Abstract
Tussilago farfara L. (Asteraceae) is widespread in Bulgaria and commonly known as ‘podbel.’ Although T. farfara is a common and widely used herb in folk medicine in the past and today, a study of alkaloid content from Bulgarian populations, which is the aim of this study, has not been done yet. Conventional processing of the dried and powdered plant material (Farfarae folium) including a mild reduction with Zn/HCl of the methanol extracts to convert N-oxides to tertiary bases resulted in the preparation of crude alkaloid mixture (CAM) of pyrrolizidine alkaloids (PAs). The alkaloids were identified by gas chromatography–mass spectrometry analysis of the CAM. A total of four PAs were detected in Farfarae folium herbal substance. Senkirkine and senecionine were found to be major alkaloids, together with integerrimine and seneciphylline, as minor components. The alkaloid content was relatively low (0.0055%) or 55 µg/g. All of the above-mentioned alkaloids were unsaturated at 1,2 position and belonged to the group of the highly hepatotoxic macrocyclic diesters. The content of PAs has to be controlled in processes of T. farfara herbal gathering and herb production, quality control of food, nutrient supplements and other coltsfoot based products. The good botanical identification of T. farfara and morphologically closer PA content species is a prerequisite for quality monitoring and control of plant based products. Microscopic differentiation of the leaves of coltsfoot and butterbur (Petasites spp.) was established and described.
Introduction
Tussilago farfara L. (Asteraceae) is a commonly known medicinal plant in Bulgaria with the most common name ‘podbel’ (bottom is white). This folk name shows the distinct colour of the upper and lower surface of the leaves, namely, white to greyish white lower surface. This well-distinguishable morphology is the basis of phytonims and in the other Slavic folk botany.[Citation1] Coltsfood is a perennial plant widespread in Bulgaria on embankments (soil banks), wet shores, screes, sewers and abandoned places on clay (loamy) soils, in the valleys and foothills up to 1200 m (1800 m) above sea level. It is a pioneer species which is colonizing by anemochory on disturbed and ruderal areas.[Citation2,Citation3] T. farfara is a member of monotypic genera in Bulgaria and is well distinguished in the field.[Citation4,Citation5] Ethnobotany provided data for coltsfoot as a valuable medicinal plant that has been used in folk remedies as herbal tea for a wide range of disorders, such as gastrointestinal and urinary ailments and mainly for pulmonary complaints. The plant is considered a good remedy for blood purification, especially for rheumatism and skin irritations. The fresh crushed leaves are used for external application for the treatment of wounds, burns, injuries, inflammation of the eye, based on the antibacterial and anti-inflammatory properties of the plant. The leaves have been used for smoking as herbal tobacco against asthma.[Citation6,Citation7] Coltsfoot is considered as a honey plant with importance in Europe.[Citation3,Citation8] Although the use of the leaves as edible greens has been reported from several areas of the Balkans [Citation9,Citation10] and neighbouring regions,[Citation11–14] till now, in Bulgaria, such data are not observed.[Citation15]
Flowers and leaves are the parts from coltsfoot that are being used. They are prepared as a powder, extract, infusion and juice (from fresh leaves). Mostly leaves are used daily (it is assumed to be dried leaves, Farfarae folium) as a part of a multi-botanical combination (6–24 mg/daily amount).[Citation16]
The main compounds of the plant are the polysaccharides with anti-inflammatory and immunostimulating activities (mucopolysaccharides, pectin, inulin), flavonoids with anti-inflammatory and antispasmodic action (quercetin, kaempferol and their glycosides, hiperoside), terpenes (tussilagon, α- and β-amyrin, faradiol, bisabolene epoxide), sterols and phenolic acids.[Citation17–20] Pyrrolizidine alkaloids (PAs), including senkirkine, senecionine, seneciphylline, integerrimine, tussilagine and isotussilagine may be present in variable amounts, usually very minor, depending on the source.[Citation20] PAs are of special interest currently, because several of them have been shown to cause toxic reactions in humans, primarily veno-occlusive liver disease, when ingested with foods or herbal medicines. Several PAs have been regarded as both hepatotoxic and carcinogenic.[Citation21–24]. Over the past decade, a number of monitoring studies in Europe on food, honey, feed, herbal teas and tea varieties have been conducted, which showed the presence of PAs in these products.[Citation25–29] In many countries the use of such plants in herbal products is prohibited or restricted.[Citation21,Citation30]
PAs are toxins, exclusively biosynthesized by plants. They are typical plant secondary metabolites against herbivores. PAs are mainly found in Boraginaceae (all genera), Asteraceae (Senecioneae and Eupatorieae) and Fabaceae (Crotalaria).[Citation24] Considering that PAs are natural constituents of a number of plants used for medicinal purposes and that PAs might be part of the food chain, the Committee on Herbal Medicinal Products decided to prepare a public statement on the use of herbal preparations containing PAs,[Citation22] which is currently in process of the risk assessment final report. In Bulgaria the PAs profile has been investigated only for Senecio spp.[Citation31,Citation32] Data are available about PA content in T. farfara from Europe and China – senkirkine, senecionine, tussilagine, isotussilaginine, tussilaginine, syneilesine, acetylsyneilesine, integerrimine, seneciphylline and neosenkirkine.[Citation20,Citation33–36]
Although T. farfara is a common and widely used herb in folk medicine in the past and today, a study for the PA content from Bulgarian populations, which is the aim of this study, has not been done yet. The presence of PAs requires good botanical identification of the herbal substance for future monitoring.
