Abstract
The honeybees (Apis mellifera) population is declining. The involved causes may be pathogens (mites, viruses and bacteria) and parasites, due to honeybee's compromised immune system, leading to various bee-associated infections. Therefore, the present study assessed the comparative efficacy of plant extracts, including neem (Azadirachta indica) and Barbaka (Vitex trifolia) against gut bacteria and ectoparasitic mite Varroa destructor of honeybee A. mellifera (Hymenoptera: Apidae). The in vitro activities of the plant extracts were determined by using standard methods against five bee gut bacterial isolates, including the well-known bee pathogenic bacteria Paenibacillus larvae. Miticides were also assessed in field against honeybee mites. The obtained results from the phytochemical screening of Barbaka and neem extracts efficiency showed inhibitory zones with diameters of 23 mm with 60 mg/mL against P. larvae and 14 mm with 60 mg/mL against Escherichia coli, respectively. None of the extracts proved to be effective against Salmonella enterica and the neem extract showed intermediate activity against Bacillus subtilis and Staphylococcus hominis. Likewise, Barbaka plant extracts were not effective against B. subtilis. Similarly, the relative treatment efficacies of neem and Barbaka extracts, together with conventional miticides against honeybee Varroa mites, varied significantly. However, the effect of Barbaka and neem extracts on the mite-infested colonies was lower than the effect of other treatments, but it was also higher than in the control colonies. This study concluded that Barbaka and neem extracts have antibacterial and miticidal activity and are reasonably safe. However, more trials have to be conducted, in order to validate these results.
Introduction
Honeybees Apis mellifera populations all over the world are getting fewer at alarming rates.[Citation1] This disappearing of the bees may lead to serious consequences. Gut microflora and parasitic mites are likely to cause this phenomenon,[Citation2] but little is known about the honeybee gut bacteria, where there might be potential pathogens. However, a well-known honeybee pathogenic bacteria (Paenibacillus larvae) that affects honeybee larvae causes a significant decrease in the honeybee population.[Citation3] The bacterium P. larvae is the causative agent of the American foulbrood, which is a very serious disease of honeybee broods around the world.[Citation4,Citation5] A wide range of antibiotics have been found to control P. larvae, but several problems, connected with its continuous use, might occur. Such problem may be their accumulation in bee products,[Citation6] which might not only reduce their quality for human utilization, but also affect the health and decrease the lifespan of bees. In this background, the substitution of conventional synthetic antibiotics with natural ones is very important. The mite Varroa destructor is a damaging parasite of the honeybee A. mellifera and has now become one of the most important worldwide pests that beekeepers have to face.[Citation7] Several acaricides have been developed to control the Varroa infestations. However, so far, no chemical product has succeeded in eradicating the pest. On the contrary, cases of resistance development towards certain acaricides by V. destructor have been observed.[Citation8–10]
Similarly many natural products have been tested against Varroa infestation in honeybee colonies; among these, thymol, formic acid and oxalic acid have shown promising effects.[Citation11–13] The neem tree (Azadirachta indica) contains several biologically active constituents, such as azadirachtin,[Citation14] meliantriol,[Citation15] salanin,[Citation16], nimbin and nimbidin.[Citation17] Neem has known insecticide properties, causing melanization in third instars larvae of Drosophila melanogaster.[Citation18] In the same way, Vitex trifolia L. (Verbenaceae), commonly called Barbaka, is a stout and small tree, found in several parts of India, which is traditionally used by the tribes and native medical practitioners for the treatment of various ailment, including liver disorders, tumours, rheumatic pain, inflammation and tuberculosis. It is reported to possess larvicidal, wound healing, anti-HIV, anticancer, trypanocidal, antibacterial and antipyretic activities.[Citation19] Biological assays of V. trifolia extracts have shown antifeedant and antifungal activities against the insect pest Spodoptera frugiperda (Lepidoptera: Noctuidae) and fungal plant pathogen Fusarium sp.[Citation20] However, studies about their potential effects against honeybee pathogen, including bacteria and Varroa mite, are lacking. So, the aim of the present study was to determine the efficacy of plant extracts of neem and Barbaka as natural alternatives for the control of bee pathogens.
