?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The uncontrolled discharge of heavy metals generated by industrial processes into the environment threatens the living organisms. The widespread and unrestricted use of heavy metals results in their accumulation. Due to their adverse effects, heavy metals can be considered as an emergent pollutant group. In industrial wastewaters Pb2+ ion concentrations vary between 200 and 250 mg/L. Removal of heavy metals from wastewaters by an adsorption process can be economic and efficient, depending on the used adsorbent, in comparison with other methods. The present study aimed to examine the ability of a diatomite to remove lead (II) ions from aqueous solutions under various conditions. The effects of optimum adsorbent mass, contact time, different temperatures and pH were investigated. Pb(NO3)2 solution in a batch system was used in the present study. First, the specifications of the sorbent were determined and four different sorbent amounts were used. After this, the effect of contact time on the process of adsorption was determined. Finally the effect of different temperatures and pH on the adsorption was evaluated by using five different pH values and a temperature range from 20 to 40 °C. The maximum obtained removal efficiency was 98.09% by using 1.5 g/L diatomite for 2 min at pH 6 and 25 °C. The adsorption isotherm of lead (II) was described with Langmuir isotherm. The paper also discussed thermodynamic parameters, including enthalpy and entropy, and concluded that the adsorption was an endothermic process under natural conditions.
Introduction
Rapid industrialization has led to an increased disposal of heavy metals into the environment.[Citation1–3] The big flux of metallic substances in the aquatic environment is a result of the tremendous increase in the use of the heavy metals over the past few decades.[Citation3–9] The presence of Pb (II), Cu (II), Cd (II), Ni (II) and Zn (II) metals in wastewater is of great concern, because some of these metals, if present beyond certain concentrations, can be a serious health hazard, which can cause many disorders in the normal functions of human beings and animals.[Citation10] Pb (II) is one of the most toxic and long-standing environmental contaminants and a serious health hazard, which can cause many disorders, such as rise in blood pressure, kidney damage, miscarriages and abortions, brain damage and others.[Citation11–14]
The maximum lead concentration allowed by the Turkey legislation is 0.001 mg/L in drinking water. The current limit, according to the US Environmental Protection Agency (EPA), for drinking water, is 0.015 mg/L.[Citation15] The wastewater from the mining, metal, dyestuff, electric and petroleum industries contains undesired amounts of lead (II) ions. In industrial wastewaters, lead ion concentrations vary between 200 and 250 mg/L.[Citation16]
Several methods, such as electrochemical reduction, ion exchange, reverse osmosis and membrane separation, have been applied to remove heavy metal ions from industrial effluents. Comparatively, one of the most widely used methods for removing heavy metal ions and organic pollutants from aqueous environments is the adsorption with a variety of solid adsorbents.[Citation17–22] Diatomite is fine-grained, low-density biogenic sediment, which essentially consists of amorphous silica derived from opalescent frustules of diatoms. Active centres of diatomite, having active hydroxyl groups on its surface, which are responsible for adsorption, may be characterized as the following: (1) insulated free silanol groups (–SiOH), (2) free dual silanol group (–Si(OH)2), (3) –Si–O–Si bridges with oxygen atoms on the surface.[Citation23] Due to the presence of silanol groups that spread over the matrix of silica, diatomite can react with many polar functional groups.[Citation24] They are used prevalently as an adsorbent of heavy metals and dyes.[Citation25]
The aim of the present investigation was to study the adsorption of lead (II) ions onto diatomite. We particularly studied the effect of contact time, temperature, adsorbent amount and different pH of diatomite on adsorption capacity. This paper presents the kinetics and equilibrium data, and an evaluation of the ability of isothermal and kinetic models to describe the process' behaviour.
Materials and methods
Samples
The raw diatomite used in this study was collected as a natural resource from Ankara, in the middle Anatolia part of Turkey. Inorganic elementary compositions of the diatomite was determined through X-ray fluorescence (XRF) analysis. The diatomite sample was calcined at 960 °C for 5 h in an electric muffle furnace to eliminate the carbon and a small amount of other organic elements, such as H, N, S and O. Recording the weight loss of the sample and the ignition residues was used for XRF analysis to determine the content of each inorganic element. The diatomite was washed with distilled water several times to remove fine particles and other impurities. After that it was dried at 100 °C for 24 h. All diatomite samples were kept in polyethylene zip lock bag in desiccator until they were used.
