Abstract
American foul brood (AFB) disease is a deleterious bacterial disease worldwide, caused by spore forming bacterium Peanibacillus larvae that affects honeybee larvae and causes a significant decrease in the honeybee population. Following symptomatical and bacteriological approaches combined with 16S rDNA sequencing, an assessment has been made to evaluate the presence of AFB disease in North-West Pakistan as no record for bee-associated bacterial disease from Pakistan is available. A total of 1276 samples from 1520 bee colonies (15 apiaries) were collected, of which 476 samples (37.30%) were found with symptoms of AFB. Biochemical and 16S rDNA analysis indicated that all these farms have Peanibacillus larvae infection. It is concluded that the prevalence of AFB bacterial disease to such an extent in these regions of Pakistan will devastate the apicultural industries in a large scale across the country.
Introduction
The distribution of infectious AFB (American foul brood) bacterial brood disease of honeybees is worldwide [Citation1] caused by Paenibacillus larvae that create substantial economic losses to beekeepers.[Citation2] It can destroy the entire colonies when the spores spread through the hive and once established in a region the eradication/suppression becomes very hard.[Citation3] Contaminated spores of the bacteria potently transmit a disease to the healthy brood. P. larvae spores stay practicable for many years in dried larvae and other hive products.[Citation4] Early diagnosis is therefore important to control the spread of AFB disease.
In recent years apiculture has become a profitable job in Pakistan. Approximately 7000 beekeepers are rearing the Apis mellifera species (non-native) in up-to-date beehives. A total of 300,000 colonies of Apis mellifera produce 7500 metric tons of honey each year. Honeybee flora is found throughout in Northern areas, Federally Administered Tribal Areas (FATA) and Azad Jammu and Kashmir (AJK) which can potentially support 1,000,000 honeybee colonies.[Citation5] Sidr honey is made from the nectar of the honeybees collecting from the blossoms of berry trees (Ziziphus nummularia, Ziziphus jujuba and Ziziphus mauritiana var.), fortunately berry trees are produced in abundance in the study area.[Citation6] These regions are important for the honey agro-food chain consisting of beekeepers, honey extraction plants, buyers, traders, exporters and carriers. Therefore, for the last few years honey has become one of the most important and profitable agricultural products for export in Pakistan. Furthermore, this study area has suffered heavily due to the onset of the Global War on Terrorism. Although the area represents one of the country's richest zones of biodiversity and it is a strong source of indigenous bee flora. Most of the population of the area is rural with a low literacy rate. Thus they are more dependent upon natural resources.[Citation6] Visual declining of honeybee population all over the globe especially in the study areas have brought attention to the need of understanding the pathogenic aspects of honeybee associated microbes. One of the major threats to bee farms may be due to the growth of pathogenic bacteria, which has many effects, including an economical level.[Citation2] To our knowledge, this is the first study in Pakistan with reference to the incidence of AFB disease or its related epidemiological aspects.
Materials and methods
Survey
Bacterial disease AFB was observed during the berry (Ziziphus spp) flowering seasons (August to October) in three districts: Bannu, Karak and Kohat. Five locations were selected in each district (). These locations represented the agro-ecological zones in south-eastern Kohat and central Karak and Bannu where berry plants are grown as major honey crops. It was also investigated that the surveyed colonies had not been treated for any bacterial diseases before sampling. Suspicious and diseased colonies were visually inspected.[Citation7,Citation8] A number of diseased brood cells that could be detected upon careful visual inspection using matchstick test were recorded.[Citation9] The irregular brood pattern and punctured cell caps were considered as AFB visual signs before sampling.[Citation10] Both ropey larval remains and dried-in scales were counted as AFB symptoms and included in the total number of AFB diseased cells. The use of any antibiotic against AFB disease and sterilization of the bee hive tools were strictly monitored.
Collection of samples
AFB-infected samples were collected by cutting out a piece of comb about 20 cm2, containing much of the dead or discoloured brood with separate disposable blade to prevent cross-contamination between samples. Brood comb was then wrapped in a paper bag and placed in a wooden box for further analysis. Ropey larval remains on wooden stick were used to examine cell contents and were separately packed for laboratory diagnosis. All samples were legibly labelled containing the information regarding sample type, bee farm's name, hives number, location and dates of the collection.
