Abstract
In the present study, the potential hazard of Bacillus thuringiensis (Bt) kurstaki HD1 spore-crystal mixture (spore/δ-endotoxin) on parasitization performance and longevity of female egg parasitoid Trichogramma evanescens Westwood was evaluated. For this purpose, Bt kurstaki HD1 was incubated at 30 °C in T3 medium at 200 rpm for seven days. Lyophilized spore-crystal mixture (5000 µg mL−1) was mixed with 50% honey solution and supplied to 0–24 h old T. evanescens adults as a nutrient to ensure the ingestion of the toxins by the parasitoids. The results indicated that spore-crystal mixture of Bacillus thuringiensis subsp. kurstaki (Btk) HD1 did not induce considerable decrease in parasitization performance and longevity of T. evanescens adults. Thus, it can be concluded that Btk HD1 products can safely be used together with egg parasitoid T. evanescens in integrated pest management system to compensate the deficiency of each control tactic alone.
Introduction
Pest control strategies are among the major parameters for increasing the productivity in agriculture. Chemical pesticides are one of the most effective control methods and hence are used to control many pest insects. The use of chemical pesticides is advantageous, because they do not require information about the ecological origin of the insects and temporarily suppress the pest population. However, they cause serious adverse effects on the nature and also on the human health.[Citation1] Using non-selective insecticides may presumably cause outbreak of insect pests, due to decreasing population of their natural enemies. Also, the widespread use of chemicals gives rise to development of pest resistance, while reducing the natural enemy complexes. Hence, they disrupt the natural ecosystems that often exist between pests and their natural enemies.[Citation2,Citation3] Due to these considerations, many alternative control tactics have been developed. Inundative release of Trichogramma spp. and use of microbial insecticides are among the alternatives to chemical control tactics and are common components of integrated pest management systems.[Citation4–8]
Egg parasitoid Trichogramma species are well known biological control agents and are widely used commercially in controlling lepidopterous insects pests.[Citation9] Commercial formulations of various Bacillus thuringiensis (Bt) strains are also efficiently used as biocontrol agents against many pest insects.[Citation10,Citation11] However, little concern has been given for antagonism between the two control tactics.[Citation12] Although no direct Bt poisoning of Trichogramma spp has reported, there is still a possibility that they may adversely affect the next generations of native parasitoids.[Citation12] Because of all these reasons, it is obligatory to make transition to the integrated pest management strategies. Scientists from different fields of study have tried to develop environmentally friendly and sustainable control tactics as a component of integrated pest management (IPM) systems. In this respect, Takada et al. [Citation13] concluded that the combination of microbial insecticides and natural enemies give better results than each of the methods used alone. However, Bt products should be compatible with the natural enemy populations. That is, before applying these two methods together, the effectiveness of Bt products on the natural enemies must be well specified. Thus, safety of microbial insecticides on natural enemies should be an indispensable component of the integrated pest management strategies. In the present study, the side effects of relatively high dose (5000 µg mL−1) of B. thuringiensis kurstaki HD1 spore/δ-endotoxin were assessed on the longevity and parasitization performance of egg parasitoid Trichogramma evanescens in order to determine whether these two strategies can be used together or not.
Materials and methods
B. thuringiensis strain
B. thuringiensis subsp. kurstaki HD1 (Btk), (Instituto de Biotecnologia, Universidad Nacional Autonoma de Mexico) was used in the experimental procedures.
Preparation of spore-crystal mixture, freeze drying and electron microscopy
Btk HD1 was grown in 150 mL T3 medium (3 g triptone, 2 g triptose, 1.5 g yeast extract, 0.005 g MnCl2, 6 g NaH2PO4, 7.1 g Na2HPO4) and incubated for seven days at 30 °C at 200 rpm to induce sporulation.[Citation14] The bacterial suspension was centrifuged at 4 °C and 15,000 × g for 10 min to obtain spore-crystal mixtures. The pellet was washed twice with sterile distilled water (dH2O) and centrifuged at 15,000 × g for 10 min. Subsequently, spore-crystal mixture was freeze dried using Labconco–Welch freeze-drier. For electron microscopy, the spore-crystal sample was suspended in dH2O on a microscope slide and fixed after air drying at room temperature. The sample was sputter coated with 10 nm Au/Pd layer using a SC7620 mini-sputter coater and viewed using a scanning electron microscope (LEO440) at 20 kV beam current.