Materials and methods
Plant material
Leaves of T. farfara were collected in the vicinity of Smolyan (Central Rhodopes floristic region), Bulgaria. Ruderal habitat, Date: 20/06/2009. Leg.: Det.: A. Nedelcheva. Voucher: AMN 13-43/09. Coordinates: 41°34'56″ N 24°40'37″ E. Herbal substance: air-dried leaves, Farfarae folium.
Extraction and identification
Air-died, powdered material (450 g) was exhaustively extracted with MeOH. The combined MeOH extracts, after evaporation to dryness, were acidified with 5% HCl, filtered and extracted with CH2Cl2. The aqueous acid solution was stirred with Zn dust (24 h, 10% of plant weight), then filtered and made alkaline with 25% NH4OH to pH 9. The alkaline solution was extracted with CH2Cl2 to afford 25 mg crude alkaloid mixture (CAM), which was additionally purified by preparative thin layer chromatography (TLC) and analysed by means of gas chromatography–mass spectrometry (GC-MS). The identification of the alkaloids was made by comparing the mass fragmentation patterns and relative retention time with those of reference compounds and literature sources.
The GC-MS analysis was performed with GC Hewlett Packard 6890 plus MS detector Hewlett Packard 5973. Column: HP 5-MS, (30 m × 0.25 mm i.d df = 0.25 µm); conditions: injector 260 °C, temp. program 100 °C (2 min) to 280 °C, 5 °C/min, isothermal at 280 °C for 20 min; split ratio 1:50; carrier gas: He const. flow 0.8 mL/min, sample 1 mg CAM. Relative retention index (RRI) was calculated by model mixture from normal hydrocarbons (C16, C18 … C32), which was used as reference mixture.[Citation37,Citation38] TLC: aluminium sheets, silica gel 60 F254 (Merck), bands detected under ultraviolet light or by Dragendorff reagent; mobile phase acetone: 25% NH4OH (2:0.3) and (2:0.5); preparative TLC: silica gel GF254 (Merck), mobile phase CHCl3–MeOH (9:1).
In many cases PAs have been reported to exist partially as highly water-soluble N-oxides. Conventional processing of the dried and powdered plant material including a mild reduction with Zn/HCl of the methanol extracts to convert N-oxides to tertiary bases resulted in the preparation of CAM. The procedure is justified by the fact that in the human body, pyrrolizidine N-oxides are reduced by the intestinal flora and show the same toxicity as the corresponding tertiary alkaloids.
Botanical characteristic
Field collected rosette leaves were used in this study. Plant samples were softened and free-hand sections were prepared. For morphological investigations related size measures, 20 plant individuals from each population or samples were studied. Plant samples preparing for microscope observation followed a standard technology for microscopy in the identification of herbal medicines.[Citation39–42]
Dry herbal substances were analysed and photographed using the light microscope Olympus BX with digital camera Olympus SP-510UZ and stereomicroscope Stemi 2000/ Carl Zeiss and Axio Cam. C. Image analysis was performed with digital image processing software ZEN Carl Zeiss.
Results and discussion
A total of four PAs were detected in Farfarae folium herbal substance. Senkirkine and senecionine were found to be major alkaloids, together with integerrimine and seneciphylline, as minor components. The alkaloid content was low (0.0055%) or 55 µg/g. All of the above-mentioned alkaloids are unsaturated at 1,2 position and belong to the group of the macrocyclic diesters ( and ).
