Abstract
The swine pathogen Streptococcus suis is a worldwide emerging threat to public health. Efforts to develop an effective vaccine require the economic production of sufficient quantities of bacteria. To address this issue, the goal of the present study was to optimize culture conditions by reducing the accumulation of lactate through optimization of the medium components and feeding strategy. Sucrose (5 g/L) and a mixture of yeast extract and peptone (4 g/L each) were the most effective carbon and nitrogen sources, respectively, for S. suis ST171 fermentation. Further, from the results of S. suis ST171 fermentation with different ratios of carbon/nitrogen, 2 g/L sucrose was used as a carbon source and a mixture of 4 g/L yeast extract and 4 g/L peptone was used as a nitrogen source. Using these optimized conditions, S. suis ST171 fermentation with glucose-stat feeding strategy cultures accumulated a low concentration of lactate (3.47 g/L), which was accompanied by a high cell yield (2.712) and viability (1.192 × 1010 colony forming units/mL).
Introduction
Streptococcus suis is a Gram-positive, facultative anaerobic bacterium [Citation1] that is economically important, because it causes a wide range of diseases in pigs,[Citation2] including meningitis, septicaemia, pneumonia, endocarditis and arthritis.[Citation3,Citation4] Moreover, zoonotic infection of humans with S. suis, particularly serotype 2, causes serious diseases in many countries engaged in intensive swine production.[Citation5] Two large outbreaks of S. suis human infections occurred in China in 1998 and 2005, causing 229 infections and 52 deaths because of toxic shock syndrome and meningitis.[Citation6,Citation7] The emergence of S. suis as a zoonotic agent is causing great public concern worldwide, particularly because a vaccine is not available.[Citation4,Citation8] The vaccine strain S. suis ST171 is a special strain for producing live vaccine against septic pig streptococcus disease.[Citation9] Although S. suis ST171 is an attenuated strain that prevents infection, it is unsuitable for practical use, because it cannot be obtained in sufficient yields. Increased cell yields will likely reduce production costs, expand the market for the vaccine [Citation9] and help protect the swine production industry and public health.
Lactate is the primary metabolite that inhibits the growth of S. suis ST171 cultures.[Citation9] The Embden–Meyerhof–Parnas (EMP) pathway, the pentose phosphate pathway and the tricarboxylic acid (TCA) cycle operate in a number of Streptomyces species.[Citation10,Citation11] The excretion of lactate is not caused from the increased expression of lactate dehydrogenase (ldhA), but from the accumulation of pyruvate.[Citation12] For example, the cell density of S. suis ST171 cultures is significantly improved by decreasing the accumulation of lactate and the excretion of lactate is decreased by optimization of the culture conditions, including the concentration of dissolved oxygen (DO), initial and residual glucose, and regulation of the strain growth rate.[Citation9]
The formation of by-products by Escherichia coli is strongly affected by the composition of the culture medium.[Citation13] Replacing of glucose with mannose or fructose, as well as supplementing the medium with yeast extract, methionine or glycine reduces the accumulation of acetate in E. coli cultures.[Citation14,Citation15] Protein synthesis is sensitive to decreasing of C/N ratios and the carbon flux between protein and oil synthesis is regulated by the composition of the medium.[Citation16] The levels of the transcripts of certain genes, required for the uptake and metabolism of carbon sources and the formation of by-products, are affected by the C/N ratio as well.[Citation17]
Moreover, an appropriate feeding method is important for achieving high cell densities of S. suis cultures.[Citation9,Citation18] The rate of formation of by-products by E. coli, such as acetate, is directly related to the growth rate or to the rate of glucose consumption.[Citation13] The excretion of by-products decreases when the glucose concentration is maintained at a low level.[Citation19] In cultures of S. suis vaccine strain SD11, lactate accumulation decreases and cell density increases with the application of a pH-feedback feeding strategy.[Citation18] Further, a strategy that combines exponential feeding and pH-stat feeding of E. coli cultures that produce tumour necrosis factor-related apoptosis-inducing ligand (TRAIL), prevents the accumulation of acetate. Also, the dry cell weight and active soluble TRAIL were increased by 58.54% and 47.37%, respectively, when compared with a constant feeding rate strategy.[Citation20] A useful approach involves applying glucose pulses to the feed and observing the changes in the DO concentration. The DO-stat feeding avoids substrate overfeeding and O2 limitation.[Citation19]
In the present study, the excretion of lactate during a fermentation process with S. suis ST171 was reduced by optimizing the composition of the medium, including the carbon and nitrogen sources, and the C/N ratio. In addition, the effect of intermittent feeding, DO-feedback feeding and glucose-stat feeding on S. suis ST171 fermentation were investigated to select an appropriate feeding strategy for a high cell density cultivation of S. suis.