Materials and methods
Bacterial cultures
Five bacterial strains were used in this study (Bacillus subtilis KRK9D, Escherichia coli KT05, P. larvae KRK17, Salmonella enterica KT1C and Staphylococcus hominis BN6G), which were previously isolated from the gut of honeybees A. mellifera in Northern Pakistan. All these isolates were characterized by using bacteriological and 16s rDNA sequence analysis. Preserved glycerol stock of these bacteria was subjected for susceptibility testing.
Preparation of plant extract
For antibacterial activity, the crude aqueous extracts of neem (A. indica) and Barbaka (V. trifolia) were prepared by completely following the protocol of Khan et al.,[Citation21] with some modifications, as the serial dilutions (20, 40 and 60 mg/mL) for susceptibility tests were prepared and filtered through a 0.22 mm microbial syringe-driven filters (FPV203013, JETBIOFIL) by using a 20 mL master A+ disposable syringe (Wenzhou Wuzhou, China) into screw-capped 250 mL bottles and stored at −20 °C. Neem and Barbaka combined leaf and bark crude aqueous extracts were used against five bacterial isolates from honeybee gut and parasitic mites V. destructor.
Bacterial susceptibility test
Clinical and Laboratory Standards Institute (CLSI) standard for susceptibility tests were strictly followed during the trials. For each bacterial culture (B. subtilis KRK9, E. coli KT05, P. larvae KRK17, S. enterica KT1C and S. hominis BN6G), a suspension of the bacteria with a concentration equivalent to McFarland standard No. 0.5 was lawn evenly by using a sterile swab onto a brain–heart infusion (BHI) agar plate and dried at room temperature. After the plate had dried, sterile 6 mm paper discs (Whatman No. 1), soaked with 10 µL of the plant extracts with concentrations of 20, 40 and 60 mg/mL were placed on the plates, with sterile water as a negative control. The plates were incubated for 18–24 h at 37 °C. After incubation, the diameters of the clear zones (mm) were measured by using a ruler and were recorded. The tests were repeated three times.[Citation22] Amikacin 30 μg (CT0107B) discs were used for positive control, as a standard antibiotic.
Estimation of mite infestation
The levels of mite infestation were studied by using 1980 colonies from 45 apiaries in the districts of Karak, Kohat and Bannu. Assessments of mite infestation (Varroa genus) levels were done before the trial with different miticides, by opening of 100 cells of sealed brood. For the assessment of mite population in debris (bees waste material, fallen on the bottom board), mite-collecting homemade trays were placed on the bottom of the bee colony. The trays were left for a 24-h period and the mites that fell in it were counted and used as a measure for the mite population.[Citation23]
Establishment of experimental colonies
The research was carried out in Ghulam honeybee model farm, near Kohat University of Science and Technology, Kohat, Pakistan. The farm contains 120 A. mellifera colonies, all naturally infected with V. destructor. Twenty-four queen right honeybee colonies (five frames) in Langstroth hives that had been standardized for bee frames and Varroa infestation levels were used, each fitted with a modified bottom board and mite collection tray. These colonies were placed at approximately five meters apart.
Treatment trials of Varroa mite
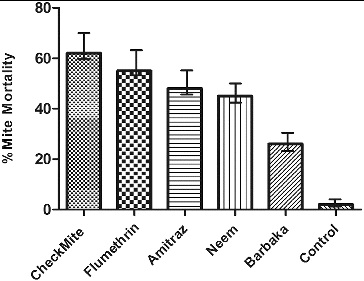
A total of 24 colonies were divided into six groups of four colonies. Groups A, B and C received the acaricides CheckMite, Flumethrin and Amitraz, respectively, which were used as recommended in the label. The liquid plant extracts (neem and Barbaka) were applied (30 mL) on groups D and E, respectively, and were trickled directly onto the adult bees, in between two frames, by using a syringe, as recommended.[Citation24,Citation25] Group F was run in parallel as a control and did not receive any treatment. Groups A, B and C received one strip of each acaricide, placed in the middle of each colony, at weekly intervals for one month. The mite mortality was determined with each treatment during a three-week period (1–9 Feb; 10–18 Feb and 19–27 Feb, 2013) and the mite drop mean values were compared. Apistan follow-up treatments were given to all of the colonies at the end of the experiment between 28 Feb to 8 March and 9–17 March, 2013. Apistan strips were removed from the colonies after four weeks, and the dropped dead mites were counted. The efficacy of each treatment was dependent on the number of dead mites – the larger the number of dead mites, the greater the effect of these treatments.[Citation26]
All of the obtained data were analyzed statistically by using least significant difference (LSD) tests. Comparisons between means were made by using the least significant difference at 0.05 probabilities.
