?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The aim of the present study was to construct a recombinant strain of Escherichia coli LY13-05 that simultaneously expresses D-hydantoinase (Dhase) and D-decarbamoylase (Dcase) and exhibits a high-efficiency substrate conversion with a specific yield rate, close to those reported in the literature for other bacteria. The cultivation conditions, fermentation properties and immobilized recombinant strain performance were investigated. The results indicated that E. coli LY13-05 cultured in Terrific Broth (TB) medium containing 1% lactose and induced at 22 °C revealed the strongest total Dhase/Dcase activity. A fermentation with 30 g/L DL-p-hydroxyphenyl hydantoin (DL-HPH) as the substrate was conducted for 12 h using the immobilized strain. The cell density in the broth reached up to 1.9 g/L; the yield of D-HPG was 29.10 g/L; the yield of D-p-hydroxyphenylglycine (D-HPG)/HPH was 97.0%; the specific productivity was 1.276 g/g h and the productivity was 2.43 g/L h. The immobilized strain exhibited certain advantages in substrate conversion efficiency during the production of D-HPG and the specific yield rate, which were close to or better than the yield of D-HPG from other reported strains. Also, compared with the free cells, the immobilized E. coli LY13-05 cells had higher conversion efficiency and yield. Moreover, the immobilized strains had better thermal stability, higher repetition application time and longer storage duration, which will be more practical for industrial production.
Introduction
D-p-hydroxyphenylglycine (D-HPG) is an intermediate of a variety of pharmaceutical, chemical and chemical industry materials. It plays a vital role in the production of cephalosporin, penicillin and other beta-lactam semisynthetic antibiotics; thus, it has a good applicational prospect. In recent years, the production of D-HPG has been transferred from chemical to enzymatic methods. Among these methods, the one that uses a single strain with a dual enzyme system reveals the best efficiency.[Citation1–3] Dual enzyme method can generate D-hydantoinase (Dhase) and D-decarbamoylase (Dcase) at the same time to complete the asymmetric ring opening and hydrolysis reaction in order to obtain D-HPG from the substrate DL-p-hydroxyphenyl hydantoin (DL-HPH). Sometimes, in order to obtain higher yield of D-HPG, racemases, such as hydantoin racemase, are also used.[Citation4] The dual enzyme method can complete two consecutive enzyme-catalyzed reactions in a single cell during the process of substrate conversion, which avoids the oxidation of intermediates when they cross the membrane twice. This method improves the substrate conversion efficiency. In addition, it is also characterized with mild reaction conditions, simple processes, high specificity and productivity, and less pollution and energy consumption. Therefore, this method is suitable for industrial production.[Citation5–8] But the biotransformation by means of co-expression of Dhase/Dcase in the practical production of D-HPG is not yet well developed. Some problems remain in the large-scale industrial production. For example, most of the strains used in the production are screened wild strains such as soil bacteria from the genera Bacillus and Pseudomonas. Their enzyme activity and yield rate are low. Their genetic stability is uncertain and the batch quality of fermentation is unstable.[Citation9–11] In addition, this method requires the preparation of crude enzyme solution by means of microbial fermentation. Improper control will easily lead to infection. Moreover, 5-(2-methylthioethyl)hydantoin is also needed as an induction agent during the reaction process. Due to the strong smell, high cost and pollution, the application of this method has been limited in the industrial production.[Citation12,Citation13]
Therefore, in order to solve the difficulties in D-HPG production, in the present study, we used the dual enzyme method for the co-expression of Dhase/Dcase with the use of lactose as the inducer, by a recombinant immobilized Escherichia coli LY13-05 strain. Its fermentation conditions were optimized for obtaining the maximum amount of immobilized cells and completing a continuous substrate conversion for the production of D-HPG. This method could be implemented with low cost, less pollution and loss of enzyme activity, high stability of the enzyme activity, high efficiency of the substrate conversion, simple purification, high product quality and yield rate during the production of D-HPG. In addition, the calcium alginate embedding method is one of the most researched and widely used methods for cells immobilization. It has less microbial toxicity, produces higher cell density and is very convenient to solidify, as it has low mechanical strength.[Citation14] Sodium alginate has been successfully used for enzyme immobilization of lipase, glucose oxidase, tyrosinase and catalase.[Citation15,Citation16] Immobilization of E. coli LY13-05 strain with calcium alginate was used and the properties of the immobilized cells were studied in this paper. The present study will lay a theoretical foundation for the earlier realization of D-HPG industrial production by an enzymatic method.