Materials and methods
Bacteria and culture medium
The strain S. suis ST171, used in this study, was obtained from China Institute of Veterinary Drugs Control (CVCC 563) and stored at the culture collection of the Shandong Binzhou Animal Science & Veterinary Medicine Institute.
The seed medium contained the following components: 5 g/L sucrose, 5 g/L yeast extract, 3 g/L MgSO4·7H2O, 3 g/L KH2PO4 and 0.1 g/L vitamin B1. The fermentation medium for S. suis ST171 contained the following components: 10 g/L sucrose, 5 g/L yeast extract, 2 g/L MgSO4·7H2O, 2 g/L KH2PO4 and 0.1 g/L vitamin B1. The pH of the seed and fermentation media was adjusted to 7.0 with 4 mol/L NaOH.
Culture methods
Automated microbiology growth analysis systems
A 250-mL baffled flask, containing 50 mL of seed medium was inoculated with a single colony of S. suis ST171 and cultivated at 37 °C for 6 h. Thirty microlitres of this culture was inoculated into 400 µL of the inoculum medium in an Automated Microbiology Growth Analysis System (Bioscreen C, Oy Growth Curves Ab Ltd, Finland) containing 300 μL of fermentation medium and was cultivated at 37 °C for 8 h.
Ten litre fermenter
A single colony of S. suis ST171 was inoculated into a 250-mL baffled flask containing 50 mL of seed medium and cultivated at 37 °C with shaking at 200 rpm for 8 h. The 10-L fermenter containing 6 L of fermentation medium was inoculated aseptically with the culture grown in the baffled flask (2%, v/v). The temperature was maintained at 37 °C and the pH was adjusted to 7.0 with 4 mol/L NaOH. The DO level was maintained at approximately 20% saturation by adjusting the agitation and aeration rates. When the initial sucrose was depleted, the feeding medium was added to the fermenter to meet the specific experimental requirements described below.
Carbon and nitrogen sources. Feeding media and feeding strategies
Different carbon and nitrogen sources were used during the study. Glucose, sucrose, lactose and galactose (4 g/L) were added into the fermentation medium in order to be used as carbon sources for S. suis ST171 fermentation process. Further, we obtained results from the fermentation processes by using yeast extract (5 g/L), peptone (5 g/L), beef extract (5 g/L) and a mixture of yeast extract (2.5 g/L) and peptone (2.5 g/L) as nitrogen sources. To determine the most appropriate fermentation conditions, we added different concentrations of the carbon and nitrogen sources, which gave the best results during the fermentation process. The used concentrations were 2, 5, 8 and 10 g/L for the chosen carbon source and 1, 2, 4 and 5 g/L for the chosen nitrogen sources. The used C/N ratios for the next trial were 1:1, 1:2, 1:4 and 1:10. Cell density, viable count [colony forming units (CFU)/mL] and accumulation of lactate (g/L) were measured during the study.
In addition, the sucrose solution and glucose solution were selected as feeding media to investigate their effect on S. suis ST171 fermentation. The effect of intermittent feeding, DO-feedback feeding and glucose-stat feeding on S. suis ST171 fermentation were investigated to select the best feeding strategy for a high cell density cultivation.