Table 1. GC-MS data of established PAs.
Figure 1. Structure of pyrolizidine alkaloids (PAs) in Tussilago farfara. Senkirkine (A), Senecionine (B), Integerrimine (C) and Seneciphylline (D).
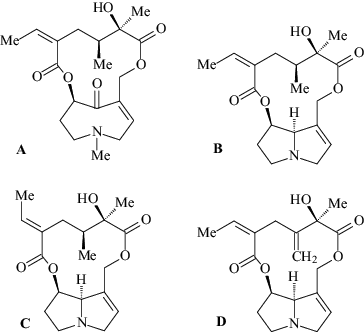
All recorded alkaloids belong to senecionine- and otonecine-type PAs and occur particularly in the Senecioneae (Asteraceae), but are also found in Crotalaria spp. (Fabaceae).[Citation21] All four found PAs were indicated for coltsfoot in previous studies.[Citation35] However, most of the studies were done on the first two alkaloids (senkirkine and senecionine), which are dominant and promising as markers in the determination of PA compounds in coltsfoot.
Known previous data on the quantity of PAs in coltsfoot leaves are obtained by different methods and approaches in the studies, which makes it difficult to compare them. However, the data are widely ranging from 0 to 368 µg/g.[Citation33,Citation36] For 20 natural populations from Poland, Adamczak et al. [Citation33] reported (senkirkine and senecionine) content in the range of 0.06–1.04 μg/g. According to these data, PAs, found in the studied Bulgarian population of T. farfara, were close to the lower range limit and could be perceived as population with low alkaloid content. Factors that affect the accumulation of alkaloids are still under discussion, but it is outlined that variability of PAs is mainly genetically determined and it can be increased by environmental factors.[Citation33]
The presence of the double bond is an important determinant in the hepatotoxicity of PAs, because only those with 1,2-unsaturation are hepatotoxic. The toxicity of PAs is due to metabolic activation. The liver is the most active site where the PAs are oxidized into extremely reactive pyrrole derivatives, which are capable of irreversible covalent binding to biopolymers, such as nucleic acids and functional proteins within cells. Some of the reactive metabolites might survive long enough to be transported into the bloodstream from the liver to other organs. All the toxic effects of the PAs are due to the alkylating activity of these pyrrole derivatives. Pyrrolizidine diesters such as senkirkine, senecionine, integerrimine and seneciphylline have been shown to act as bifunctional alkylating agents that may cross-link DNA. They are more toxic than the monofunctional pyrrolizidine monoesters.[Citation22] The German Commission E had set a standard for the use of the herb, in which the daily dose should not contain more than 1 μg PAs, with limited duration of administration of six weeks total per year and 0.1 µg/day (no restriction, for medicinal products only) in order to avoid any risks for consumers.[Citation21,Citation22,Citation25] Currently the European Medicines Agency (EMA) set the amount of PAs within the daily dose <0.035 μg for adults (body weight of 50 kg). For children the usage daily amount would be 0.014 μg PAs/day (body weight of 20 kg).[Citation21,Citation22]
The weight of one air-dried leaf sample (medium size) was 0.4 g ± 0.15 g that relative to the study population means 22 μg. PA content in air-dried matter and hypothetically would provide 0.44 μg PAs for adults and 1.1 μg PAs for children. In both cases, the values are higher than the requirements of the EMA, according to which exposure to PAs should be kept as low as practically achievable. Member States should take steps to ensure that the public are protected from exposure and the following thresholds should be applied.[Citation22]
There are several documented case studies where the use of the leaves of other species that may be confused with coltsfoot has led to toxic effect and disability in patients. Leaves of Adenostyles alliariae (Gouan) Kern and Petasites hybridus (L.) P.Gaertn., B.Mey. & Scherb. have been used as wrong herbal substances.[Citation43]
Coltsfoot collected from Bulgaria may be adulterated with leaf material of various species from the genus Petasites (), in which rosette leaves appear before blossoming too. Butterbur is also known as PAs dangerous herb [Citation44,Citation45] and contains PAs integerrimine, petasitenine, senkirkine, senecionine, neopetasitenine, neoplatyphylline, isotussilagine, tussilagine and seniciphylline.[Citation35] Very rarely leaves of Arctium can be wrongly collected. The plants are clearly distinguishable with a field guide. Substitution of Petasites species can occur with dried leaf material, as they are difficult to distinguish. Their leaves can be difficult to distinguish macroscopically in cut leaf material, but are readily discernible using microscopy.