Culturing of bacteria
Suspensions were prepared from the preserved samples by mixing diseased material with 9 mL of sterile water in test tubes. Afterwards different dilutions were made i.e. 1/10, 1/100, 1/1000. 100 µL aliquots of the diluted sample were inoculated in previously prepared brain-heart infusion (BHI) Agar supplemented with thiamine (BHI broth 18.5 g, agar 5 g, thiamin hydrochloride 0.1 g/L, distilled water 400 mL) plates.[Citation11] The plates were incubated for 24–48 h at 37 °C. The colonies in master plates were differentiated morphologically and various colonies were picked for pure culture, streaked in new BHIT agar plate and incubated at 37 °C until the growth of pure colony was observed.
Microscopic examination
To observe the morphology of P. larvae like spores two drops of water were mixed with the sample. The suspension was then transferred by means of loop and smeared on a glass slide as a thin film, the smear was then air dried and fixed by heat. Carbol fuchsin was used as a staining agent and then applied to the smear for 30 sec. After remaining the excess of the stain, the slide was then gently dried. The bacteria were examined under oil immersion lens of the light microscope at 1000× magnification. The spore of P. larvae measured as 1.3 × 0.6 μm in size and was appeared to be ellipsoidal in shape.[Citation8,Citation10]
Biochemical tests
For further confirmation of visible AFB signs, various biochemical tests were performed for the identification of bacterial isolates with the help of Bergey's Manual. From each farm, five randomly selected samples were processed. The most important tests used for this purpose were indole test, catalase test, citrate utilization test, nitrate reduction test, citrate utilization, hydrolysis of starch, hydrolysis of casein and hydrolysis of gelatin.[Citation8]
Colony polymerase chain reaction (PCR) and gel electrophoresis
Colony PCR of selected isolates were carried out for the amplification of the 16S rDNA gene. A well-isolated overnight colony was picked and directly subjected to the PCR amplification [Citation12] Two specific primers for the 16S rDNA amplification were used, as forward (5'-GGC TCA GAA CGA ACG CT GG CG GC-3') and reverse primer (5'-CCC ACT GCT GCC TCC CGT AGG AGT-3'.[Citation13,Citation14] The reaction mix (25 µL) was made by adding 12.5 µL PCR master mix, 2.5 µL of each primer, 7.5 µL ultrapure nuclease free water and a determined number of the bacteria from a single colony were added. PCR amplification was carried by using thermal cycler (BioRed, USA).
PCR was carried out with program as follows; pre-denaturation step at 94 °C for 4 min, followed by denaturation at 94 °C for 40 sec, annealing at 65 °C for 1 min and extension 72 °C for 1 min (30 cycles), final denaturation step at 94 °C for 30 sec. PCR product (10 µL) was analysed after gel electrophoresis and 40 µL were purified with PCR clean up kit (Invitrogen Inc., USA). The DNA estimation was carried out by using Qubit dsDNA Hs assay Kit USA with Qubit 2.0 Fluorometer (Invitrogen, Life Technology, USA).