Analysis of fermentation products
The DO, pH and temperature were measured automatically with electrodes attached to the fermenters. The optical density was monitored at 600 nm using an Automated Microbiology Growth Analysis System and a spectrophotometer (722N, INESA, China). The viable bacterial count in 1 mL of fermentation broth was determined as described previously.[Citation9] The concentrations of glucose and lactate were monitored using an SBA-40E Biosensor Analyser (Biology Institute of Shandong Academy of Sciences, China). The concentrations of sucrose, lactose and galactose were determined using an Agilent 1206 (Agilent Technologies, Santa Clara, CA, USA) high-pressure liquid chromatography system.
Statistical analysis
All experiments were conducted in triplicate and the data were averaged and are presented as the mean ± standard deviation. One-way analysis of variance followed by Dunnett's multiple comparison tests were used to determine significant differences. Statistical significance was defined as p < 0.05.
Results and discussion
Effect of carbon source on S. suis ST171 fermentation
Carbon sources
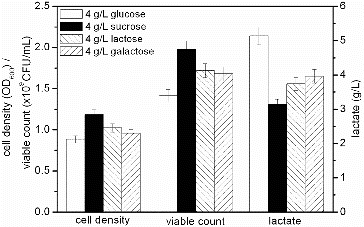
Certain carbohydrates serve as carbon sources to increase the growth rate and synthesis of cellular components of S. suis. Glucose is the most frequently used raw material for industrial fermentations, because it is relatively inexpensive and the most effective carbon and energy source.[Citation21] The lac and gal gene clusters, which are arranged tandemly in the genome of Streptococcus gordonii, encode the components of the tagatose pathway (lacABCD) and the sugar phosphotransferase system. The permease-designated EIILac transports lactose and galactose, whereas EIIGal transports galactose.[Citation22] Glucose, sucrose, lactose and galactose were added into the fermentation medium in order to be used as carbon sources for S. suis ST171 fermentation process. The results displayed in indicated that when glucose was used as the carbon source, the cell density (0.884) and viable count (1.42 × 109 CFU/mL) were the lowest and the accumulation of lactate (5.04 g/L) was the highest. The highest cell density (1.185) and viable count (1.98 × 109 CFU/mL) and the lowest accumulation of lactate (3.14 g/L) occurred when using sucrose as the carbon source. Compared with galactose, growth in the presence of lactose yielded an increase in cell density (1.023) and viable count (1.72 × 109 CFU/mL) by 6.89% and 2.38%, respectively. Also, the accumulation of lactate (3.74 g/L) decreased by 5.56%. The highest cell density and viable count were obtained with sucrose because of the low concentration of lactate. It has been proven that the accumulation of lactate is reduced in the presence of low concentrations of nicotinamide adenine dinucleotide hydrogen (NADH) that is generated primarily by the EMP pathway and TCA cycle [Citation23] and NADH levels are low when carbon flow into these metabolic pathways is reduced.[Citation24] The accumulation of lactate is lower in the presence of sucrose because of its complicated metabolic pathways.[Citation25] We selected sucrose as the carbon source for S. suis ST171 fermentations. In S. suis SD11 fermentation, cell density and viable count, obtained with sucrose, were higher than that with glucose.[Citation18]
Sucrose concentration
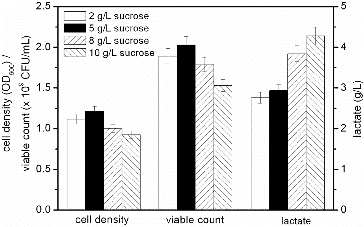
The results of fermentations using different concentrations of sucrose are presented in . The accumulation of lactate was increased by increasing the concentration of sucrose. The highest cell density (1.213) and viable count (2.13 × 109 CFU/mL) were obtained by using 5 g/L sucrose. Growth of bacteria in the presence of excess amount of a carbon source under aerobic conditions generates acidic by-products in the so-called ‘overflow’ metabolism.[Citation26] Cell density and viable count decreased when the concentration of sucrose was above 5 g/L. Although low concentrations of lactate were accumulated in low sucrose concentrations, the latter did not satisfy the requirement for the growth amount of the S. suis vaccine strain SD11.[Citation18] Compared with the 5 g/L surcose, the cell density and viable count obtained at 2 g/L sucrose were decreased by 8.17% and 11.26%, respectively. The cell density and viable count obtained with 2 g/L sucrose were higher than those obtained with 8 and 10 g/L sucrose.