Table 2. Microscopic differentiation of the leaves of coltsfood (Tussilago farfara) and Petasites spp.
Botanical characteristic and herbal substance identification of Tussilago farfara
Surface view
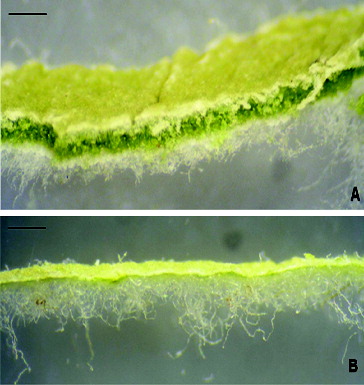
Upper epidermis of polygonal cells with dense cuticular striations and large anomocytic stomata, 35 µm long; the density of indumentums on the upper surface varies with leaf age: young leaves have tufts of long uniseriate covering trichomes up to 600 µm long (). Adult leaves were generally glabrous. Lower epidermis was densely tomentose and most covering trichomes uniseriate ≥1 mm long, base of few small cells, followed by one larger spherical cell, and one extremely long, twisted and slightly thick-walled terminal cell; the indumentum obscures the epidermal cells unless it has been removed by processing; if removed, cells with wavy anticlinal walls and numerous large anomocytic stomata – 35 µm long could be seen and under low magnification the aerenchyma of the mesophyll was visible through the surface ().
Transverse section
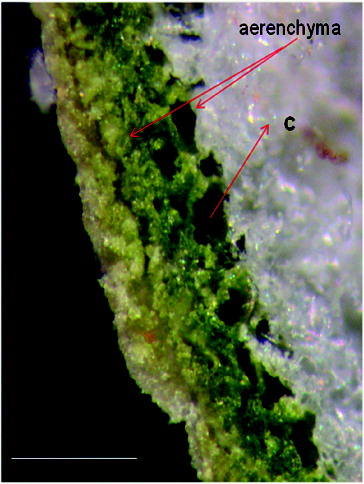
Bifacial; palisade cells in three or four rows; spongy mesophyll consists of aerenchyma with very large cavities separated by narrow cell layers; sphaerocrystals of inulin may occur ().
Powder
Primarily fragments of the long terminal cells of the covering trichomes, frequently in tangles; upper epidermis with polygonal cells, stomata and cuticular striations; lower epidermis with wavy cell walls and stomata; few bundles of fibres from the petiole.
Established morphological characteristics of coltsfoot confirm the essential ones described for this species microscopic characteristic.[Citation39,Citation41] Microscopic differentiation of the leaves of coltsfoot and butterbur (Petasites spp.) was established and described. The aerenchyma spaces (cavities) in Petasites are not as large as in T. farfara ().
Conclusions
The present data were the first study of alkaloid content from Bulgarian populations of coltsfoot (T. farfara). Our investigations indicated that the Bulgarian natural population of T. farfara was with relatively low level of toxic PAs. The content of PAs has to be controlled in the processes of T. farfara herbal gathering and herb production, quality control of food, nutrient supplements and other coltsfoot based products. The good botanical identification of T. farfara and PA content of morphologically close species is a prerequisite for quality monitoring and control of plant based products.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Kolosova V. Leksika i simvolika slavyanskoi narodnoi botaniki: Etnolingvisticheski aspect [Lexicon and symbolism of Slavic folk botany: the ethnolinguistic dimension]. Moscow: Indrik; 2009. Bulgarian.
- Assyov B, Petrova A, Dimitrov D, Vassilev R. Konspekt na vishata flora na Bulgaria Horologi I florin elementi [Conspectus of the Bulgarian vascular flora. Distribution maps and floristic elements]. Sofia: Bulgarian Biodiversity Foundation; 2012. Bulgarian.
- Kuzmanov B. Tussilago L. In: Peev D, Kozhuharov S, Anchev M, editors. Flora na Republica Bulgaria [Flora of the Republic of Bulgaria]. Sofia: Marin Drinov; 2012. p. 411–412. Bulgarian.
- Akcin EO. Morphological and anatomical characteristics of Cichorium intybus L., Tragopogon latifolius Boiss. and Tussilago farfara L. (Asteraceae). Int J Nat Eng Sci. 2007;1(3):81–85.
