, dairy goats and buffaloes. EFSA J. 2014;12(5):3666.
- Boekhout T, Robert V. Yeasts in food: beneficial and detrimental aspects. Hamburg: Taylor & Francis; 2003.
- Bekatorou AP, Psarianos C, Koutinas AA. Production of food grade yeasts. Food Technol Biotechnol. 2006;44:407–415.
- Damtew W, Emire SA, Aber AB. Evaluation of growth kinetics and biomass yield efficiency of industrial yeast strains. Arch Appl Sci Res. 2012;4(5):1938–1948.
- Jiru TM. Evaluation of yeast biomass production using molasses and supplements [dissertation]. Addis Ababa: Taylor & Francis; 2009.
- Roman W, editor. BiologiaetIndustria, Yeasts. Vol. 1. The Hague: Taylor & Francis; 1957.
- Crueger W, Crueger A. Biotechnology: a textbook of industrial microbiology. 2nd ed. Sunderland: Taylor & Francis; 1990.
- Beudeker RF, Dam HWV, Plaat JBVD, Vellenga K. Developments in baker's yeast production. In: Verachtert H, Mot RD, editors. Yeast biotechnology and biocatalysis. New York: Taylor & Francis; 1990; p. 103–146 .
- Evans IH. Yeast strains for baking: recent developments. In: Spencer JFT, Spencer DM. Yeast technology. Berlin Heidelberg: Taylor & Francis; 1990; p. 13–54.
- Reed G, Nagodawithana T. Yeast technology. New York: Taylor & Francis; 1991.
- Nilsson A, Norbeck J, Oelz R, Blomberg A, Gustafsson L. Fermentative capacity after cold storage of baker's yeast is dependent on the initial physiological state but not correlated to the levels of glycolytic enzymes. Int J Food Microbiol. 2001;71:111–124.
- Zamani J, Pournia P, Seirafi H. A novel feeding method in commercial baker's yeast production. J Appl Microbiol. 2008;105:674–680.
- Lodder J, Kreger-Van Rij NJW. The yeasts: a taxonomic study. Amsterdam: Taylor & Francis; 1952.
- Velagapudi VR, Wittmann C, Lengauer T, Talwar P, Heinzle E. Metabolic screening of Saccharomyces cerevisiae single knockout strains reveals unexpected mobilization of metabolic potential. Process Biochem. 2006;41:2170–2179.
- Vicente MA, Fietto LG, Castro IM, Santos ANG, Coutrim MX, Branda˜o RL. Isolation of Saccharomyces cerevisiae strains producing higher levels of flavoring compounds for production of cachaça the Brazilian sugarcane spirit. Int J Food Microbiol. 2006;108:51–59.
- Bergander E. Biochemie und Technologie der Hefe [Biochemistry and technology of yeast.] Leipzig: Taylor & Francis; 1959.
- Arroyo-López F, Orlić S, Querol A, Barrio E. Effects of temperature, pH and sugar concentration on the growth parameters of Saccharomyces cerevisiae, S. kudriavzevii and their interspecific hybrid. Int J Food Microbiol. 2009;13:120–127.
- Hengstmann U, Chin K, Janssen PH, Liesack W. Comparative phylogenetic assignment of environmental sequences of genes encoding 16S rRNA and numerically abundant culturable bacteria from anoxic rice paddy soil. Appl Environ Microbiol. 1999;5(11):5050–5058.
- Hegde S, Bhadri G, Narsapur K, Koppal S, Oswal P, Turmuri N, Jumnal V, Hungund B. Statistical optimization of medium components by response surface methodology for enhanced production of bacterial cellulose by Gluconacetobacterpersimmonis. J Bioprocess Biotech. 2013;4:1–142.
- Chen PS, Toribara TY, Warner H. Microdetermination of phosphorus. Anal Chem. 1956;28,1756–1758.
- Švec I, Hrušková M. Wheat flour fermentation study. Czech J Food Sci. 2004;22:17–23.
- Rahimpour A, Jahanshahi M, Peyravi M. Development of pilot scale nanofiltration system for yeast industry wastewater treatment. J Environ Health Sci Eng. 2014;12:1–55.
- Jahan N, Azmuda N, Khan A. Isolation and identification of indigenous bakers' yeast. Bangladesh J Microbiol. 2007;24(1):65–66.
- Bobye B, Dayo-Owoyemi I. Comparative evaluation of the sensory properties of doughs fermented with yeasts isolated from orange. Asian Network Sci Inform Biotechnol. 2009;8(3):389–392.
- Ma'aruf A, Asyikeen ZN, Sahilah A, Mohd. Khan A. Leavening ability of yeast isolated from different local fruits in bakery product. SainsMalaysiana. 2011;40(12):1413–1419.
- Vicente A, Castrillo J, Teixeira J, Ugalde U. On-line estimation of biomass through pH control analysis in aerobic yeast fermentation systems. Biotechnol Bioeng. 1997;58(4):445–450.
- Perepelitsa N, Kaiser S, Eibl D. Application of the Finesse glass bioreactor for fermentation of Saccharomyces cerevisiae [Internet]. Zurich: Taylor & Francis; [cited 2015 Feb 12]. Available from: http://finesse.com/media/121717/ApplicationnoteyeastsV4.pdf2012.
- Oslan S, Salleh A, Rahman R, Basri M. Locally isolated yeasts from Malaysia: identification, phylogenetic study and characterization. Acta Biochim Pol. 2012;59(2):225–229.
- El-Gendy N, Madian H, Abu Amr S. Design and optimization of a process for sugarcane molasses fermentation by Saccharomyces cerevisiae using response surface methodology. Int J Microbiol. 2013;2013:Article ID 815631. Available from: http://dx.doi.org/10.1155/2013/815631
- Blonskajaa V, Kamenevb I, Zubc S. Possibilities of using ozone for the treatment of wastewater from the yeast industry. Proc Estonian Acad Sci Chem. 2006;55(1):29–39.
- Hahn-Hägerdal B, Karhumaa K, Larsson CU, Gorwa-Grauslund M, Görgens J, van Zyl WH. Role of cultivation media in the development of yeast strains for large scale industrial use. Microb Cell Fact. 2005;4(31):1–16.
- Rodrigues RCLB, Lu C, Lin B. Fermentation kinetics for xylitol production by a Pichiastipitis D-Xylulokinase mutant previously grown in spent sulfite liquor. Appl Biochem Biotechnol. 2008;148:199–209.
- Estela-Escalante W, Rychtera M, Melzoch K, Hatta-Sakoda B. Effect of aeration on the fermentative activity of Saccharomyces cerevisiae cultured in apple juice. Rev MexIngQuím. 2012;11(2):211–226.
- Briggs DE, Boulton CA, Brooks PA, Stevens R. Brewing: science and practice. Cambridge: Taylor & Francis; 2004.
- Jørgensen H, Olsson L, Rønnow B, Palmqvist EA. Fed-batch cultivation of baker's yeast followed by nitrogen or carbon starvation: effects on fermentative capacity and content of trehalose and glycogen. Appl Microbiol Biotechnol. 2002;59:310–317.