Sequencing of 16S ribosomal DNA
The pure quantified PCR products were sequenced using either forward or reverse primers and Big Dye Terminator Kit (Applied Biosystems Inc., USA) by Genetic Analyzer (ABI 377 Applied Biosystems Inc., USA). Bacterial isolates were recognized as the similar species when their 16S rDNA sequences shared ≥97% similarity with 16S rDNA gene.[Citation15]
Results and discussion
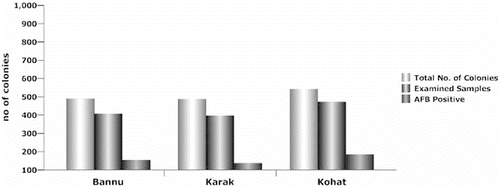
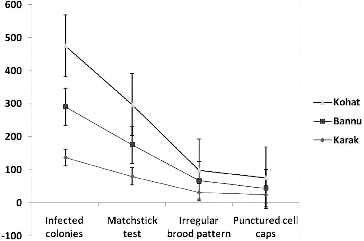
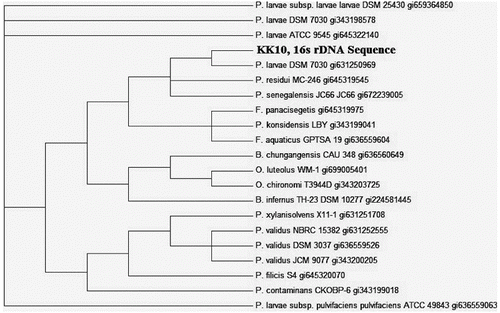
The prevalence of AFB, as shown in , was favoured to be in all the districts uniformly distributed. A total of 1276 examined samples 476 were found positive for AFB visible signs and confirmed by biochemical and 16S rDNA sequencing ( and ), indicating 37.30% incidence. District Kohat has the highest level of incidents 39%, followed by Bannu 37% and Karak 34.5% (). The study area map, showing the locations of beekeeping practices due to berry trees are higher contaminated than the rest areas in the districts (). The visual identification by matchstick test provided significant difference than the other two tests: irregular brood pattern and punctured cell caps (). These results also showed that good visual test for clinical AFB symptoms which is of basic importance for determining the risk of disease development as clinical symptoms of AFB are of more value in spreading the disease within colonies via drifting. Similarly contaminated pollen could also be a source of AFB infection [Citation16] for that reason the incidence of AFB disease in the three study areas were prevalent as the migratory bee-keeping activities in these areas are very common. Despite the prolonged use of antibiotics against AFB disease, the signs of AFB remain visible. Similarly hive tools sterility may contribute in AFB of honeybee spreading as no sterility measures were adopted by the local bee keepers (data not shown). 16S rDNA gene was amplified using colony PCR to strengthen the morphological and biochemical evidences. Pure PCR product was sequenced and maximum parsimony tree was constructed using MEGA 6 after BLAST and multiple alignments using ClustalW with sequences from the most closely related (≥97% similarity) to known taxa. The tree shows the relationship between strain KK10 and related taxa ().[Citation17,Citation18]
The even distribution of AFB among the above three regions may prove challenging for the health of bee farms in these and other regions of the country. On the other hand, it strongly indicates that samples of infected brood from individual colony are extremely efficient in detecting the visually diseased colonies. However, the three tests indicated that there is a substantial difference of AFB among three districts. This result suggests that regional survey is most important before any control scheme.[Citation19,Citation20] From the present study, it appears that district Kohat honeybee farms () have higher level of AFB prevalence which may produce shocking effects on honey productions, because colony health is equally important for the production of honey and other by-products of honeybees. The untreated foul brood disease not only destroys the hive bee population, but also wipes out an apiary because colonies which showed no visible AFB signs may be infected but not yet showing signs of disease.[Citation21] Such a finding is surprising as this was the area where the prevalence of AFB was not expected by beekeepers for several years. The higher level of AFB incidents in district Karak and Bannu () may be due to migratory beekeeping activities in these regions as it has become one of the strongest berry honey production areas in the province of Khyber Pakhtunkhwa ().