Effect of nitrogen sources on S. suis ST171 fermentation
Nitrogen sources
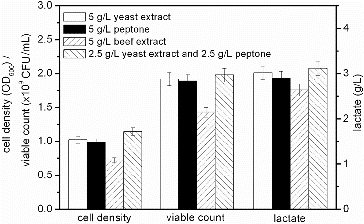
Nitrogen is an absolute requirement for cell growth and is assimilated by the synthesis of glutamine and glutamate.[Citation27] The results of the fermentation processes by using yeast extract, peptone and beef extract as nitrogen sources, are displayed in . The accumulation of lactate was increased with higher cell densities, which was in accordance with the study, described previously, which indicated that the concentration of lactate was directly proportional to the cell density.[Citation28] The cell density and viable count obtained when using beef extract were lower compared with the values obtained when using yeast extract and peptone. Cell density (1.023) and viable count (1.92 × 109 CFU/mL) obtained when using yeast extract were increased by 3.86% and 1.59% compared with the values obtained when using peptone, respectively. When a mixture of yeast extract and peptone was used, the highest cell density (1.147) and viable count (1.98 × 109 CFU/mL) were obtained. Yeast extract and peptone are rich in free amino acids, which support cell growth with a low consumption rate of the carbon source and minimize the accumulation of by-products.[Citation29] We selected a 1:1 mixture of yeast extract and peptone as a nitrogen source for S. suis ST171 fermentations.
Mixture of yeast extract and peptone concentration
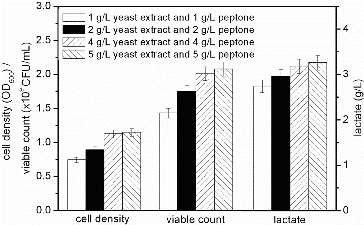
shows the effects of varying concentrations of 1:1 ratio of yeast extract combined with peptone. The cell density, viable count and accumulation of lactate increased when increasing the concentrations of these nitrogen sources. In the presence of mixture containing 1 g/L of each component, the cell density, viable count and lactate accumulation were 0.745, 1.42 × 109 CFU/mL and 2.74 g/L, respectively. With 4 g/L of each component, these values were 1.127, 2.01 × 109 CFU/mL and 3.18 g/L, respectively. Compared with the obtained values when using 4 g/L of each component, the obtained values when using 5 g/L of each component were increased by 0.98%, 1.49% and 1.89%. Because there was not a significant difference between the values when using 4 or 5 g/L mixtures, we used the 4 g/L mixture to reduce the costs for further analysis.
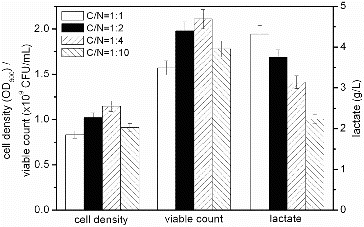
Effect of the C/N ratio on S. suis ST171 fermentation
The C/N ratio is more important than the nitrogen concentration for increasing the cell density and desired product concentration in fermentations.[Citation30] Next, we maintained a constant amount of the nitrogen source mixture (4 g/L yeast extract and 4 g/L peptone) and added varying amounts of sucrose, so that the C/N ratios by weight [ = sucrose/(yeast extract + peptone)] were 1:1, 1:2, 1:4 and 1:10 (). As the C/N ratio increased, so did the production of lactate by S. suis ST171. On the other hand, the cell density and viable count decreased. These findings are consistent with the effect of the C/N ratio on acetate accumulation in cultures of E. coli.[Citation17] With 1:1 and 1:10 ratios of C/N, the lactate concentrations were 4.32 g/L and 2.23 g/L, respectively. The highest cell density (1.147) and viable count (2.08 × 109 CFU/mL) were obtained when the C/N ratio was 1:4; that is, 2 g/L sucrose and a mixture of 4 g/L yeast extract and 4 g/L peptone. The ratio of C/N affects the levels of transcription of genes (pfkA and pykF) related to the transport of carbohydrates. For example, as the C/N ratio increases, the expression of TCA cycle genes, such as gltA, icdA, fumC, sdhC and mdh increase, which also increases the accumulation of by-products.[Citation17] Due to the higher nitrogen source in the fermentation medium with C/N ratio maintained at 1:4, higher cell density and viable count were obtained at 2 g/L sucrose, which indicated that sucrose of 2 g/L was used as carbon source for S. suis ST171 fermentation.