Materials and methods
Strains and plasmids
The strain E. coli LY12-15 was purchased from Hangzhou Tianhe Microorganism Reagent Co., Ltd. Both plasmid and competent cells for the preparation of the recombinant bacterial strain were preserved in our laboratory.
The Dhase and Dcase genes are encoded separately in the chromosome of wild Pseudomonas aeruginosa LY11-12, preserved in our laboratory. The two genes were constructed into the T7 promoter-driven recombinant plasmid pLY11-3 and pLY11-5, respectively.
Culture medium and culture medium ingredients
The strain E. coli LY13-05 was inoculated into four different production media: Luria–Bertani (LB) medium, which included 10 g/L tryptone, 5 g/L yeast extract and 10 g/L NaCl, pH 7.0; Terrific Broth (TB), prepared with 12 g/L tryptone, 24 g/L yeast extract, 4 g/L glycerol, 170 mmol/L KH2PO4 and 0.72 mol/L K2HPO4; yield HPG medium (YH), which had 15 g/L tryptone, 7.5 g/L yeast extract, 1 g/L glucose, 2 g/L KH2PO4, 4 g/L K2HPO4, 7 g/L Na2HPO4·12H2O, 1.2 g/L (NH4)2SO4 and 0.2 g/L NH4Cl, pH 7.0; corn steep liquor, sodium glutamate, yeast extract culture medium (CSY), which contained 30 g/L corn steep liquor, 20 g/L sodium glutamate and 5 g/L yeast extract, pH 7.0.
Reagents and enzymes
T4 DNA ligase and XbaI/XhoI restriction enzymes were purchased from Takara Biotechnology (Dalian) Co., Ltd. RNA enzyme (Sigma R4875) was purchased from Sigma Company. Sodium alginate was purchased from China Henan Anli Fine Chemical Co., Ltd. The rest of the reagents were commercially available and were of analytically pure grade.
The construction of Dhase/Dcase-co-expressing recombinant E. coli LY13-05
Dhase/Dcase-co-expressing plasmid pEY6 was constructed, according to the method described by Hu et al. [Citation17], and then transferred into E. coli LY12-15. The products were identified by polymerase chain reaction (PCR) and dual enzyme digestion. The recombinant bacterial strain E. coli LY13-05 with co-expression capacity of Dhase/Dcase was obtained.
The optimization of recombinant bacterial strain E. coli LY13-05 fermentation conditions
E. coli LY13-05 was activated, cultured and inoculated into LB, TB, YH or CSY medium. The samples were collected for the determination of total Dhase/Dcase activity at the designated time (fermentation for 15 h and sample determination every 3 h) during the fermentation process to explore the optimal fermentation medium for E. coli LY13-05.
Based on the optimal culture conditions used in the previous study from Li et al., [Citation10] E coli cells in the present study were cultured at 37 °C with a rotation speed of 150 rpm and when the optical density of 600 nm (OD600) reached up to 0.7–0.8, lactose at the dose from 0% to 2% (w/v) was added for the expression induction. The cells were cultured for another 3–4 h at 30 °C after the induction. The samples were collected for determination of the inducer dosage effect on the activity of Dhase/Dcase.
In the optimal culture medium, after the addition of the optimal dosage of the inducer, lactose, the cells were cultured for another 3–4 h at 18 °C–36 °C after induction. The samples were collected to explore the effect of temperature on the total Dhase/Dcase activity.