Scales contain millions of spores which are highly transferable and drive disease transmission within and between colonies. Once the apiary is contaminated, the AFB control measures are very difficult [Citation22] due to a high number of infectious spores because bacterial spores remain viable for long periods up to 35 years [Citation23] and are highly tolerable to climatic conditions.[Citation24] A foetal infection was initiated when 10 spores via contaminated larval food were administrated.[Citation24–29] The present finding further emphasizes the role of spore source in the transmission of AFB between colonies (data not shown). The bee equipment were not properly sterilized which may lead to the significant correlation of AFB infection within and between the colonies. Moreover, beekeeper always remains the principal spreading cause of the disease because if the combs or hive equipment are transferred from an AFB-infected colony to a healthy colony, it becomes contaminated. Furthermore, it is also reported that bees robbing honey from infected colonies also transmit the disease.[Citation30] However, worker bees themselves are the main spreading cause of brood disease. Additionally, the worker honeybee can get in touch through the same places (nectars, water sources, etc.) where bees from infected colonies usually forage.[Citation16] Similarly, during dearth conditions rubbing behaviour of bees can spread AFB between colonies. So supplementary sugar feeding should be the preventive measures to minimize the spread of bacterial disease in beekeeping [Citation31]
In addition to the clinical symptoms related with antibiotic treatment of AFB, bee farms using antibiotics for the control of AFB showed comparatively less prevalence than the farms that do not (Table S1 in the Online Supplementary Appendix). Although antibiotics are not efficient against the infectious spores, it suppresses the AFB visual symptoms and masks the disease but cannot alleviate AFB.[Citation24] Similarly, overuse of antibiotic can lead to the problem of resistance.[Citation32–34] As widespread resistance to oxytetracyclin (well-known antibiotic used for 50 years) has been investigated by treating different P. larvae isolates.[Citation35] So regular treatment, following manufacture instructions of antibiotic, may reduce the chances of AFB which affect the colony health, hence using antibiotics at six-month interval, showed significant symptoms of AFB disease (Table S1). According to Reynaldi et al., regular contact to effective antibiotics also plays a key role in the AFB disease.[Citation36] After 60 days of inoculation; colonies treated with tilmicosin were found to have no signs of AFB disease while in control colonies visible signs of the disease appeared after 120 days of the antibiotic treatment.[Citation36] However, the use of high concentrations of chemical and autoclaving hive equipment is not feasible for beekeeping practices.[Citation37] On the other hand, plant-based antibiotic proved viable and not harmful to the ecological environment.[Citation38] It was reported that among 50 plant extracts from the genus hypericum 14 were highly affective compounds, used against the vegetative form of P. larvae.[Citation39]
Additionally, political and economic disruption in the study areas caused by international war against terrorism resulted in most of the beekeepers to keep their bees not properly handled.[Citation40] Existing bee colonies showed pathogenic load of bacteria and increased demand for honey in the gulf countries is cited as critical issues to be considered immediately. Like in Europe, a remarkable decline in the bee hives was observed during the early 1990s caused by the Soviet collapse, rather than from widespread ecological factors.[Citation41] The socio-economic status of beekeepers drastically changed with the ending of the Soviet Union. Likewise, many beekeepers in northwest Pakistan were engaged in static jobs like shops and left the migration of bees from one floral area for the next due to current war events. These not only minimize the bee colonies [Citation42] but boost the bacterial disease load in the existing bee farms.
Conclusions
All solutions for the betterment of beekeeping are difficult to quantify. However, bacterial disease and no sterilization of hive tools all have measurable effects on honeybee population. Proper management of existing bee farms may recover the population of Apis mellifera and increase the productivity of honey and their by-products to a great extent. Therefore, every beekeeper should be vigilant for any sign of AFB and to make sure that the bees are healthy and disease free. While inspecting the presence of AFB, the matchstick test could be considered reliable to fully describe AFB visual symptoms in beekeeping. Furthermore, among molecular techniques for bacterial identification, denaturing gradient gel electrophoresis (DGGE) should provide comparatively better results. If AFB is suspected or confirmed, then contaminated wax combs should be burned. Similarly, honey from outside source and sugar should not be fed to bees as these may contain P. larvae spores.
Supplemental data
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/13102818.2015.1040454
Table_S1.pdf
Download PDF (172.9 KB)Acknowledgements
Staff, especially Nadia Ashraf, at Jamil-ur-Rehman Centre for Genome Research ICCBS University of Karachi is highly acknowledged for their help and support. We also thank the local beekeepers for sharing their original information and providing samples to us. Authors are thankful to Dr Sheikh Mahiuddin Department of Chemistry University of Karachi and Gul Nawaz and Farhan Shafique at KUST for correction and academic support. We thank Mr Junaid Ahmad Kori who has dedicated a lot of time to helpful discussion.
Disclosure statement
The authors declare that they have no conflict of interests.
References
- Antúnez K, D'Alessandro B, Piccini C, Corbella E, Zunino P. Paenibacillus larvae larvae spores in honey samples from Uruguay: a nationwide survey. J Invertebr Pathol. 2004;86:56–58.
- Genersch E, Forsgren E, Pentikäinen J, Ashiralieva A, Rauch S, Kilwinski J, Fries I. Reclassification of Paenibacillus larvae subsp. pulvifaciens and Paenibacillus larvae subsp. larvae as Paenibacillus larvae without subspecies differentiation. Int J Syst Evol Microbiol. 2006;56:501–511.