- Warakomska Z, Kolasa Z. The flowering biology and apicultural value of coltsfoot (Tussilago farfara L. f. Asteraceae). Ann Univ Mariae Curie Sklodowska. 2003;LVIII:1–8.
- Ahtarov B, Davidov B, Yavashev A. Materiali za Balgarski botanichen rechnik [Materials for the Bulgarian botanical glossary]. Sofia: Bulgarian Academy of Science, Royal Court Printing House; 1939. Bulgarian.
- Ivancheva S, Stancheva B. Ethnobotanical inventory of medicinal plants in Bulgaria. J Ethnopharm. 2000;69(2):165–172.
- Koleva II, Beek TA, Soffers AEMF, et al. Alkaloids in the human food chain – natural occurrence and possible adverse effects. Mol Nutr Food Res. 2012;56:30–52.
- Redžić S. Wild edible plants and their traditional use in the human nutrition in Bosnia and Herzegovina. Ecol Food Nutr. 2006;45:189–232.
- Redzić S. Use of wild and semi-wild edible plants in nutrition and survival of people in 1430 days of siege of Sarajevo during the war in Bosnia and Herzegovina (1992–1995). Coll Antropol. 2010;34(2):551–570.
- Dénes A, Papp N, Babai D, et al. Wild plants used for food by Hungarian ethnic groups living in the Carpathian Basin. Acta Soc Bot Pol. 2012;81(4):381–396.
- Dogan Y. Traditionally used wild edible greens in the Aegean Region of Turkey. Acta Soc Bot Pol. 2012;81(4):329–342.
- Dogan Y, Baslar S, Ay G, Mert HH. The use of wild edible plants in western and central Anatolia (Turkey). Econ Bot. 2004;58(4):684–690.
- Kizilarslan C, Ozhatay N. An ethnobotanical study of the useful and edible plants of Izmit. Marmara J Pharm. 2012;16:194–200.
- Nedelcheva A. An ethnobotanical study of wild edible plants in Bulgaria. Eurasia J Biosci. 2013;7:77–94.
- European Food Safety Authority. Panel on Dietetic Products, Nutrition and Allergies (NDA); Scientific opinion on the substantiation of health claims related to Tussilago farfara L. and function of the upper respiratory tract and immune health (ID 2497) pursuant to Article 13 of Regulation (EC) No 1924/2006 on request from the European Commission. EFSA J. 2009;7(9):1301.
- Didry N, Pinkas M, Torck M. Phenolic components from Tussilago farfara. Ann Pharm Fr. 1980;38:237–241.
- Didry N, Pinkas M, Torck M, Dubreuil L. Sur la composition chimique et l'activité du Tussilage [Components and activity of Tussilago farfara]. Ann Pharm Fr. 1982;40:75–80. French.
- Ryu JH, Jeong YS, Sohn DH. A new bisabolene epoxide from Tussilago farfara, and inhibition of nitric oxide synthesis in LPS-activated macrophages. J Nat Prod. 1999;62:1437–1438.
- Xue SY, Li ZY, Zhi HJ, et al. Metabolic fingerprinting investigation of Tussilago farfara L. by GC–MS and multivariate data analysis. Biochem Syst Ecol. 2012;41:6–12.
- European Food Safety Authority. Panel on Contaminants in the Food Chain (CONTAM); Scientific opinion on pyrrolizidine alkaloids in food and feed. EFSA J. 2011;9(11):2406.
- European Medicines Agency. Public statement on the use of herbal medicinal products containing pyrrolizidine alkaloids (PAs). EMA/HMPC/893108/2011; 2013–2015. [cited 2015 Apr 1]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Public_statement/2013/11/WC500154224.pdf
- Joint FAO/WHO Codex Alimentarius Commission E. Codex Alimentarius: Discussion paper on pyrrolizidine alkaloids. Hague: Food and Agriculture Organization of the United Nations; 2011.
- Wiedenfeld H. Plants containing pyrrolizidine alkaloids – toxicity and problems. Food Addit Contam. 2010;28(3):282–292.
- Determination of Pyrrolizidine Alkaloids in Food and Feed Project (Germany). Pyrrolizidine alkaloids in herbal teas and tea varieties: Federal Institute for Risk Assesment (BfR) (Germany); 2013. (Opinion No. 018/ Q12).