Determination of total Dhase/Dcase activity
A total of 1 mL of fermentation broth was harvested and centrifuged at 12,000 rpm for 1 min for collecting the bacteria. The collected bacteria were washed with an appropriate volume (1 mL) of physiological saline once and then were centrifuged again (12,000 rpm for 1 min). The cell pellets were resuspended with 1.0 mL of 0.1 mmol/L phosphate buffer (pH 8.0) and 400 µL of the bacterial suspension were mixed with 400 µL of saturated DL-HPH solution (3% concentration, v/v). The mixture was incubated at 40 °C with a rotation speed of 150 rpm for 30 min in a water bath shaker. The fermentation reaction was terminated by adding 800 µL of 10% (v/v) trichloroacetic acid. Another 800 µL of the fermentation broth were transferred to an Eppendorf tube and centrifuged. After centrifugation, 100 µL of the supernatant were diluted to 1 mL with double distilled water (ddH2O). The concentration of D-HPG (g/L) was determined by high-performance liquid chromatography (HPLC).[Citation18] The total Dhase/Dcase activity was calculated by using EquationEquation (1)(1)
(1) :
(1)
(1) where C is the concentration of D-HPG (g/L). One unit of enzyme activity is defined as the required amount of the enzyme for generating 1 mol of the product in 1 min under the catalytic conditions.
Preparation of recombinant immobilized E. coli LY13-05 cells
Under the optimal culture conditions, when E. coli LY13-05 had the highest Dhase/Dcase activity, the cells were centrifuged and collected for the preparation of immobilized cells.
The 2.5% (w/v) sodium alginate and 5% (w/v) CaCl2 solutions were prepared first. After centrifugation, the cell pellets were washed with 0.5% (w/v) saline and then the cell suspension was subjected to centrifugation for two more times (12,000 rpm for 1 min). The recombinant bacterial suspension was adjusted to a concentration of 3 × 107 colony-forming units (CFU) by physiological saline and then transferred into a triangle bottle for future use. The recombinant bacterial suspension was mixed with sodium alginate solution to form a cell–alginate suspension. The cell–alginate suspension was collected with a 50-mL syringe and added into the CaCl2 solution dropwise. Under constant stirring conditions, gel beads with a diameter of approximately 2 mm were formed. After cross-linking in an aseptic environment for 30 min, the cross-linked products were stored in a refrigerator at 4 °C overnight for further hardening.[Citation16,Citation19]
Production of D-HPG by immobilized E. coli LY13-05 cells
One litre of 30 g/L DL-HPH solution (pH 9.0) was prepared and then mixed with the immobilized E. coli LY13-05 cells in nitrogen environment.[Citation20] The fermentation was conducted for 0–16 h with a shaking speed of 150 rpm at 37 °C. The growth curve of the immobilized cells during the fermentation process was established. The concentration of D-HPG products (D-HPG yield (g/L)) was determined by HPLC and compared with that from the non-immobilized E. coli LY13-05 cells under the optimal conditions.
After the immobilized cells’ fermentation, the fermentation broth was harvested after centrifugation and then subjected to vacuum distillation at 55 °C–65 °C. The residue was dissolved in 300 mL of 1 mol/L HCl. The suspension was sequentially subjected to filtration, pH adjustment to 5.0 (isoelectric points), crystallization and filtration. Finally, the D-HPG product with high purity was obtained. The purity of D-HPG product was calculated by specific rotation ([a]20D, c 1, 1 N HCl) and optical purity (%); specific rotation was measured at 589 nm on an automatic digital polarimeter (SAC-I 5951, ATAGO-China Guangzhou Co., Ltd).
The fermentation time (h) where the product concentration was the highest was used for calculation, and the cell of the strain harvest in the time was centrifuged and then weighted. The cell density (g/L), yield (%), productivity (g/L h), specific productivity (g/g h) and optical purity (%) were calculated by using EquationEquations (2)(2)
(2) –(Equation6
(6)
(6) ):
(2)
(2)
(3)
(3)
(4)
(4)
(5)
(5)
(6)
(6)
Application performance of immobilized E. coli LY13-05 cells
The total Dhase/Dcase activity in immobilized E. coli LY13-05 cells at 35 °C was determined and defined as 100%. Then the cells were kept at 35 °C–60 °C for 1 h and the dual enzyme activity was determined again to explore the thermal stability of the immobilized E. coli LY13-05 cells.[Citation21] The relative activity (%) was calculated by using EquationEquation (7)(7)
(7) :
(7)
(7)
After the first fermentation of the immobilized E. coli LY13-05 cells, the cells were rinsed with 0.5% (w/v) CaCl2 and sterile distilled water. The cleaned cells were immediately subjected to the next batch of fermentation. Under the identical reaction conditions, the content of D-HPG product was determined. The same fermentation was repeated 10 times to explore the repeated application performance of the immobilized E. coli LY13-05 cells.