- Bastos EMA, Simone M, Jorge DM, Soares AEE, Spivak M. In vitro study of the antimicrobial activity of Brazilian propolis against Paenibacillus larvae. J Invertebr Pathol. 2008;97:273–281.
- Pohorecka K, Skubida M, Bober A, Zdańska D. Screening of Paenibacillus larvae spores in apiaries from Eastern Poland. Nationwide survey. Part I. Bulletin of the Veterinary Institute in Pulawy. 2012;56:539–545.
- Waghchoure-Camphora ES, Martin SJ. Population changes of Tropilaelaps clareae mites in Apis mellifera colonies in Pakistan. J Apicultural Res. 2009;48:46–49.
- Adnan M, Ullah I, Tariq A, Murad W, Azizullah A, Khan A, Ali N. Ethnomedicine use in the war affected region of northwest Pakistan. J Ethnobiol Ethnomed. 2014;10:1–16.
- Hansen H, Brodsgaard C. American foulbrood: a review of its biology, diagnosis and control. Bee World. 1999;80:5–23.
- De Graaf D, Alippi A, Brown M, Evans J, Feldlaufer M, Gregorc A, Hornitzky M, Pernal SF, Schuch DM, Titera D, Tomkies V, Ritter W. Diagnosis of American foulbrood in honey bees: a synthesis and proposed analytical protocols. Lett Appl Microbiol. 2006;43:583–590.
- Munawar MS, Raja S, Waghchoure ES, Barkat M. Controlling American foulbrood in honeybees by shook swarm method. Pakistan J Agric Res. 2010;23:53–58.
- Masry SHD, Kabeil SS, Hafez EE. New Paenibacillus larvae bacterial isolates from honey bee colonies infected with American foulbrood disease in Egypt. Biotechnol Biotechnol Equip. 2014;28:271–276.
- Gochnauer T. Growth, protease formation, and sporulation of Bacillus larvae in aerated broth culture. J Invertebr Pathol. 1973;22:251–257.
- Hartung F, Werner R, Hoque MI, Alam SS, Khan S, Paul AR, Mühlbach H-P. Association of phytopathogenic bacteria with top-dying disease of sundri tree (Heritiera fomes) in Bangladesh. Angew Bot. 1998;72:48–55.
- Khan IA, Khan A, Asif H, Jiskani MM, Mühlbach H-P, Azim MK. Isolation and 16s rDNA sequence analysis of bacteria from dieback affected mango orchards in southern Pakistan. Pak J Bot. 2014;46:1431–1435.
- Young J, Downer H, Eardly B. Phylogeny of the phototrophic rhizobium strain BTAi1 by polymerase chain reaction-based sequencing of a 16S rRNA gene segment. J Bacteriol. 1991;173:2271–2277.
- Mukhopadhyay J, Braig HR, Rowton ED, Ghosh K. Naturally occurring culturable aerobic gut flora of adult Phlebotomus papatasi, vector of Leishmania major in the old world. PloS one. 2012;7:e35748.
- Šekulja D, Pechhacker H, Licek E. Drifting behavior of honey bees (Apis Mellifera Carnica Pollman, 1879) in the epidemiology of American foulbrood. Zbornik Veleučilišta u Rijeci. 2014;2:345–358.
- Nei M, Kumar S. Molecular Evolution and Phylogenetics. New York (NY): Taylor & Francis; 2000.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729.
- Datta S, Bull JC, Budge GE, Keeling MJ. Modelling the spread of American foulbrood in honeybees. J R Soc Interface. 2013;10:20130650.
- Ritter W. Early detection of American foulbrood by honey and wax analysis. Apiacta. 2003;38:125–130.
- Reybroeck W, Daeseleire E, De Brabander HF, Herman L. Antimicrobials in beekeeping. Vet Microbiol. 2012;158:1–11.
- Shimanuki H. Identification and control of honey bee diseases. Agricultural Research and Extension Service, Taylor & Francis; 1983.
- Chaudhary O. Present status of pests and diseases of honeybees Apis mellifera L. In: Dr SK Garg, Dr MS Jakhar, editors. Taylor & Francis. 2014:p. 2097.
- Genersch E. American foulbrood in honeybees and its causative agent, Paenibacillus larvae. J Invertebr Pathol. 2010;103:S10–S19.