Results and discussion
Plants are natural reservoirs of valuable phytochemicals. A lot of plants have been explored around the world for their antimicrobial activities.[Citation27] Therefore, in the present study, neem and Barbaka plants were selected and their crude extracts prepared, as these plants have been used against different insect pests.[Citation28,Citation29] The antibacterial activity of neem and Barbaka plants extracts was determined by the presence or absence of inhibitory zones. The results revealed variability among the zones of inhibition (). The antibacterial activity was evaluated for the honeybee pathogen P. larvae, as well as for the non-pathogenic bacteria E. coli and B. subtilis; however, all the isolates used in this study were previously isolated from the alimentary canal of honeybee A. mellifera. The human pathogenic bacteria S. enterica [Citation30] were also obtained while researching the bee gut for bacterial isolations. It is possible for S. enterica to be also responsible for the decline in bee populations in the study area. The extracts of both plants showed antibacterial activity against at least four of the tested isolates. All concentrations of the tested plants extracts were inactive against S. enterica KT1C, whereas the extract of Barbaka also showed no inhibitory activity against B. subtilis KRK9D. The results demonstrated that the zones of inhibition of bacterial growth were dose-dependent, because they became larger when the concentrations of the extracts were increased. No antimicrobial activity was observed with the negative control.
Table 1. Susceptibility of honeybee gut bacteria to extracts of neem and Barbaka, measured by the growth inhibitory zone.
The obtained results for the severity of mite infestation before treatment among the studied 1980 colonies in the districts of Karak, Kohat and Bannu were uneven – 73.26%, 60.90% and 53.47%, respectively (). This indicated that the presence of Varroa is a major problem for beekeepers in these areas. All of the treatments caused significant mite mortality (P < 0.05 in all cases), when compared to the control colonies (), but the treatments’ efficacies varied significantly. LSD tests indicated significant differences among the means of all groups (A, B, C, D, E) and control (F). However, the efficacy of the treatment with Barbaka extract was lower than the other treatments, but it was higher when compared to the control colonies (). The number of mites that fell on the sticky board during the first collection period was significantly higher than that observed during the second and third collection periods.
Table 2. Regional-wise infestation of Varroa mite in different colonies.
In several studies, different bacterial isolates were exposed to a variety of plant-based chemicals,[Citation19] but the ways to control the honeybee pathogenic bacteria P. larvae are very limited. Although the used plant extracts were not active against the isolates of S. enterica in the present results, both plant extracts successfully inhibited the growth of the Gram-positive bacteria S. hominis and P. larvae and one of the Gram-negative bacterium (E. coli) used in this experiment. In a previous study, 34.30 mg/mL acetone extract of neem showed 8 and 9 mm inhibitory zones of E. coli and Staphylococcus aureus, respectively.[Citation31] In this study, P. larvae showed significant growth decline when treated with both plant extracts. These findings were comparable to the effects of two aqueous extracts of Eucalyptus cinerea and Minthostachys verticillata, which showed 100% efficacy against P. larvae strains [Citation32]; therefore, the present research was further supported by previous studies.[Citation33,Citation34] The present study showed that the applications of botanical extracts were efficacious against bee gut bacteria and Varroa and, thus, were recommended for further control measures of mite and American foulbrood (AFB) disease of honeybee.