The average lead (II) ions concentration in the lead plating industry wastewater was found to be 98 mg/L, therefore, an initial lead (II) concentration of 100 mg/L was selected in our study. Lead (II) nitrate (Pb(NO3)2) with 99% purity was purchased from Merck (Germany). The molecular weight of Pb(NO3)2 is 331.21 g/mol. A stock solution of lead (II) (1000 mg/L) was prepared by dissolving Pb(NO3)2 in twice-distilled water. Prior to adding the adsorbents, the pH of each solution was adjusted to the required value by adding 0.02 mol/L HCl or 0.5 mol/L NaOH. All used chemicals were purchased from Merck (Merck, Whitehouse Station, NJ). Reagent and all solutions were prepared in distilled water.
Equipment
The metal solution was filtered through 0.45 µm membrane filters after settling. The filtrates were then analysed using an inductively coupled plasma spectrometer (Optima 2100DV ICP, Perkin-Elmer, Boston, MA). During characterization of natural diatomite samples, XRF spectrometer (Spectro XLAB 2000) was used for chemical analysis. All pH measurements were carried out using a pH meter (WTW, Weilheim, Germany).
Adsorption kinetics
To investigate the mechanism of lead (II) adsorption on diatomite, the pseudo-first-order and pseudo-second-order equations were used to find out the adsorption mechanism.
Pseudo-first-order equation is one of the most popular and empirical models for adsorption kinetics, which was presented by Lagergren at the end of the nineteenth century.[Citation26] This model has the following formulation:(1)
(1) After definite integration by applying the initial conditions t = 0 to t and qt = 0 to qt, EquationEquation (1)
(1)
(1) becomes
(2)
(2)
Another simple and well-known kinetic model, which has been used extensively in recent years, is pseudo-second-order equation. This model was presented empirically by Ho and McKay and theoretically by Haerifar and Azizizan.[Citation26] The pseudo-order model can be represented in the following equation:(3)
(3)
By integrating EquationEquation (3)(3)
(3) , for the boundary conditions t = 0 to qt = 0 and t = t to qt = qt, it gives
(4)
(4)
Equations parameters – k1,k2 – the rate constants of pseudo-first/second-order adsorption (mg/g h), k2 qe2 – the initial rate (mg/g h), qe – calculated adsorbed amount of adsorbent, t – time (h), q – adsorbed amount of adsorbent and R2 – linear regression correlation coefficient.[Citation27]
Adsorption isotherms
Adsorption isotherms can be obtained by performing a series of batch experiments to examine the adsorbent capacity for the target adsorbate.
The Langmuir isotherm was theoretically derived, supposing that the adsorption takes place on fixed homogenous adsorption sites of equal energy, forming a monolayer surface coverage, with no interactions between adsorbed molecules. The Langmuir model can be described by the equation,(5)
(5) where q (mmol/g) and Ce (mg/L) are the equilibrium concentration of adsorbate on the adsorbent surface and the adsorbate concentration in the solution, respectively. The constant k is the equilibrium constant, which represents the affinity between adsorbate and adsorbent, and qm is the maximum amount of the adsorbate, adsorbed on the surface.[Citation28]
The Freundlich equation can be written as
(6)
(6) where KF is a constant, indicative of the relative adsorption capacity of the adsorbent (mg1−(1/n) L1/n g−1), qe is the amount of adsorbate per unit mass of adsorbent at equilibrium (mg/L) and n is a constant, indicative of the intensity of the adsorption. The Freundlich expression is an exponential equation and, therefore, assumes that when the adsorbate concentration increases, the concentration of adsorbate on the adsorbent surface also increases.[Citation29]
The Elovich equation can be written as
(7)
(7) where a is the initial sorption rate constant (mmol/g min) and the parameter β is related to the extent of surface coverage and activation energy for chemisorption (g/mmol).[Citation30]
The Temkin isotherm, considering the effect of the adsorption heat that decreases with the coverage of the adsorbent and the adsorbate–adsorbent interaction, is given as
(8)
(8) where θ is the fractional coverage, R is the universal gas constant (kJ/mol K), T is the temperature (°C), ΔQ = (−ΔH) is the variation of adsorption energy (kJ/mol) and K0 is the Temkin equilibrium constant (L/mg).[Citation31]
If the adsorption obeys Temkin equation, the variation of adsorption energy and the Temkin equilibrium constant can be calculated from the slope and the intercept of the plot θ versus ln Ce.[Citation29]
The Redlich–Peterson isotherm model is represented by incorporating the features of the Langmuir and Freundlich isotherms into a single equation
(9)
(9) where KRP (L/g) and a (L/mg) are the Redlich–Peterson model isotherm constants and β is the Redlich–Peterson model exponent.[Citation32]
Adsorption procedures
Adsorption studies of lead (II) ions were investigated using diatomite. All batch experiments were carried out in series of 250 mL Erlenmeyer flasks. We placed 0.1, 1.5, 2 and 5 g/L of diatomite in 250 mL Erlenmeyer flasks, containing 100 mL of lead (II) ion solution at desired temperature and pH value. Then the suspensions were placed in a shaker with invariant rotation rate of 250 rpm and taken for a constant sorption time of 60 min. The effects of adsorbent mass, contact time, temperature and pH on the adsorption of lead (II) were studied using the experimental conditions.