Determination of δ-endotoxin and colony forming units
The number of spores was estimated by determining the colony forming units (CFUs). One millilitre of Btk HD1 culture was incubated at 80 °C for 10 min and dilutions (101, 102, 103, 104, 105) were plated on Luria Bertani agar medium (5 g L−1 yeast extract, 10 g L−1 peptone, 10 g L−1 NaCI, 15 g L−1 agar). In order to determine the δ-endotoxin concentration, 1 mL of the culture medium was centrifuged for 10 min at 10,000 × g and the pellet was washed twice with 1 mol L−1 NaCl and twice with distilled water. The pellet was then suspended in 1000 µL of 50 mmolL−1 NaOH (pH 12.5).[Citation7,Citation15] After 3 h incubation at 30 °C, the total protein in the supernatant was measured using the Bradford [Citation16] method. The toxin yield of Btk HD1 was calculated by dividing δ-endotoxin (µg mL−1) to CFU (spores mL−1).
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis
Protein gel electrophoresis was conducted as described by Sambrook et al. [Citation17] The spore-crystal mixture of Btk HD1 was resuspended in equal amount of sample buffer (4 mL 10% SDS, 2 mL glycerol, 1.2 mL of 1 mol L−1 Tris (pH 6.8), 0.01% w/v Bromophenol Blue, 10 mL β-Mercaptoethanol, 2.8 mL dH2O) and boiled at 95–100 °C for 5–10 min. The samples were then loaded and separated by SDS-PAGE under reducing conditions using a continuous gel containing 12% separating gel and 5% stacking gel. The gel was stained with Coomassie Brilliant Blue R250 and analysed. The used molecular weight marker was SM0431, Fermantas.
Insect cultures
Ephestia kuehniella (Lepidoptera: Pyralidae) were reared on a mixture of wheat flour, wheat bran and glycerol. The insect culture was maintained at constant temperature (27 °C ± 1 °C), 14L:10D photoperiod and 60% ± 5% relative humidity.[Citation18–20] Egg parasitoid T. evanescens (Hymenoptera: Trichogrammatidae) was obtained from Adana Agricultural Pest Control Institute and cultured in Biocontrol Laboratory of Biology Department at Erciyes University.
Bioassay
Freshly laid eggs of the host E. kuehniella were fixed on 1.5 cm × 10 cm cardboards using 10% gum arabic. Then the eggs were exposed to a 0–24 h old adult female T. evanescens in sterile glass tubes (1.5 cm ×16 cm). T. evanescens adults were supplied daily with 5000 µg mL−1 spore-crystal mixture in a final concentration of 50% honey.[Citation21–24] Although low LC50 doses are enough to control most lepidopterous pests (465.59 µg g−1 for E. kuehniella),[Citation7] much higher doses are required for coleopteran pests in storage conditions, as it was indicated in a previous study (5749.50 µg g−1 for B.thuringiensis subsp. tenebrionis).[Citation7] Considering the required doses for all pest populations in storage conditions, the highest amount of spore/δ-endotoxin concentration was selected to ensure whether T. evanescens adults remained active without suffering any side effects. The control group was supplied with sterile distilled water in 50% honey in place of the spore/δ-endotoxin. Fifty fresh host eggs on cardboards were supplied for every T. evanescens female separately and were replaced daily. The parasitized eggs on each of the treated cardboards were counted daily during their lifespan. The longevity of each adult female was also determined in this experiment. For each treatment, 10 T. evanescens were used separately to determine the daily egg laying patterns of adult wasps. The treatments were conducted in triplicate.