Neem plant extract yielded a low efficacy of only 45% () against bee mites. This may be due to the crude nature of the neem extracts, because the activity of the ingredients/compounds of neem plant against different mite and insect pests was reported worldwide.[Citation35,Citation18] Ariana et al. [Citation36] reported laboratory evaluations of thyme, savory, rosemary, marjoram, dillsun and lavender essences, which caused a mortality up to 95% and 97%. Only neem oil provided Varroa jacobsoni control comparable to the known varroacide formic acid, but it was not as effective as the synthetic product Apistan (τ-fluvalinate). Melathopoulos et al. [Citation37] reported that neem oil spray treatments had no effect on adult honeybee populations, but they reduced the amount of sealed brood in colonies by 50% and caused queen loss at higher doses. Some experts reported the compatibility of neem oils with bees, and found that after 10 days of treatment, pupae could not release their skins and young bees emerged with damaged wings. In some studies, the compound azadirachtin in neem extract was proven to lead to developmental disturbances,[Citation38,Citation39] but in the present research, effects of miticides on bees and their brood could not be observed.
In the past, the effect of neem oil was examined against Varroa mite in honeybee colonies. Neem oils, emulsified in water, showed 50%–90% death rate among mites and had no effect on the bee population.[Citation37] Accordingly, in the present findings, the mite death rates were comparable with the results of Melathopoulos et al. [Citation37–40] and supported the potential applications of neem against Varroa. The administration of water-diluted neem seed extract was efficacious against poultry red mite Dermanyssus gallinae at the selected mite-infested locations. A single application led to more than 80% efficacy. However, the second treatment increased the dramatic reduction of the mite population.[Citation41] The mite mortality in the present study was not as good as the one reported in the above research. The difference may be due to the species and methods of administration.
Imdorf A [Citation42] reported thymol, along with cineole and citronellal, as control agents against the tracheal mite Acarapis woodi (Rennie) and Varroa. At the end of the 28-day treatment period, mite mortality in the colonies, receiving thymol and cineole, was 56.4% and 49.1%, respectively, compared with a natural mite fall of 28% in the control colonies, whereas, mite mortality after treatment with neem and Barbaka extracts observed in the present findings were 45% and 26%, respectively, compared with 2% mite fall in the control colony. V. trifolia may have a potential use against bee mites, but the low mite death rate of 26% showed that there is a need of alternative treatment methods. The application of the three synthetic acaricides in this study was operative, but their effectiveness may correlate to the problem of resistance that varied for mites obtained from different sites.[Citation43]
Conclusions
Plant extracts are a rich source of natural chemical compounds that have been utilized in various applications on the basis of their biological activities. The aqueous plant extracts of neem and Barbaka showed positive results against honeybee pathogenic microbes and, thus, can facilitate the management of American foulbrood disease caused by P. larvae. The present study was a preliminary attempt to investigate the antibacterial properties of neem and Barbaka. However, further research is needed in order to identify and characterize the active molecules responsible for their antibacterial properties and to determine their potential use in beekeeping industries.
Acknowledgements
Staff at Department of Biological Sciences Gomal University D.I. Khan is highly acknowledged for their help and support. We also thank all beekeepers, especially Mr Ghulam Khan, for the help in providing us colonies for the Varroa treatment. The authors are also obliged to Mr Naeemullah Khan, PMS Officer and M.Phil. Scholar from the Department of English University of Peshawar, for language correction.
Disclosure statement
The authors declare that they have no conflict of interests.
References
- Core A, Runckel C, Ivers J, et al. A new threat to honey bees, the parasitic phorid fly Apocephalus borealis. Plos One. 2012;7:e29639.
- Di Prisco G, Pennacchio F, Caprio E, et al. Varroa destructor is an effective vector of Israeli acute paralysis virus in the honeybee, Apis mellifera. J Gen Virol. 2011;92:151–155.
- Antúnez K, D'Alessandro B, Piccini C, et al. Paenibacillus larvae larvae spores in honey samples from Uruguay: a nationwide survey. J Invertebr Pathol. 2004;86:56–58.