The effect of different adsorbent masses on the adsorption was determined by investigation at various adsorbent masses in the range of 0.1–5 g/L.
The adsorption of Pb(II) ions on diatomite was investigated as a function of time in the range of 0–60 min.
The effect of different temperatures was determined by investigation at various temperatures between 20 and 40 °C.
The effect of pH on the lead (II) adsorption onto diatomite was studied in the range of 2–6 for an initial lead concentration of 100 mg/L.
Four isotherm equations were utilized in the study, namely, Langmuir, Freundlich, Temkin and Redlich–Peterson.
Each experiment was replicated three times and the mean values were used in the analyses. If the standard errors were greater than 0.01, the test was repeated to check for errors.
Results and discussion
Characterization of diatomite
The quantitative chemical analysis of diatomite obtained by XRF technique revealed that the Ankara diatomite consisted mainly of SiO2 (71.40%). It also comprised 11.60% Al2O3, 7.45% Fe2O3, 0.75% TiO2, 0.90% Na2O, 1.80% K2O and 6.1% loss on ignition.
Effect of adsorbent amount
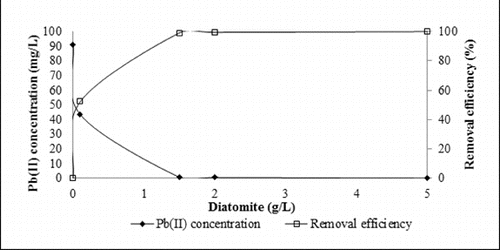
The effect of various doses of diatomite on lead (II) removal was studied. Adsorbent dosage is an important parameter because of its strong effect on the capacity of the adsorbent at a given initial concentration of the adsorbate.[Citation33,Citation34] As it can be seen in the percentage of lead removal increased with the increasing of the amount of diatomite. shows the effect of adsorbent amount to the removal efficiency. Lead (II) removal efficiency increased from 52%–99% with increasing the amount of the adsorbent between 0.1 and 5 g/L. The lead (II) removal efficiency stabilized after 1.5 g/L. Therefore, the amount of 1.5 g/L was selected as the optimum dosage of the used adsorbent.
Effect of temperature and thermodynamic study
An amount of 1.5 g/L diatomite was added to 100 mL of aqueous solution of lead (II), having an initial concentration of 100 mg/L. The removal percentage of lead (II) increased when the temperature increased from 20 to 40 °C. Because of this reason, the study was conducted at room temperature conditions (25 °C).
Additionally, the results of the thermodynamic calculations are shown in . The results show that ∆H values were positive and the process was endothermic.
Table 1. Thermodynamic parameters.
Effect of contact time
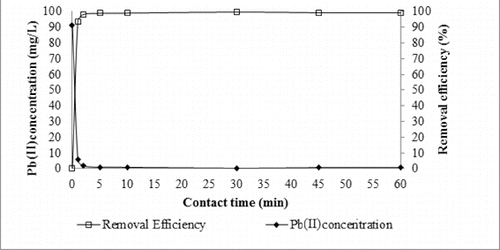
The effect of contact time was studied using a constant concentration (1.5 g/L) of lead (II) solution at 25 °C. The removal of lead (II) by adsorption using diatomite was rapid during the initial period of contact time and then became slower, with the increase of contact time. This is due to the strong attractive forces between the lead molecules and the adsorbent. The effect of contact time on the adsorption of lead (II) ions on diatomite is presented in . The removal efficiency reached equilibrium at 2 min with 98.09% maximum adsorption capacity. The removal percentage of lead (II) remained unchanged when the time increased from 2 to 60 min.