Statistical analysis
Mean daily parasitization per female of both control and treatment groups were analysed using one-way analyses of variance (ANOVA). Means were separated at the 5% significance level by using Tukey honest significant differences post-test for each group. Females' parasitization rate, as well as control and treated groups' longevity, was compared with independent samples t-test for each day.[Citation25]
Results and discussion
Scanning electron micrography of Btk HD1 spore-crystal mixture
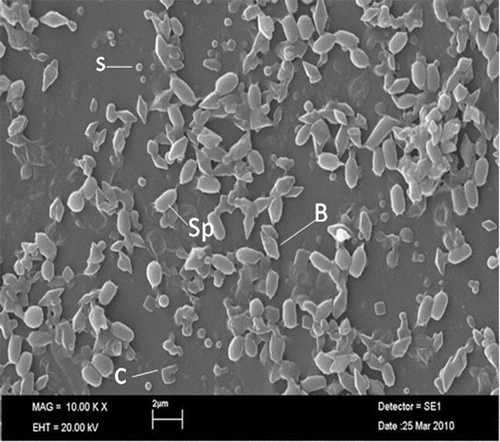
Spore-crystal sample was examined under scanning electron microscope, in order to be shown in detail. It was evident that Btk HD1 produced bipyramidal, spherical and cubic crystal proteins with different sizes (). Morphology of Cry proteins were also detected by some other researchers of Btk HD1.[Citation5–7] These types of Cry proteins have specific toxicity against pest insects belonging to Lepidoptera, Diptera and Coleoptera.[Citation26]
Biomass and δ-endotoxin determination
CFU values and δ-endotoxin production of Btk HD1 were estimated as 38.67 ± 0.88 × 105 spore mL−1 and 620 µg mL−1 ± 4.82 µg mL−1, respectively. Also, the toxin yield was calculated as 16.03 µg/105 spores mL−1. Similar results were obtained in a study carried out by Yilmaz et al. [Citation7] Also, Saadaoui et al. [Citation27] estimated the δ-endotoxin production of Btk HD1 as 669 µg mL−1 for 1010 spore L−1.
SDS-PAGE analysis
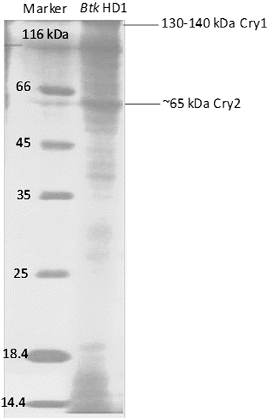
Molecular weights of cuboidal, spherical and bipyramidal crystals of Btk HD1 were reported as 65, 130 and 130–140 kDa, respectively.[Citation28,Citation29] In the present study, the characteristic banding patterns of Cry1 (130–140 kDa) and Cry2 (65–70 kDa) proteins were confirmed by the SDS-PAGE analysis ().
Longevity and parasitization performance
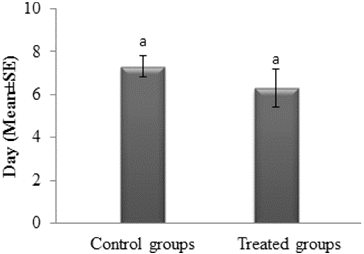
Numerous studies have been documented on the deleterious effects of chemical pesticides on parasitoid wasps and have stressed that the application of chemicals and the release of the parasitoid wasps should not be coincided at the same time period.[Citation13,Citation30–33] On the other hand, some researchers reported that Bt products have little or no detrimental effect on Trichogramma wasps [Citation22,Citation24,Citation34–37] and can safely be used simultaneously in the same field. In the present study the average longevity of T. evanescens was calculated as 7.3 and 6.3 d for the control and treated groups, respectively (t = 0.977; df = 18; P = 0.341) (). Although the present doses were tenfold higher than the doses reported by Salama and Zakı [Citation36] (500 µg mL−1), deleterious effects on longevity of T. evanescens adults were not observed.
Figure 3. Longevity of T. evanescens females after exposition to Btk HD1 spore-crystal mixture (5000 µg mL−1).