- Brodsgaard CJ, Ritter, W, Hansen, H. Response of in vitro reared honey bee larvae to various doses of Paenibacillus larvae larvae spores. Apidologie. 1998;29:569–578.
- Bamrick JF, Rothenbuhler WC. Resistance to American foulbrood in honey bees. IV. The relationship between larval age at inoculation and mortality in a resistant and in a susceptible line. J Insect Pathol. 1961;3:381–390.
- Genersch E, Ashiralieva A, Fries I. Strain-and genotype-specific differences in virulence of Paenibacillus larvae subsp. larvae, a bacterial pathogen causing American foulbrood disease in honeybees. Appl Environ Microbiol. 2005;71:7551–7555.
- Woodrow A. Susceptibility of honeybee larvae to individual inoculations with spores of Bacillus larvae. J Econ Entomol. 1942;35:892–895.
- Woodrow A, Holst E. The mechanism of colony resistance to American foulbrood. J Econ Entomol. 1942;35:327–330.
- Lindström A, Korpela S, Fries I. The distribution of Paenibacillus larvae spores in adult bees and honey and larval mortality, following the addition of American foulbrood diseased brood or spore-contaminated honey in honey bee (Apis mellifera) colonies. J Invertebr Pathol. 2008;99:82–86.
- Andi MA, Ahmadi A. Influence of vitamin C in sugar syrup on brood area, colony population, body weight and protein in honey bees. Intl J Biosc. 2014;4:32–36.
- Boligon AA, Brum TFd, Zadra M, Piana M, Alves CFdS, Fausto VP, Júnior Vdos S, Vaucher Rde A, Santos RC, Athayde ML. Antimicrobial activity of Scutia buxifolia against the honeybee pathogen Paenibacillus larvae. J Invertebr Pathol. 2013;112:105–107.
- Alippi AM, López AC, Reynaldi FJ, Grasso DH, Aguilar OM. Evidence for plasmid-mediated tetracycline resistance in Paenibacillus larvae, the causal agent of American foulbrood (AFB) disease in honeybees. Vet Microbiol. 2007;125:290–303.
- Lodesani M, Costa C, Man MC. Limits of chemotherapy in beekeeping: development of resistance and the problem of residues. Bull Univ Agric Sci Vet Med Cluj-Napoca Anim Sci Biotechnol. 2009;62:1–7.
- Evans J. Diverse origins of tetracycline resistance in the honey bee bacterial pathogen Paenibacillus larvae. J Invertebr Pathol. 2003;83:46–50.
- Reynaldi FJ, Albo GN, Alippi AM. Effectiveness of tilmicosin against Paenibacillus larvae, the causal agent of American foulbrood disease of honeybees. Vet Microbiol. 2008;132:119–128.
- Dobbelaere W, De Graaf D, Reybroeck W, Desmedt E, Peeters J, Jacobs F. Disinfection of wooden structures contaminated with Paenibacillus larvae subsp. larvae spores. J Appl Microbiol. 2001;91:212–216.
- Batabyal L, Sharma P, Mohan L, Maurya P, Srivastava C. Larvicidal efficiency of certain seed extracts against Anopheles Stephensi, with reference to Azadirachta indica. J Asia Pac Entomol. 2007;10:251–255.
- Hernández-López J, Crockett S, Kunert O, Hammer E, Schuehly W, Bauer R, Crailsheim K, Riessberger-Gallé U. In vitro growth inhibition by hypericum extracts and isolated pure compounds of Paenibacillus larvae, a lethal disease affecting honeybees worldwide. Chem Biodivers. 2014;11:695–708.
- Sammataro D, Weiss M. Comparison of productivity of colonies of honey bees, Apis mellifera, supplemented with sucrose or high fructose corn syrup. J Insect Sci. 2013;13(19):1–13.
- vanEngelsdorp D, Meixner MD. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J Invertebr Pathol. 2010;103:S80–S95.
- Mill AC, Rushton SP, Shirley MD, Smith GC, Mason P, Brown MA, Budge GE. Clustering, persistence and control of a pollinator brood disease: epidemiology of American foulbrood. Environ Microbiol. 2013;16(12):3753–3763.