Effect of pH
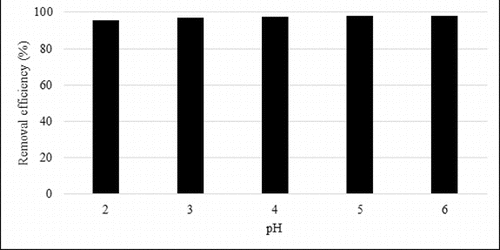
The pH of the solution is one of the most important parameters affecting the adsorption process. As it is known, change in the pH value could cause a change in the adsorption capacity. Therefore, the effect of different pH values was investigated in the present study. The studies were conducted at 25 °C using a temperature-controlled shaker. The experimental results of applying different pH values on the adsorption of lead (II) are presented in . The figure displays a general trend of increasing lead (II) removal with increasing solution pH until maximized at pH 6. The pH of the solution influenced the degree of ionization of the lead (II) nitrate. The pH level of an aqueous solution is an important variable for the adsorption of metals on the adsorbents. H+ ions completely cover the diatomite surface under acidic conditions and the lead (II) ions cannot compete with them for adsorption sites.[Citation13] The competition with the hydrogen ions decreases with the increasing of the pH and in this way the positively charged Pb (II) ions can be adsorbed at the negatively charged sites of the adsorbent.[Citation13,Citation35] Under these circumstances, the adsorbing cation might be Pb2+, Pb(OH)+, Pb(OH)2, Pb(OH)3−, Pb(OH)42−.[Citation36,Citation37] At pH lower than 8, the Pb (II) ions were the dominant species. Pb(OH)2 was present at pH higher than 8.[Citation36] Adsorption occurs at a relatively low pH (∼6.0) for Pb (II) ions.[Citation38,Citation39] Based on these results, diatomite exhibited a good capacity for removing lead (II) from solution at pH values ranging from 4 to 6. The optimum adsorption was detected at pH value of 6.
Figure 3. Effect of pH on lead (II) adsorption.
The effect of pH can be explained by considering the surface charge on the adsorbent material.[Citation40] At low pH values (pH 2–4), the low adsorption observation was explained due to increase in positive charge (protons) density on the surface sites and thus, electrostatic repulsion occurred between the metal ions (M2+ :Pb2+) and the edge groups with positive charge (Si-OH2+) on the surface, as follows [Citation41,Citation42]:
(10)
(10)
Adsorption isotherm
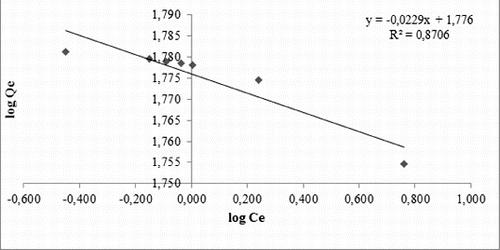
In this paper, several adsorption isotherm models, such as those of Langmuir, Freundlich, Temkin and Redlich–Peterson, were applied to the experimental data. EquationEquation (5)(5)
(5) was used to obtain Langmuir isotherm and shows Langmuir isotherm of experimental data. The equation was used to calculate the value dimensionless equilibrium parameter RL, which is a dimensionless constant referred to as separation factor or equilibrium parameter. Langmuir isotherm indicated favourable conditions for the adsorption of diatomite, because RL was between zero and one,
(11)
(11) where Qmax is the maximum adsorbent adsorption capacity, KL is the capacity of adsorption and aL is the energy of adsorption.
Figure 4. Lead (II) adsorption onto diatomite according to the Langmuir isotherm model.
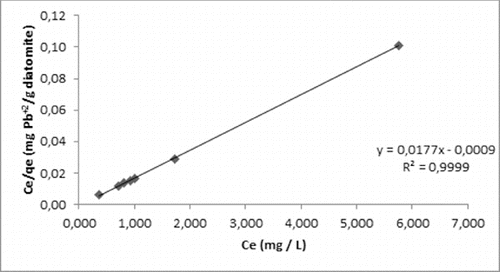
The maximum diatomite adsorption capacity was found to be 56.49 mg lead ions/g diatomite by using the Langmuir isotherm ().