Effect of feeding substrate on S. suis ST171 fermentation
Feeding media
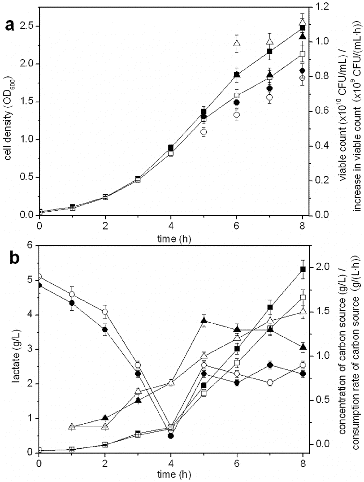
Fed-batch processes are employed to achieve high cell densities, whereas the composition of the feeding medium is important for cell growth.[Citation30] shows the results of S. suis ST171 fermentation with different feeding media, which indicated that the lactate concentrations were 5.32 and 4.51 g/L at the end of fermentation in the presence of glucose or sucrose, respectively. Cell density (2.478) and viable count (0.835 × 1010 CFU/mL) were 1.16-fold and 1.05-fold higher during the use of glucose, respectively, when compared with sucrose. Due to the increased accumulation of lactate, the increase in viable count and the consumption rate of carbon source with glucose were lower than these with sucrose during the late fermentation period, which was consistent with the reduction of production rate of desired product with higher excretion of acetate.[Citation31] Because of the more complex metabolism of the sucrose, this carbon source did not satisfy the requirement of cell growth during the late fermentation period, which led to lower cell density and viable count, although lactate concentrations were lower. Therefore, glucose was selected as the feeding medium.
Feeding strategy
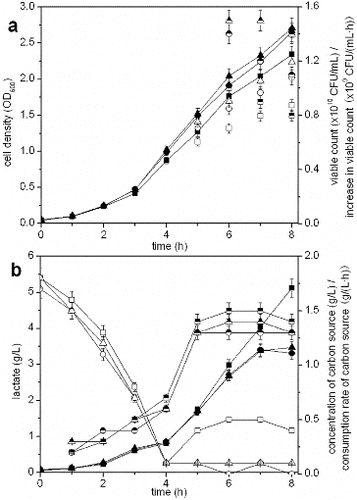
By adjusting the feeding rate of glucose in fed-batch fermentation, the concentration of glucose is maintained below a certain critical value required for the formation of by-products.[Citation13] This is why the accumulation of by-products is decreased by maintaining a low glucose concentration.[Citation31] The results from using intermittent feeding, DO-feedback feeding and glucose-stat feeding are presented in . The concentration of glucose was maintained at approximately 0.5 g/L using intermittent feeding and at a low level (0.1 g/L) with DO-feedback and glucose-stat feeding, but glucose starvation occurred with DO-feedback feeding. Using intermittent feeding, the lowest cell density (2.343) and viable count (0.875 × 1010 CFU/mL) and the highest accumulation of lactate (5.12 g/L) were obtained. Compared with cell density (2.652), viable count (1.078 × 1010 CFU/mL) and accumulation of lactate (3.31 g/L) obtained using DO-feedback feeding, these respective values were increased by using glucose-stat feeding, which were 2.712, 1.192 × 1010 CFU/mL and 3.47 g/L, respectively. The lactate that was accumulated using DO-feedback feeding was reused during the late fermentation period and the production rate of lactate using glucose-stat feeding was lower compared with that of intermittent feeding. Lactate that accumulated during growth is recaptured because of the change in expression levels of ldhA and Dld (encoding NAD+-dependent and NAD+-independent lactate dehydrogenases) in the stationary phase.[Citation32] The consumption rate of glucose with intermittent feeding was higher, compared with those of DO-feedback and glucose-stat feeding. The glucose consumption rate was the lowest with DO-feedback feeding. Glucose-stat feeding maintains a low lactate concentration and serves as a sufficient source of carbon to support cell growth.[Citation31] Here, the highest cell density and viable count were obtained using glucose-stat feeding.