The prepared E. coli LY13-05 immobilized cells were dissolved in TB culture medium containing 0.5% (w/v) CaCl2, and stored in a refrigerator at 4 °C. The cells were taken out every two days for the conduction of fermentation tests. The content of D-HPG product was determined to explore the storage period of the immobilized E. coli LY13-05 cells.
Similarly, the thermal stability, repeated application performance and storage period of free E. coli LY13-05 cells were determined and the comparison between free E. coli LY13-05 cells and immobilized E. coli LY13-05 cells was conducted.
Data analysis
All tests in this paper were repeated three times or more. One-way analysis of variance (ANOVA) was used in the statistics and analyzed using SPSS 13.0 statistical software. All data were presented as mean ± standard error. Comparisons among groups were performed using a two-sample t-test, a value of p < 0.05 was considered statistically significant.
Results and discussion
The optimization of immobilized E. coli LY13-05 cells fermentation conditions
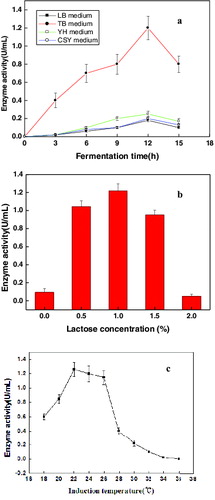
The results of E. coli LY13-05 cells cultured in different media at various induction temperatures and inducer dosages are shown in . The E. coli LY13-05 cells cultured in the ordinary LB medium revealed the weakest total Dhase/Dcase activity. The strongest total Dhase/Dcase activity was obtained in TB culture medium ((a)). The optimal TB culture medium could result in the largest induction amount of the protein. In contrast, the total Dhase/Dcase activity of E. coli LY13-05 cells in YH and CSY media was not on a higher level than the activity observed in TB medium; thus, both media were not suitable for the cultivation of E. coli LY13-05 cells. In addition, the total Dhase/Dcase activity of E. coli LY13-05 cells was reached up to the highest level (1.22 U/mL) in the presence of 1% lactose as the inducer ((b)) and the total Dhase/Dcase activity of E. coli LY13-05 cells reached 1.26 U/mL under an induction temperature of 22 °C ((c)). Thus, the E. coli LY13-05 cells, induced in TB medium with 1% lactose at 22 °C, had the strongest total Dhase/Dcase activity. The obtained results from the previous research of Li et al. [Citation10] showed that the highest total Dhase/Dcase activity was 0.89 U/mL, which was lower than the activity obtained in the present research. The higher enzyme activity obtained in our study is beneficial to lower the cost and improve the production efficiency.
Comparison of D-HPG production in free and immobilized E. coli LY13-05 cells
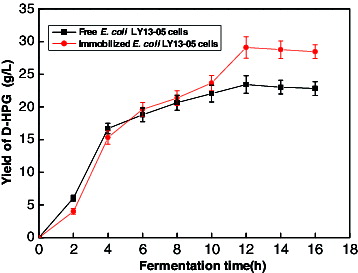
Free and immobilized E. coli LY13-05 cells were included to the fermentation broth after adding 30 g/L DL-HPH substrate. As shown in , with the extension of the fermentation time, the yield of D-HPG increased due to the transformation of the DL-HPH substrate by both free and immobilized E coli LY13-05 strain. The D-HPG yield of the immobilized E. coli LY13-05 cells was slower than that of the free cells during the first 0–6 h. After 6 h, the conversion rate of the immobilized cells exceeded that of the free cells, so that higher yield of D-HPG was achieved from the immobilized E. coli LY13-05 cells. After fermentation for 12 h, the yield of D-HPG from the immobilized cells was 29.10 ± 1.65 g/L, while the yield of D-HPG from the free cells was 23.42 ± 1.35 g/L. After data analysis by one-way ANOVA, the difference between the two groups reached the significant level, p < 0.05. In conclusion, the results of the study showed that the fermentation product D-HPG had a higher yield when immobilized E. coli LY13-05 cells were used.