EquationEquation (6)(6)
(6) was used in order to apply the experimental data to Freundlich isotherm. shows Freundlich isotherm of the experimental data. The 1/n value falls between 0 and 1, which is an indication for the favourable adsorption of lead ions on the diatomite. EquationEquation (8)
(8)
(8) was used for the Temkin's model of adsorption.
Figure 5. Lead (II) adsorption onto diatomite according to Freundlich isotherm model.
Redlich–Peterson's model of adsorption was applied to the experimental data by using EquationEquation (9)(9)
(9) . The exponent (β) varied between 1 and 0.
In this study, the β value was found to be 1.0334. β value close to 1 conformed Langmuir isotherm. The adsorption was on a single layer.
And also, first, second, pseudo-first- and pseudo-second-order kinetics were utilized. The results are shown in . According to the results, this study was fitted to both the pseudo-second-order kinetic.
Table 2. The kinetic equations and R2 of the kinetic models.
In thermodynamic study, the negative value of change in Gibbs free energy (ΔG) indicated that adsorption process of lead (II) on diatomite was spontaneous under natural conditions.
It has been reported that Enteromorpha prolifera activated carbon,[Citation43] Polygonum orientale Linn activated carbon,[Citation44] barley straw [Citation45] activated tea waste,[Citation46] coffee residue [Citation47] activated carbon from apple pulp [Citation48], sawdust activated carbon,[Citation49] pine cone activated carbon [Citation50] and volcanic tuff [Citation39] have the capacity to adsorb and accumulate heavy metals. In these studies, the pH ranged from 5 to 6 and the temperatures ranged from 25 to 30 °C (). The removal efficiency (98.09%) bigger than that of the other studies (e.g. P.orientale Linn activated carbon – 96.8%; barley straw – 88%; activated carbon from apple pulp – 43%).
Table 3. Comparison of maximum monolayer adsorption capacities for Pb(II) removal.
Boudrahem et al.[Citation47] reported that the best removal efficiency of lead (II) was at about 63 mg/L of adsorbent at 25 °C with pH 5.8.
It is obvious from that the adsorption capacities of diatomite adsorbent were bigger than the previously reported ones, which suggested that this is a trustworthy adsorbent for the removal of lead (II) from aqueous solutions.
Conclusions
In this study, the adsorption of Pb2+ ion on diatomite taken from Ankara was investigated. In this regard, the adsorption mechanism was evaluated during batch adsorption tests, under various conditions, in order to determine the optimum conditions. The effect of adsorbent dosage, time, temperature and pH on lead (II) removal from water was determined. The optimum parameters in 100 mL of total solution were determined to be 1.5 g/L adsorbent, 2 min stirring time, 25 °C and pH 6 for Pb2+. The adsorption isotherm of lead (II) was described by using the Langmuir isotherm. The kinetic data could be modelled by a pseudo-second-order (R2 = 1) kinetics equation. It was concluded that when optimum conditions are ensured, diatomite could be a satisfactory adsorbent and can be used in practical applications for heavy metal ion removal. It may be also concluded that diatomite may be used as economic, ecological and abundant source for the removal of lead (II) and it may be an alternative to more costly materials, such as ion-exchange resins and activated carbon.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Amuda OS, Giwa AA, Bello LA. Removal of heavy metal from industrial wastewater using modified activated coconut shell carbon. Biochem Eng J. 2007;36:174–181.
- Kadirvelu K, Namasivayam C. Activated carbon from coconut coirpith as metal adsorbent: adsorption of Cd(II) from aqueous solution. Adv Environ Res. 2003;7:471–478.
- Kailas L. Wasewar. Adsorption of metals onto tea factory waste: a review. Int J Rec Res App Stu. 2010;3(3):303–322.
- Rahmani A, Zavvar Mousavi H, Fazli M. Effect of nanostructure alumina on adsorption of heavy metals. Desalination. 2010;253:94–100.
- Alawa B, Srivastava A, Srivastava JK, Palsaniya J. Adsorption of heavy metals from industrial downstream using polypyrrole as a polycomposite material. Novus Int J Eng Tech. 2014;3(1):35–46.
- Vinodhini R, Narayanan M. The impact of toxic heavy metals on the hematological parameters in common carp (Cyprinus carpio L.). Iranian J Environ Health Sci Eng. 2009;6(1):23–28.
- Yang H, Rose NL. Distribution of Hg in the lake sediments across the UK. Sci Total Environ. 2003;304:391–404.