- Masry SHD, Kabeil SS, Hafez EE. New Paenibacillus larvae bacterial isolates from honey bee colonies infected with American foulbrood disease in Egypt. Biotechnol Biotechnol Equip. 2014;28(2):271–27.
- Müller S, Garcia-Gonzalez E, Elke Genersch E, et al. Involvement of secondary metabolites in the pathogenesis of the American foulbrood of honey bees caused by Paenibacillus larvae. Nat Prod Rep. Forthcoming 2015.
- Antúnez K, Harriet J, Gende L, et al. Efficacy of natural propolis extract in the control of American Foulbrood. Vet Microbiol. 2008;131:324–331.
- Anderson, DL, Trueman, JWH. Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp Appl Acarol. 2000:24,165–189.
- Boot WJ, van Baalen M, Sabelis MW. Why do Varroa mites invade worker brood cells of the honey bee despite lower reproductive success? Behav Ecol Sociobiol. 1995;36:283–289.
- Martin SJ. Acaricide (pyrethroid) resistance in Varroa destructor. Bee World. 2004;85:67–69.
- Moretto G, Leonidas Jde M. Infestation and distribution of the mite Varroa destructor in colonies of Africanized bees. Braz J Biol. 2003;63(1):83–86.
- Gregorc A, Jelenc J. Control of Varroa jacobsoni Oud. in honeybee colonies using Apilife-VAR. Zb Vet Fak Univ Ljubljana. 1996;33:255–259.
- Lindberg CM, Melathopoulos AP, Winston ML. Laboratory evaluation of miticides to control Varroa jacobsoni (Acari: Varroidae), a honey bee (Hymenoptera: Apidae) parasite. J Econ Entomol. 2000;93:189–198.
- Fassbinder C, Grodnitzky J, Coats J. Monoterpenoids as possible control agents for Varroa destructor. J Apic Res. 2002;41:83–88.
- Nakanishi K. Structure of the insect antifeedant azadirachtin. In: Runeckles V, editor. Recent advances in phytochemistry. Vol. 9. Springer; 1975. p. 283–298.
- Lavie D, Jain MK, Shpan-Gabrielith S. A locust phagorepellent from two Melia species. Chem Commun (Camb). 1967;18:910–911.
- Warthen J. Adult house fly feeding deterrent from neem seeds: Agricultural Research (Northeastern Region), Science and Education Administration, US Department of Agriculture; 1978.
- Chiu SF. The active principles and insecticidal properties of some Chinese plants, with special reference to Meliaceae. Proceedings of the 2nd International Neem Conference, Schriftenreihe der Gesellschaft fur Technische Zusammenarbeit (Publication series of the society for technical cooperation), Eschborn, Germany. Rauischholzhausen; 1984. p. 255–262.
- Anjum SI, Yousf M, Ayaz S, et al. Toxicological evaluation of chlorpyrifos and neem extract (Biosal b) against 3rd instars larvae of Drosophila melanogaster. J Anim Plant Sci. 2010;20:9–12.
- Anandan R, Jayakar B, Karar B, et al. Effect of ethanol extract of flowers of Vitex trifolia Linn on CCl4 induced hepatic injury in rats. Pak J Pharm Sci. 2009;22:391–394.
- Hernandez M, Heraso C, Villarreal M, et al. Biological activities of crude plant extracts from Vitex trifolia L (Verbenaceae). J Ethnopharmacol. 1999;67:37–44.
- Khan AV, Ahmed QU, Shukla I, et al. Antibacterial activity of leaves extracts of Trifolium alexandrinum Linn. against pathogenic bacteria causing tropical diseases. Asian Pac J Trop Biomed. 2012;2:189–194.
- Judaki A, Panahi J, Havasian MR, et al. Study of the inhibitory effect of Quercus coccifera's aqueous extract on Staphylococcus aureus and Pseudomonas aeruginosa in vitro. Bioinformation. 2014;10:689–692.
- Fries I, Aarhus A, Hansen H, et al. Comparison of diagnostic methods for detection of low infestation levels of Varroa jacobsoni in honey-bee (Apis mellifera) colonies. Exp Appl Acarol. 1991;10:279–287.