Figure 2. Comparison of D-HPG production from free and immobilized E. coli LY13-05 cells. Note: Error bars represent standard errors of the means; data analyses were done using standard deviation.
In order to comprehensively understand the fermentation performance of E. coli LY13-05 cells for the production of D-HPG, the immobilized E. coli LY13-05 strain was compared with other reported microbial strains with a Dhase/Dcase production capacity. As shown in , a fermentation with 30 g/L DL-HPH as substrate was conducted using the immobilized strains for 12 h; the cell density in the broth reached up to 1.9 g/L, the yield of D-HPG was 29.10 g/L, the yield of D-HPG/HPH was 97.0%, the specific productivity was 1.276 g/g h and the productivity was 2.43 g/L h. While, from the other reported strain fermentations, the highest D-HPG yield was 28.80 g/L, the highest yield (substrate conversion rate) was 96.9%, the highest specific productivity was 0.221 g/g h and the highest productivity was 2.21 g/L h.[Citation5,Citation10,Citation22,Citation23] The result indicated that, on the same culture conditions, the immobilized E. coli LY13-05 strain had its certain advantages in substrate conversion efficiency and yield during the production of D-HPG. The yield of D-HPG from the immobilized E. coli LY-1305 strain was better or close to the other reported strains.
Table 1. Comparison of D-HPG production from one-step transformation in different microorganisms with the co-expression capacity of Dhase/Dcase.
As mentioned previously, after purification by using centrifugation, vacuum distillation, HCl, adjustment of pH, crystallization and filtration, 25.61 g/L D-HPG product was obtained, the production rate of D-HPG reached up to 88%, the specific rotation of D-HPG product was obtained, [a]20D was −155.2° (c 1, 1 N HCl) and the optical purity reached up to 99.5%. Moreover, HPLC results illustrate that part of the intracellular substances from cell conversion will penetrate into the reaction system, which results in increased difficulty of product purification. In contrast, for the immobilized cells, however, there were no such defects.[Citation24] Thus, more pure product of D-HPG can be obtained by immobilized cells fermentation.
The application performance of immobilized E. coli LY13-05 cells
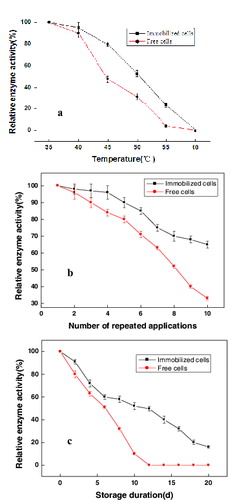
The thermal stability, repeated application stability and storage period of the immobilized E. coli LY13-05 cells were studied. As shown in , the immobilized cells had higher thermal stability than the free cells. With the increase of the temperature, the relative activity revealed a reduction and even a complete inactivation at 60 °C ((a)). With the increase in the number of applications, the immobilized cells exhibited a decrease in relative activity, but the decline was slower than that in the free cells. The relative enzyme activity of the immobilized cells remained 85% after six repeated applications and the immobilized gel beads were still intact even after 10 repeated applications ((b)). With the increase of storage duration, the relative activity in both free and immobilized cells revealed a decreasing trend and the half-life of both free and immobilized cells were 6 and 12 days of storage, respectively ((c)). Thus, the immobilized cells had better storage properties. In conclusion, the results of the study showed that the application performance of the immobilized E. coli LY13-05 cells was better than that of the free cells. Compared with the free cells, the immobilized cells improved their thermal stability by 33%, had an 18% higher relative enzyme activity after repeated applications and a 60% better relative enzyme activity during storage. Thus, the immobilized E. coli LY13-05 cells had better performance than the free E. coli cells.
Figure 3. Performance of immobilized and free E. coli LY13-05 cells. Thermal stability (a), repeated application stability (b) and storage duration performance (c). Note: Error bars represent standard errors of the means; data analyses were done using standard deviation.
At present, enzyme immobilization during the process of D-HPG production by using biological enzyme method, for increase of the stability of Dhase and Dcase, is a commonly used method. Yet, few researches of cell immobilization have been done so far.[Citation19,Citation25] When compared with immobilized enzymes, immobilized cells are less sensitive to the environment and have higher stability,[Citation26] therefore they may have more applicational prospects in industry.