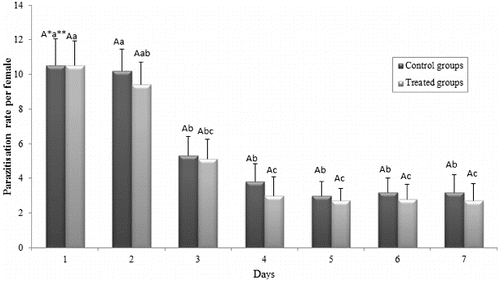
Results of the current study indicated that T. evanescens female adults parasitized most of the host eggs in the first and second days of the experiment. Daily parasitism per female started to decrease from the third day for both the control and the treated groups (). For the control groups: F = 9.128; df = 6; P ≤ 0.0001; for Btk HD1 treated groups: F = 8.758; df = 6; P ≤ 0.0001. For parasitization on the first day: t = 0.095; df = 18; P = 0.926; on the second day: t = 0.986; df = 18; P = 0.337; on the third day: t = 0.495; df = 18; P = 0.627; on the fourth day: t = 0.594; df = 18; P = 0.560; on the fifth day: t = 0.271; df = 18; P = 0.789; on the sixth day: t = 0.499; df = 18; P = 0.624; on the seventh day: t = 0.356; df = 18; P = 0.726.
Figure 4. Parasitization of T. evanescens per female after exposition to Btk HD1 spore-crystal mixture (5000 µg mL−1).
Researchers tested the parasitization performance and longevity of several other egg parasitoids, such as Trichogramma ostrinia, Trichogramma cacoecia, Trichogramma pratissolii, Trichogramma pretiosum and Trichogramma brassica, treated with Bt and reported no negative effect,[Citation24,Citation34–37] as is the case in the current study. In a similar manner, studies with larval parasitoids Cotesia plutellae (Hymenoptera: Braconidae), Hyposoter exiguae (Hymenoptera: Ichneumonidae) and Microplitis croceipes (Hymenoptera: Braconidae) indicated no decrease in parasitization performance between the control and treated groups.[Citation38–40] In a study carried out by Vaez et al., [Citation12] it was indicated that the application of 9.8 × 105 IU L−1 Bt spores to the eggs of the host organism affected negatively the searching efficiency and palpation of Trichogramma brassicae adults. On the other hand, Ruiu et al. [Citation23] reported 16.3% mortality rate on housefly pupae parasitoid Muscidifurax raptor (Hymenoptera: Pteromalidae) fed with 2 × 109 spores/g of Btk strain HD1 in 60% sucrose. Also, Dunbar and Johnson [Citation41] and Salama et al. [Citation42] reported a negative effect of Bt treatment on longevity and parasitization performance of various hymenopteran parasitoids.
Conclusions
The results of the present study, conducted on T. evanescens after application of relatively high dose (5000 µg mL−1) of Btk HD1 spore-crystal mixture, indicated that Bt products and Trichogramma wasps can safely be used in IPM strategies. Thus, the controlling deficiency of the inundative release of parasitoids could desirably be compensated by using Bt products. The data obtained from the present study and many other supporting works did not indicate any adverse effects of Bt products, when used in combination with T. evanescens in controlling important pest species. However, comprehensive preliminary studies should be conducted, considering the reported side effects of Bt on other various insect parasitoids, before starting the treatments in the field or storage conditions, for ensuring that Bt products do not show adverse effects on parasitoids and, consequently, for determining the tolerable doses.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ayvaz A. Effects of gamma radiation to some biological properties of Mediterranean Flour Moth Ephestia kuehniella Zeller Lepidoptera: Pyralidae and egg parasitoid Trichogramma evanescens Westwood Hymenoptera: Trichogrammatidae [dissertation]. Ankara: Taylor & Francis; 2001.
- Ehler LE, Eveleens KG, van Den Bosch R. An evaluation of some natural enemies of cabbage lopper on cotton in California. Environ Entomol. 1973;2:1009–1015.
- Eveleens KG, van Den Bosch R, Ehler LE. Secondary outbreak induction of beet armyworm by experimental insecticide applications in cotton in California. Environ Entomol. 1973;2:497–503.
- Joung K, Cote JC. A review of the environmental impacts of the microbial insecticide Bacillus thuringiensis. Taylor & Francis 2000;29:1–16.
- Azizoglu U, Yılmaz S, Ayvaz A, Karabörklü S, Akbulut M. Characterization of local Bacillus thuringiensis isolates and their toxicity to Ephestia kuehniella (Zeller) and Plodia interpunctella (Hübner) larvae. Egypt J Biol Pest Control. 2011;21:143–150.