- Khorasgani FC. Removal of Cd (II) and Cr (VI) from electroplating wastewater by coconut shell. Int J Env Eng Man. 2013;4(4):273–280.
- Gaikwad RW. Removal of Cd (II) from aqueous solution by activated charcoal derived from coconut shell. Elec J Env Agricult Food Chem. 2004;3(4):702–709
- Padilla OE, Leyva RR, Flores CJV. Binary adsorption of heavy metals from aqueous solution onto natural clays. Chem Eng J. 2013;225:535–546.
- Shi W, Shao H, Li H, Shao M, Du S. Progress in the remediation of hazardous heavy metal-polluted soils by natural zeolite. J Hazard Mater. 2009;170:1–6.
- Dursun S, Pala A, Argun ME. Lead pollution removal from water via clinoptilolite fixed bed column. Poster session presented at: Blacksea international environmental symposium; 2008 Aug 25–29; Giresun, Turkey.
- Argun ME, Dursun S. Activation of pine bark surface with NaOH for lead removal. J Int Env App Sci. 2007;2(1,2):5–10.
- Oliver MA. Soil and human health: a review. Eur J Soil Sci. 1997;48:573–592.
- Cechinel MAP, Souza SMAGU, Souza AAU. Study of lead (II) adsorption onto activated carbon originating from cow bone. J Clean Prod. 2014;65:342–349.
- Sag Y, Ozer D, Kutsal TA. Comparative study of the biosorption of lead (II) ions to Z. ramigera and R. arrhizus. Process Biochem. 1995;30:169–174.
- Darvishi CSR, Shams KG, Khataee AR, Jorfi S. Silica nanopowders/alginate composite for adsorption of lead (II) ions in aqueous solutions. J Taiwan Inst Chem Eng. 2014;45:973–980.
- Dong L, Zhu Z, Ma H, Qiu Y, Zhao J. Simultaneous adsorption of lead and cadmium on MnO2-loaded resin. J Environ Eng Sci. 2010;22:225–229.
- Wang Y, Wang X, Wang X, Liu M, Wu Z, Yang L, Xia S, Zhao J. Adsorption of Pb(II) from aqueous solution to Ni-doped bamboo charcoal. J Ind Eng Chem. 2013;19:353–359.
- Kousha M, Daneshvar E, Sohrabi MS, Koutahzadeh N, Khataee AR. Optimization of C.I. Acid black 1 biosorption by Cystoseira indica and Gracilaria persica biomasses from aqueous solutions. Int Biodeterior Biodegradation. 2012;67:56–63.
- Kousha M, Daneshvar E, Esmaeli AR, Jokar M, Khataee AR. Optimization of Acid Blue 25 removal from aqueous solutions by raw, esterified and protonated Jania adhaerens biomass. Int Biodeterior Biodegradation. 2012;69:97–105.
- Torab-Mostaedi M, Asadollahzadeh M, Hemmati A, Khosravi A. Equilibrium, kinetic, and thermodynamic studies for biosorption of cadmium and nickel on grapefruit peel. J Taiwan Inst Chem Eng. 2013;44:295–302.
- Zhuravlev LT. The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surf A Physicochem Eng Asp. 2000;173:1–38.
- Šlivic M, Smičiklas PS, Plećaš I. Comparative study Cu2+ adsorption on a zeolite, clay and a diatomite from Serbia. Appl Clay Sci. 2009;43:33–40.
- Caliskan N, Kul RA, Alkan S, Sogut GE, Alacabey İ. Adsorption of zinc (II) on diatomite and manganese-oxide-modified diatomite: a kinetic and equilibrium study. J Hazard Mater. 2011;193:27–36.
- Haerifar M, Azizizan S. An exponential kinetic model for adsorption at solid/solution interface. Chem Eng J. 2013;215–216:65–71.
- Azizian S. Kinetic models of sorption: a theoretical analysis. J Colloid Interface Sci. 2004; 276:47–52.
- Praus P, Turicovă MA. Physico-chemical study of the cationic surfactants adsorption on montmorillonite. J Braz Chem Soc. 2007;18:378–383.
- Hamdaoui O, Naffrechoux E. Modeling of adsorption isotherms of phenol and clorophenols onto granular activated carbon: Part I. Two-parameter models and equations allowing determination of thermodynamic parameters. J Hazard Mater. 2007;147:381–394.