- Coulombe RA. Pyrrolizidine alkaloids in foods. Adv Food Nutr Res. 2003;45:61–99.
- Jimenez JB. Phytochemical and analytical studies of feed and medicinal plants in relation to the presence of toxic pyrrolizidine alkaloids [dissertation]. Bonn (Germany): University of Bonn; 2013.
- Kempf M, Reinhard A, Beuerle T. Pyrrolizidine alkaloids (PAs) in honey and pollen legal regulation of PA levels in food and animal feed required. Mol Nutr Food Res. 2010;54(1):158–168.
- Michel R, Raezke KP. Pyrrolizidine alkaloids in honey. Brief overview regarding the occurrence, toxicological effects and risk assessment. Bremen:Intertek Food Services GmbH; 2009.
- Wawrosh C, Kopp B, Wiedenfeld H. Permanent monitoring of pyrrolizidine alkaloid content in micropropagated Tussilago farfara L.: a tool to fulfill statutory demands for the quality of coltsfoot in Austria and Germany. Acta Hort. 2000;530:469–472.
- Kostova N, Christov V, Cholakova M, et al. Pyrrolizidine alkaloids from Bulgarian species of the genus Senecio. J Serb Chem Soc. 2006;71(12):1275–1280.
- Sidjimov A, Tashev A. Pyrrolizidine alkaloids of Senecio species from Bulgaria. Ann Univ Sofia Fac Chimie. 2004;97(2):139–147.
- Adamczak A, Opala B, Gryszczynska A, Buchwald W. Content of pyrrolizidine alkaloids in the leaves of coltsfoot (Tussilago farfara L.) in Poland. Acta Soc Bot Pol. 2013;82(4):289–293.
- Dreger M, Krajewska-Patan A, Gorska-Paukszta M, et al. Content of pyrrolizidine alkaloids (senecionine and senrkine) in Tussilago farfara L. Plants cultivated in vitro. Herba Pol. 2012;58(4):62–69.
- Dreger M, Stanislawska M, Krajewska-Patan A, et al. Pyrrolizidine alkaloids – chemistry, biosynthesis, pathway, toxicity, safety and perspectives of medicinal usage. Herba Pol. 2009;55(4):127–147.
- Lebada R, Schreier A, Scherz S, et al. Quantitative analysis of the pyrrolizidine alkaloids senkirkine and senecionine in Tussilago farfara L. by capillary electrophoresis. Phytochem Anal. 2000;11(6):366–369.
- Kovats E. Gas-chromatographic characterization of organic compounds. Part 1: Retention indices of aliphatic halides, alcohols, aldehydes and ketones. Helv Chim Acta. 1958;41:1915–1932.
- Witte L, Rubiolo P, Bicchi C, Hartmann T. Comparative analysis of pyrrolizidine alkaloids from natural sources by gas chromatography-mass spectrometry. Phytochemistry. 1993;32:187–196.
- Rahfeld B. Microscopical atlas for herbal drugs. Heidelberg: Spektrum Akademischer Verlag; 2009.
- Serrano R, da Silva G, Silva O. Application of light and scanning electron microscopy in the identification of herbal medicines. In: Méndez-Vilas A, Díaz J, editors. Microscopy: science, technology, application and education. Badajoz: Formatex; 2010. p. 182–190.
- Upton R, Graff A, Jolliffe G, Langer R, Williamson E. American herbal pharmacopoeia. Boca Raton (FL): CRC Press; 2011.
- Zhao Z. Application of microscopic techniques for the authentication of herbal medicines. In: Méndez-Vilas A, Díaz J, editors. Microscopy: science, technology, application and education. Badajoz: Formatex; 2010. p. 803–812.
- Heinrich M. Safety of traditional remedies. In: Elisabetsky E, Etkin N, editors. Ethnopharmacology Vol 2, Encyclopedia of life support systems. Oxford: UNESCO-EOLSS; 2005. p. 223–241.
- Aydin AA, Letzel T. Simultaneous investigation of sesquiterpenes, pyrrolizidine alkaloids and N-oxides in Butterbur (Petasites hybridus) with an offline 2D-combination of HPLC-UV and LC-MMI-ToF-MS. J Pharm Biomed Anal. 2013;85:74–82.
- Aydin AA, Zerbes V, Parlar H, Letzel T. The medicinal plant butterbur (Petasites): analytical and physiological review. J Pharm Biomed Anal. 2013;75:220–229.