- Yılmaz S, Demirezen N, Azizoglu U, Karabörklü S, Ayvaz A, Akbulut M, Tekin S. Identification of some cry1 genes and toxicity determination in Bacillus thuringiensis isolates obtained from mills and warehouses in Turkey. Egypt J Biol Pest Control. 2011;21:189–195.
- Yılmaz S, Ayvaz A, Akbulut M, Azizoglu U, Karabörklü S. A Novel Bacillus thuringiensis strain and its pathogenicity against three important pest insects. J Stored Prod Res. 2012;51:33–40.
- Azizoğlu U. Cloning, expression and insecticidal activity of cry1 and cry2 genes from Bacillus thuringiensis SY49-1 strain [dissertation]. Kayseri: Taylor & Francis; 2014.
- Tezze AA, Botto EN. Effect of cold storage on the quality of Trichogramma nerudai (Hymenoptera: Trichogrammatidae). Biol Con. 2004;30:11–16.
- Sanahuja G, Raviraj B, Twyman RM, Capell T, Christou P. Bacillus thuringiensis: a century of research,development and commercial applications. Plant Biotech J. 2011;9:283–300.
- Sansinenea E, editor. Bacillus thuringiensis biotechnology. Netherlands: Taylor & Francis; 2012.
- Vaez N, Iranipour S, Hejazi MJ. Effect of treating eggs of cotton bollworm with Bacillus thuringiensis Berliner on functional response of Trichogramma brassicae Bezdenko. Arch of Phytopath and Plant Prot. 2013;46:2501–2511.
- Takada Y, Kawamura S, Tanaka T. Effects of various insecticides on the development of the egg parasitoid Trichogramma dendrolimi (Hymenoptera: Trichogrammatidae). J Econ Entomol. 2001;94:1340–1343.
- Travers RS, Martin PAW, Reichelderfer CF. Selective process for efficient isolation of soil Bacillus spp. Appl Environ Microbiol. 1987;53:1263–1266.
- Tounsi S, Dammak M, Rebaî A, Jaoua S. Evidence of the effect of dendotoxin ratio in Bacillus thuringiensis crystals on the toxicity against Ephestia kuehniella. Biol Control. 2006;37:243–246.
- Bradford MM. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254.
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. New York (NY): Taylor & Francis; 1989.
- Ayvaz A, O Sagdıc, S Karaborklu, I Ozturk. Insecticidal activity of the essential oils from different plants against three stored product insects. J Insect Sci. 2010;10:1–13.
- Ayvaz A, Karaborklu S, Sagdıc O. Fumigant toxicity of five essential oils against the eggs of Ephestia kuehniella Zeller and Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae). Asian J Chem. 2009;21:596–604.
- Azizoglu U, Yılmaz S, Karabörklü S, Ayvaz A. Ovicidal activity of microwave and UV radiations on mediterranean flour moth Ephestia kuehniella Zeller, 1879 (Lepidoptera: Pyralidae). Turk J Entomol. 2011;35:437–446.
- Ebrahimi M, Sahragard A, Talaei-Hassanloui R. Effect of Bacillus thuringiensis var. kurstaki on survival and mortality of immature and mature stages of Diadegma insulare parasitizing Plutella xylostella. Phytoparasitica 2012;40:393–401.
- Santos HJG, Marques EJ, Pratissoli D, Kloss TG, Machado LC, Andrade GS. Efeito de Bacillus thuringiensis (Bacillaceae) sobre parâmetros biológicos do parasitoide Trichogramma pretiosum (Trichogrammatidae). [Effect of Bacillus thuringiensis (Bacillaceae) on biological parameters of Trichogramma pretiosum (Trichogrammatidae)] Natureza on line. 2011;9:1–6. Portuguese.
- Ruiu L, Satta A, Floris I. Susceptibility of the house fly pupal parasitoid Muscidifurax raptor (Hymenoptera: Pteromalidae) to the entomopathogenic bacteria Bacillus thuringiensis and Brevibacillus laterosporus. Biol Control. 2007;43:188–194.
- Polanczyk RA, Pratissoli Vianna UR, Oliveira RGS, Andrade GS. Interaction between natural enemies Trichogramma and Bacillus thuringiensis in pest control. Acta Sci Agron. 2006;28:233–239.