Final remarks
The application of Dhase/Dcase co-expression for the production of D-HPG is not yet well developed. The co-expression of Dhase/Dcase needs the strong-smelling 5-(2-methylthioethyl)hydantoin as an inducer. The product quality is not stable and the culture is susceptible to phage pollution, so the yield from the fermentation process might be influenced.[Citation1,Citation27] In our study, a recombinant bacterial strain E. coli LY13-05 that can co-express Dhase/Dcase was established to produce D-HPG by using a dual enzyme system in a one-step manner. The fermentation performance and yield rate of its product were better or close to those of all other reported bacterial strains used in the production of D-HPG through fermentation. This method improves the substrate conversion efficiency and reduces the costs of production. The immobilized E. coli LY13-05 cells in the present study proved to have better thermal stability, higher stability after repetitious applications and longer storage duration during the reaction of substrates in water solution than the free cells, and are more conducive to the actual application of D-HPG production. In our study, the yield rate of D-HPG reached up to 88%, and the purity was 99.5%. The production of D-HPG using immobilized cells proved to be suitable for industrial production. Therefore, we are devoting our studies to carrier types and the optimization of high-density fermentation for immobilized cells. It is expected to enhance further the substrate conversion efficiency, reduce the production costs and promote the industrial application of one-step production of D-HPG by adopting the dual enzyme method.
Conclusions
In this paper, a recombinant immobilized E. coli LY13-05 strain that can simultaneously express Dhase/Dcase was constructed to produce D-HPG with the use of lactose as an inducer. The method combinedly used the dual enzyme and recombinant cells and lactose inducer to produce D-HPG by one-step. It is beneficial for the large-scale application of D-HPG production in the future.
Acknowledgements
This study was accomplished together by Department of Environment and Chemistry Engineering from Yellow River Conservancy Technical Institute and College of Life Sciences at Longyan University. Both made the equal contribution to this project.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Nanba H, Ikenaka Y, Yamada Y, Yajima K, Takano M, Takahashi S. Isolation of Agrobacterium sp. strain KNK712 that produces N-carbamyl-D-amino acid amidohydrolase, cloning of the gene for this enzyme and properties of enzyme. Biosci Biotechnol Biochem. 1998;62:857–881.
- Wiese A, Syldatk C, Mattes R, Altenbuchner J. Organization of genes responsible for the stereospecific conversion of hydantoins to α-amino acids in Arthrobacter aurescens DSM 3747. Arch Microbiol. 2001;176:187–196.
- Li Y, Kan Z, Zhu B. Advances in study of D-p-hydroxyphenylglycine synthesized by microbial enzyme method. J Biol. 2003;20:11–13.
- Wiese A, Wilms B, Syldatk C, Mattes R, Altenbuchner J. Cloning, nucleotide sequence and expression of a hydantoinase and carbamoylase gene from Arthrobacter aurescens DSM 3745 in Escherichia coli and comparison with the corresponding genes from Arthrobacter aurescens DSM 3747. Appl Microbiol Biotechnol. 2001;55:750–757.
- Park JH, Kim GJ, Kim HS. Production of D-amino acid using whole cells of recombinant Escherichia coli with seprarately and coexpressed D-hydantoinase and N-carbamoylase. Biotechnol Progr. 2000;16:564–570.
- Lee CK, Lee ZS, Yang PF. Effect of cell membrane of Agrobacterium radiobacter on enhancing D-amino acids production from racemic hydantoins. Enzyme Microb Technol. 2001;28:806–814.
- Jiang M, Shang L, Wei P, Yu R, Shen N, Ouyang P, Chang HN. Pilot-scale production of D-p-hydroxyphenylglycine from DL-5-p-hydroxyphenylhydantoin by Burkholderia cepacia JS-O2. Enzyme Microb Technol. 2007;41:407–412.
- Nozaki H, Kira I, Watanabe K, Yokozeki K. Purification and properties of D-hydantoin hydrolase and N-carbamoyl-D-amino acid amidohydrolase from Flavobacterium sp. AJ11199 and Pasteurella sp. AJ11221. J Mol Catal B Enzym. 2005;32:205–211.