- Şengil İA, Özacar M, Türkmenler H. Kinetic and isotherm studies of Cu (II) sorption onto valonia tannin resin. J Hazard Mater. 2009;162:1046–1052.
- Shahzad BK, Doan HD, Wu J. Multicomponent isotherms for biosorption of Ni+2 and Zn+2. Desalination. 2009;249:429–439.
- Redlich O, Peterson DL. A useful adsorption isotherm. J Phys Chem. 1959;63:1024.
- El-Latif MMA, Ibrahim AM, El-Kady MF. Adsorption equilibrium, kinetics and thermodynamics of methylene blue from aqueous solutions using biopolymer oak sawdust composite. J Amer Sci. 2010;6(6):267–283.
- Soni M, Yadav JS, Sharma AK, Srivastava JK. Parametric optimization for adsorption of reactive orange 16 on water hyacinth root powder. Int J Cur Res Chem Phar Sci. 2014;1(6):140–147.
- Gupta SS, Bhattacharyya KG. Adsorption of Ni (II) on clays. J Colloid Interface Sci. 2006; 295:21–32.
- Gaballah I, Kilbertus G. Recovery of heavy metal ions through decontamination of synthetic solutions and industrial effluents using modified barks. J Geochem Explor. 1998;62:241–286.
- Taty-Costodes VC, Fauduet H, Porte C, Delacroix A. Removal of Cd(II) and Pb(II) ions, from aqueous solutions, by adsorption onto sawdust of Pinus sylvestris. J Hazard Mater. 2003;105:121–142.
- Jain CK, Ram D. Adsorption of lead and zinc on bed sediments of the river Kali. Water Res. 1997;31:154–162.
- Karatas M. Removal of Pb (II) from water by natural zeolitic tuff: kinetics and thermodynamics. J Hazard Mater. 2012;199–200:383–389.
- Belaid T, Aitali S, Benamor M, Belhamel K. Synthesis and characterization of new chelating resin functionalized with pyrocatechol violet and its application as extractant for zinc (II). Mat Sci Eng. 2009;609:75–80.
- Sari A, Tuzen M, Citak D, Soylak M. Equilibrium, kinetic and thermodynamic studies of adsorption of Pb(II) from aqueous solution onto Turkish kaolinite clay. J Hazard Mater. 2007;149:283–291.
- Meroufel B, Benali O, Benyahia M, Zenasni MA, Merlin A, George B. Removal of Zn (II) from aqueous solution onto kaolin by batch design. J Wat Res Pro. 2013;5:669–680.
- Li Y, Du Q, Wang X, Zhang P, Wang D, Wang Z, Xia Y. Removal of lead from aqueous solution by activated carbon prepared from Enteromorpha prolifera by zinc chloride activation. J Hazard Mater. 2010;183:583–589.
- Wang L, Zhang J, Zhao R, Li Y, Li C, Zhang C. Adsorption of Pb (II) on activated carbon prepared from Polygonum orientale Linn.: kinetics, isotherms, pH, and ionic strength studies. Bioresour Technol. 2010;101:5808–5814.
- Pehlivan E, Altun T, Parlayıcı S. Utilization of barley straws as biosorbents for Cu2+ and Pb2+ ions. J Hazard Mater. 2009;164:982–986.
- Mondal MKJ. Removal of Pb (II) ions from aqueous solution using activated tea waste: adsorption on a fixed-bed column. J Environ Manage. 2009;90:3266–3271.
- Boudrahem F, Aissani BF, Ait A.H. Batch sorption dynamics and equilibrium for the removal of lead ions from aqueous phase using activated carbon developed from coffee residue activated with zinc chloride. J Environ Manage. 2009;90:3031–3039.
- Depci T, Kul AR, Onal Y. Competitive adsorption of lead and zinc from aqueous solution on activated carbon prepared from Van apple pulp: study in single- and multi-solute systems. Chem Eng J. 2012;200:224–236.
- Sreejalekshmi KG, Krishnan KA, Anirudhan TS. Adsorption of Pb(II) and Pb(II)-citric acid on sawdust activated carbon: kinetic and equilibrium isotherm studies. J Hazard Mater. 2009;161:1506–1513.
- Momcilovic M, Purenovic M, Bojic A, Zarubica A, Ranpelovic M. Removal of lead (II) ions from aqueous solutions by adsorption onto pine cone activated carbon. Desalination. 2011;276:53–59.