- SPSS Version 10. IL: Taylor & Francis; 2001.
- Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler DR, Dean DH. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Bio Rev. 1998;62:775–806.
- Saadaoui I, Rouis S, Jaoua S. A new Tunisian strain of Bacillus thuringiensis kurstaki having high insecticidal activity and endotoxin yield. Arch Microbiol. 2009;191:341–348.
- Sauka DH, Benintende GB. Bacillus thuringiensis: general aspects. An approach to its use in the biological control of lepidopteran insects behaving as agricultural pests. Rev Argent Microbiol. 2008;40:124–140.
- Wasano N, Ohba M. Assignment of the δ-endotoxin genes of the four Lepidoptera-specific Bacillus thuringiensis strains that produce spherical parasporal inclusions. Curr Microbiol. 1998;37:408–411.
- Sugiyama K, Katayama H, Saito T. Effect of insecticides on the mortalities of three whitefly parasitoid species, Eretmocerus mundus, Eretmocerus eremicus and Encarsia formosa (Hymenoptera: Aphelinidae). Appl Entomol Zool. 2011;46:311–317.
- Yong-yu X, Tong-xian L, Leibee GL, Jones WA. Effects of selected insecticides on Diadegma insulare (Hymenoptera: Ichneumonidae), a parasitoid of Plutella xylostella (Lepidoptera: Plutellidae). Biocontrol Sci Technol. 2004;14:713–723.
- Grützmacher AD, Zimmermann O, Yousef A, Hassan SA. The side-effects of pesticides used in integrated production of peaches in Brazil on the egg parasitoid Trichogramma cacoeciae Marchal (Hym., Trichogrammatidae). J Appl Entomol. 2003;128:377–383.
- António V, Luísa O, Patrícia G. Effects of conventional pesticides on the preimaginal developmental stages and on adults of Trichogramma cordubensis (Hymenoptera: Trichogrammatidae). Biocontrol Sci Technol. 2001;11:527–534.
- Wang ZY, Wu Y, Kang-Lai HE, Shu-Xiong BAI. Effects of transgenic Bt maize pollen on longevity and fecundity of Trichogramma ostriniae in laboratory conditions. Bull Insect. 2007;60:49–55.
- Manachini B, Lozzia GC. Studies on the effects of Bt corn expressing Cry1Ab on two parasitoids of Ostrinia nubilalis Hb (Lepidoptera: Crambidae). Bulletin 2004;27:109–116.
- Salama HS, Zakı FN. Biological effects of Bacillus thuringiensis on the egg parasitoid, Trichogramma evanescens. Insect Sci Appl. 1985;6:145–148.
- Hassan SA, Krieg A. Bacillus thuringiensis preparations harmLess to the parasite Trichogramma cacoeciae (Hym: Trichogrammatidae). Z Pflanzenk Pflanzen. 1975;82:515–52.
- Chilcutt CF, Tabashnik BE. Effects of Bacillus thuringiensis on adults of Cotesia plutellae (Hymenoptera: Braconidae), a parasitoid of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Biocontrol Sci Technol. 1999;9:435–440.
- Blumberg D, Navon A, Keren S, Goldenberg S, Ferkovich SM. Interactions among Helicoverpa armigera (Lepidoptera: Noctuidae), its larval endoparasitoid Microplitis croceipes (Hymenoptera: Braconidae), and Bacillus thuringiensis. J Econ Entomol. 1997;90:1181–1186.
- Ellen MT, Watson TF. Effect of Dipel (Bacillus thuringiensis) on the survival of immature and adult Hyposoter exiguae (Hymenoptera: Ichneumonidae). J Invertebr Pathol. 1986;47:178–183.
- Dunbar JP, Johnson AW. Bacillus thuringiensis: effects on the survival of a tobacco budworm parasitoid and predator in the laboratory. Environ Entomol. 1974;4:352–354.
- Salama HS, El-Moursy A, Zaki FN, Aboul-Ela R, Abdel-Razek A. Parasites and predators of the meal moth Plodia interpunctella Hbn. as affected by Bacillus thuringiensis Berl. J Appl Entomol. 1991;112:244–253.