- Hartley CJ, Kirchmann S, Burton SG, Dorrington RA. Production of D-amino acids from D, L-5-substituted hydantoins by an Agrobacterium tumefaciens strain and isolation of a mutant with inducer-independent expression of hydantoinhydrolying activity. Biotechnol Lett. 1998;20:707–711.
- Li Z, Hu Z, Liu J. Optimization of the enzymatic synthesis of N-carbamoyl-D-P-hydroxyphenylglycine using D-hydantoinase genetic engineering strain. Chin J Biochem Mol Biol. 2002;18:553–558.
- Kirchmann S, Vanzyl P, Brady D, Abrahams N, Rech S, Dorrington R, Burton S. A dual phase fermentation protocol for the production of hydantoinase and carbamoylase by the wild type Pseudomonas putida RU-KM3. Enzyme Microb Technol. 2007;41:539–545.
- Li Z, Hu Z, Liu J. High expression of D-hydantoinase gene from Pseudomonas putida in E. coli. Chin J Biochem Mol Biol. 2002;18:145–150.
- Wu S, Yang L, Liu Y, Zhao G, Wang J, Sun W. Enzymatic production of d-p-hydroxyphenylglycine from dl-5-p-hydroxyphenylhydantoin by Sinorhizobium morelens S-5. Enzyme Microb Technol. 2005;36:520–526.
- Taqieddin E, Amiji M. Enzyme immobilization in novel alginate–chitosan core–shell microcapsules. Biomaterials. 2004;10:1937–1945.
- Betigeri SS, Neau SH. Immobilization of lipase using hydrophilic polymers in the form of hydrogel beads. Biomaterials. 2002;17:3627–3636.
- Blandino A, Macias M, Cantero D. Calcium alginate gel as encapsulation matrix for coimmobilized enzyme systems. Appl Biochem Biotechnol. 2003;1:53–60.
- Hu X, Li Q, Cong J. [Co-expression of D-hydantoinase and N-carbamoylase in recombinant Escherichia coli]. Chin J Process Eng. 2006;6:948–953. Chinese.
- Song H, Tu C, Ouyang P. [Determination of p-hydroxy-D-phenylglycine with high performance liquid chromatography]. J Nanjing Technol Univ. 2005;24:97–99. Chinese.
- Aranaz I, Acosta N, Heras A. Synthesis of p-hydroxyphenylglicine by cell extract from Agrobaterium radiobacter encapsulated in alginate capsules. Enzyme Microb Technol. 2006;39:215–221.
- Yin BD, Chen YC, Lin SC, Hsu WH. Production of D-amino acid precursors with permeabilized recombinant Escherichia coli with d-hydantoinase activity. Process Biochem. 2000;35:915–921.
- Chen YC, Yin BD, Lin SC, Hsu WH. Production of N-carbamoyl-d-hydroxyphenylglycine by D-hydantoinase activity of a recombinant Escherichia coli. Process Biochem. 1999;35:285–290.
- Runser S, Chinski N, Ohleyer E. D-p-hydroxyphenylglycine production from DL-5-p-hydroxyphenylhydantoin by Agrobacterium sp. Appl Microbiol Biotechnol. 1990;33:382–388.
- Chao YP, Fu H, Lo T, Chen PT, Wang JJ. One-step production of D-p-hydroxyphenylglycine by recombinant Escherichai coli strains. Biotechnol Progr. 1999;5:1039–1045.
- Yu W, He XP, Cao PM, Liu F, Chen GG, Zhang YK, Wu KY, Liang ZQ. [Production of L-phenylalanine by biotransformation of cinnamic acid suing immobilized whole Rhodotorula glutinis cells]. Food Sci Technol. 2011;36:7–11. Chinese.
- Chen JT, Chao YP. Chitin-binding domain based immobilization of D-hydantoinase. J Biotechnol. 2005;117:267–275.
- Chen JB, Xu Y. [Advances on enzymatic synthesis of D-hydroxyphenylgline]. J Biol. 2007;24:8–11. Chinese.
- Zhang X, Mo Z, Wang L. [Study on characteristics of the strain Pseudomonas producing DHase and DCase]. Chin J Pharm Biotechnol. 2009;16:76–79. Chinese.