- Imdorf A, Bogdanov S. Use of essential oils for the control of Varroa jacobsoni. 1999. p. 17 (FAIR CT97-3686).
- Broedsgaard C, Jensen S, Hansen J, et al. Spring treatment with oxalic acid in honeybee colonies as varroa control. Denmark; 1999. (DIAS report – Horticulture).
- Marinelli E, Pulcini P, Morgia C, et al. Oxalic acid by Varrox® to varroa control in central Italy. APIMONDIA symposium 2004 “Prevention of residues in honey 2”, Celle, 27–28 April, 2004. Apiacta. 2004;39:39–43.
- Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–582.
- Biswas K, Chattopadhyay I, Banerjee RK, et al. Biological activities and medicinal properties of neem (Azadirachta indica). Curr Sci. 2002;82:1336–1345.
- Ganapaty S, Vidyadhar K. Phytoconstituents and biological activities of Vitex – a review. J Nat Remed. 2005;5:75–95.
- Holman EJ, Allen KS, Holguin JR, et al. A community outbreak of Salmonella enterica serotype Typhimurium associated with an asymptomatic food handler in two local restaurants. J Environ Health. 2014;77:18–20.
- Victor IU, Igeleke C. Antimicrobial properties of the extracts of locally sold garlic and neem leaf in Benin City, Nigeria. Int J Biosci. 2012;2:21–27.
- González M, Marioli J. Antibacterial activity of water extracts and essential oils of various aromatic plants against Paenibacillus larvae the causative agent of American Foulbrood. J Invertebr Pathol. 2010;104:209–213.
- Gende LB, Floris I, Fritz R, et al. Antimicrobial activity of cinnamon (Cinnamomum zeylanicum) essential oil and its main components against Paenibacillus larvae from Argentine. Bull Insectol. 2008;61:1–4.
- Flesar J, Havlik J, Kloucek P, et al. In vitro growth-inhibitory effect of plant-derived extracts and compounds against Paenibacillus larvae and their acute oral toxicity to adult honey bees. Vet Microbiol. 2010;145:129–133.
- Naqvi S, Temuri K, Nurulain S, et al. Toxicity and effect of neem fractions (RB-U, RB-b and Morgosan-OTM) on phosphatase and protein pattern of Culex fatigans (KU strain). Pak J Pharm. 1995;12:49–52.
- Ariana A, Ebadi R, Tahmasebi G. Laboratory evaluation of some plant essences to control Varroa destructor (Acari: Varroidae). Exp Appl Acarol. 2002;27:319–327.
- Melathopoulos AP, Winston ML, Whittington R, et al. Field evaluation of neem and canola oil for the selective control of the honey bee (Hymenoptera: Apidae) mite parasites Varroa jacobsoni (Acari: Varroidae) and Acarapis woodi (Acari: Tarsonemidae). J Econ Entomol. 2000;93:559–567.
- Rembold H, Sharma G, Czoppelt C, et al. Evidence of growth disruption in insects without feeding inhibition by neem seed fractions. Z Pflanzenkr Pflanzenschutz. 1980;87:290–297.
- Naumann K, Isman MB. Toxicity of a neem (Azadirachta indica A. Juss) insecticide to larval honey bees. Am Bee J. 1996;136:518–520.
- Blackwell A. Azadirachtin: an update. J Insect Physiol. 1993;39:903–924.
- Abdel-Ghaffar F, Sobhy HM, Al-Quraishy S, et al. Field study on the efficacy of an extract of neem seed (Mite-Stop®) against the red mite Dermanyssus gallinae naturally infecting poultry in Egypt. Parasitol Res. 2008;103:481–485.
- Imdorf A, Bogdanov S, Ochoa RI, et al. Use of essential oils for the control of Varroa jacobsoni Oud. in honey bee colonies. Apidologie.1999;30(2–3):209–228.
- Mozes-Koch R, Slabezki Y, Efrat H, et al. First detection in Israel of fluvalinate resistance in the Varroa mite using bioassay and biochemical methods. Exp Appl Acarol. 2000;24:35–43.