Conclusions
In summary, we showed that the accumulation of lactate in fermentation cultures of S. suis ST171 was decreased by optimizing the concentrations of medium constituents and feeding strategy. Higher cell density and viable count were also obtained. The study, therefore, illustrates a useful approach for large-scale production of a vaccine strain of S. suis ST171. This enhances the application of a vaccine, leading to prevention of the occurrence of septic pig streptococcus disease and promotion of the pig industry development.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Zhang TF, Ding Y, Li TT, Wan Y, Li W, Chen HC, Zhou R. A fur-like protein PerR regulates two oxidative stress response related operons dpr and metQIN in Streptococcus suis. BMC Microbiol. 2012;12:85.
- Segura M, Zheng H, de Greeff A, Gao GF, Grenier D, Jiang YQ, Lu CP, Maskeli DC, Oishi K, Okura M, Osawa R, Schultez C, Schwerk C, Sekizaki T, Smith H, Srimanote P, Takamatsu D, Tang JQ, Tenenbaum T, Tharavichitkul P, Hoa NT, Valentin-Weigand P, Wells JM, Wertheim H, Zhu BL, Gottschalk M, Xu JG. Latest developments on Streptococcus suis: an emerging zoonotic pathogen: part 1. Future Microbiol. 2014;9(4):441–444.
- De Greeff A, Hamilton A, Sutcliffe IC, Buys H, Van Alphen L, Smith HE. Lipoprotein signal peptidase of Streptococcus suis serotype 2. Microbiology. 2003;149:1399–1407.
- Rodríguez-Ortega MJ, Luque I, Tarradas C. Overcoming function annotation errors in the Gram-positive pathogen Streptococcus suis by a proteomics-driven approach. BMC Genomics. 2008;9:588.
- De Greeff A, Buys H, Verhaar R, Van Alphen L, Smith HE. Distribution of environmentally regulated genes of Streptococcus suis serotype 2 among S. suis serotypes and other organisms. J Clin Microbiol. 2002;40(9):3261–3268.
- Huang YT, Teng LJ, Ho SW, Hsueh PR. Streptococcus suis infection. J Microbiol Immunol Infect. 2005;38(5):306–313.
- Lun ZR, Wang QP, Chen XG, Li AX, Zhu XQ. Streptococcus suis: an emerging zoonotic pathogen. Lancet Infect Dis. 2007;7(3):201–209.
- Zheng H, Ye C, Segura M, Xu J. Mitogenic effect contributes to increased virulence of Streptococcus suis sequence type 7 to cause streptococcal toxic shock-like syndrome. Clin Exp Immunol. 2008;153:385–391.
- Cheng LK, Fu Q, Zhang SS, Miao LZ, Shen ZQ. Effect of the lactic acid by-product on fermentation cultivation of swine streptococcosis vaccine strain (ST171). Chin J Pre Vet Med. 2012;34(3):227–231.
- Hodgson DA. Primary metabolism and its control in Streptomycetes: a most unusual group of bacteria. Adv Microb Physiol. 2000;42:47–238.
- Papagiann M. Recent advances in engineering the central carbon metabolism of industrially important bacteria. Microb Cell Fact. 2012;11:50.
- Zhu HF, Shimizu K. Effect of a single-gene knockout on the metabolic regulation in Escherichia coli for D-lactate production under microaerobic condition. Metab Eng. 2005;7:104–115.
- Eiteman MA, Altman E. Overcoming acetate in Escherichia coli recombinant protein fermentations. Trends Biotechnol. 2006;24(11):530–536.
- Aristidou AA, San KY, Bennett GN. Improvement of biomass yield and recombinant gene expression in Escherichia coli by using fructose as the primary carbon source. Biotechnol Prog. 1999;15:140–145.
- Han K, Zong J, Lim HC. Relieving effects of glycine and methionine from acetic acid inhibition in Escherichia coli fermentations. Biotechnol Bioeng. 1993;41:316–324.
- Truong Q, Koch K, Yoon JM, Everard JD, Shanks JV. Influence of carbon to nitrogen ratios on soybean somatic embryo (cv. Jack) growth and composition. J Exp Bot. 2013;64(10):2985–2995.
- Kumar R, Shimizu K. Metabolic regulation of Escherichia coli and its gdhA, glnL, gltB, D mutants under different carbon and nitrogen limitations in the continuous culture. Microb Cell Fact. 2010;9:8.
- Wu YC, Miao LZ, Li M, Cheng LK. Effect of carbon source on fermentation cultivation of Streptococcosis suis vaccine strain SD11. In: Zhang TC, Ouyang P, Kaplan S, Skarnes B, editors. Proceedings of the 2012 International Conference on Applied Biotechnology. Vol. 1, Lecture Notes in Electrical Engineering. Berlin, Taylor & Francis, 2014. p. 233–240.
- Johnston WA, Stewart M, Lee P, Cooney MJ. Tracking the acetate threshold using DO-transient control during medium and high cell density cultivation of recombinant Escherichia coli in complex media. Biotechnol Bioeng. 2003;84(3):314–323.
- Shen YL, Zhang Y, Sun AY, Xia XX, Wei DZ, Yang SL. High-level production of soluble tumor necrosis factor-related apoptosis-inducing ligand (Apo21/TRAIL) in high-density cultivation of recombinant Escherichia coli using a combined feeding strategy. Biotechnol Lett. 2004;26:981–984.
- Gosset G. Improvement of Escherichia coli production strains by modification of the phosphoenolpyruvate: sugar phosphotransferase system. Microb Cell Fact. 2005;4:14.
- Zeng L, Martino NC, Burne RA. Two gene clusters coordinate galactose and lactose metabolism in Streptococcus gordonii. Appl Environ Microbiol. 2012, 78(16):5597–5605.
- Liu LM, Li Y, Li HZ, Chen J. Significant increase of glycolytic flux in Torulopsis glabrata by inhibition of oxidative phosphorylation. FEMS Yeast Res. 2006;6:1117–1129.
- De Andaa R, Larab AR, Hernandez V, Hemandez-Montalvo V, Gosset G, Bolivar F, Ramirez OT. Replacement of the glucose phosphotransferase transport system by galactose permease reduces acetate accumulation and improves process performance of Escherichia coli for recombinant protein production without impairment of growth rate. Metab Eng. 2006;8:281–290.
- Shen DQ, Feng XY, Lin DQ, Yao SJ. Effect of different carbon sources on pyruvic acid production by using lpdA gene knockout Escherichia coli. Chin J Biotech. 2009;25(9):1345–1351.
- Shin S, Chang DE, Pan JG. Acetate consumption activity directly determines to the level of acetate accumulation during Escherichia coli W3110 growth. J Microbiol Biotechnol. 2009;19(10):1127–1134.
- Reitzer L. Nitrogen assimilation and global regulation in Escherichia coli. Annu Rev Microbiol. 2003;57:155–176.
- Ryu YG, Butler M, Chater KF, Lee KJ. Engineering of primary carbohydrate metabolism for increased production of actinorhodin in Streptomyces coelicolor. Appl Environ Microbiol. 2006;72:7132–7139.
- Lau J, Tran C, Licari P, Galazzo J. Development of a high cell-density fed-batch bioprocess for the heterologous production of 6-deoxyerythronolide B in Escherichia coli. J Biotechnol. 2004;110:95–103.
- Chen N, Huang J, Feng ZB, Yu L, Xu QY, Wen TY. Optimization of fermentation conditions for the biosynthesis of L-threonine by Escherichia coli. Appl Biochem Biotechnol. 2009;158(3):595–604.
- Cheng LK, Wang J, Xu QY, Xie XX, Zhang YJ, Chen N. Effect of feeding strategy on L-tryptophan production by recombinant Escherichia coli. Ann Microbiol. 2012;62:1625–1634.
- Vadali RV, Bennett GN, San KY. Applicability of CoA/acetyl-CoA manipulation system to enhance isoamyl acetate production in Escherichia coli. Metab Eng. 2004;6:294–299.