Abstract
Chronic periodontal diseases, which mainly include gingivitis and periodontitis, have been described as the inflammation of the supporting tissues of the teeth. The main cause of periodontal diseases is the accumulation of the microbial dental plaque. If dental plaque is not eliminated by mechanical or chemical plaque control methods, mineralized dental plaque (calculus) occurs. The mineralization process and mechanisms of the dental calculus formation are similar to that of other pathologic calcifications. The presence of a certain type of microorganism is discovered in various pathological calcifications, such as kidney stones and arterial plaques. This microorganism is the nanobacterium. Thus, it may be considered as a potential risk factor for the chronic periodontal diseases. Nanobacterium is one of the most controversial issues in today's biological studies. Nanobacteria, as the smallest known self-replicating bacteria, are classified as Gram-negative organisms. Although their growth is slow, they grow best under aerobic conditions. Their doubling time is about three days with the metabolic rate, which is 10,000 times slower than that in Escherichia coli. Nanobacteria are also resistant to heat and various conditions that would normally kill other bacteria. Present data suggest that nanobacteria are only killed by ethylenediaminetetraacetic acid and tetracycline. The aim of this paper was to perform a narrative review of publications on nanobacteria since 1998 and highlight their hypothesized relationship with the periodontal disease.
Introduction
Nanobacteria, also known as calcifying nanoparticles (CNPs), were first described by Kajander and Ciftçioğlu in 1998.[Citation1] The nature of these unknown cell-culture contaminants and their possible role in human organ disease has raised a lot of attention in medical and human health research over the last decades.[Citation1]
There are significant reports in the literature that correlate with CNPs in numerous human diseases and conditions with pathological calcifications in human organs, such as dental pulp, salivary glands, kidneys and arteries.[Citation2]
The possible role of nanobacteria in the periodontal disease was first proposed based on association between the oral hygiene and the incidence of the cardiovascular disease, which was probably mediated via the oral infection inflammation pathways.[Citation3–5] Among more than 300 species of known bacteria in the oral cavity, nanobacteria seem to be good candidates with their specific role in the pathological calcification.[Citation6]
The aim of this paper was to perform a narrative review of publications on CNPs since 1998 and highlight their hypothesized relationship with the periodontal disease.
Calcifying nanoparticles: cell culture contaminants or nanobacteria
CNPs (nanobacteria, nanobacteria-like particles, nanobes) were discovered as cell culture contaminants by Kajander et al., almost 25 years ago.[Citation7] However, the question of their nature has been actively discussed since then. Nowadays, published literature suggest, with high probability that these nanoparticles have intrinsic nucleic acids and, consequently, they should have their own replication and specific protein biosynthesis system.
Mathew et al. [Citation8] showed that they may have successfully replicated, even in the absence of serum, whereas inorganic hydroxyapatite crystals did not replicate independently in the presence of serum in the culture medium. In addition, under the same culture conditions, CNPs were not detected in the control serum-containing media.
Epidemiological studies have devoted to the association of CNPs with the risk of various diseases, suggesting that the presence of CNPs is a pathological, not a physiological process. CNP antigens and anti-CNP antibodies were detected significantly more often in cases, when compared with controls. Moreover, bacterial proteins were absent in serum under physiological conditions.[Citation9–12]
Evidence shows that CNPs may participate in pathological calcifying processes of the blood vessels, even without their calcified shells.[Citation13] This simply may point to the presence of specific immunogenic bacterial protein antigens in their structure. It is also noteworthy to mention that these proteins could probably have specific toxin-like activity.
CNPs are highly susceptible to a number of chemo therapeutics agents, such as nitrofurantoin, trimethoprim, trimethoprim-sulfamethoxazole, 5-fluorouracil, cytosine arabinoside, 6-aminocaproic acid, sodium azide and potassium cyanide. This leads to a suppression of the biosynthesis of nucleic acids and proteins, as well as to a suppression of the respiratory enzyme functions, although these enzymes do not possess the usual chelating activity on CNPs. This notion can be an evidence, supporting the presence of their own system of replication and metabolism, which signifies their biological nature.[Citation14]
All these data, evidently, contradict to the hypothesis that CNPs are just physicochemical contaminants, formed from minerals and serum proteins under homeostasis conditions. The theory was proposed by Raoult et al.,[Citation15] Martel and Young,[Citation16] Martel et al.,[Citation17] Wu et al. [Citation18] and Young et al.[Citation19,Citation20]
Without oxygen, CNPs do not replicate, which insinuates that CNPs are facultative anaerobes or microaerophilic microorganisms.[Citation11] The replicative activity of CNPs is elevated during the photobiostimulation [Citation21] and also during the exposure to mercaptoethanol,[Citation11] which impels the growth of many other aerobes.[Citation11]
The published data again insinuated a living nature of CNPs, making the term ‘nanobacteria’, which was originally proposed by Kajander et al.,[Citation7] valid. The main controversy of establishing CNPs as living organisms, is the absence of a fairly accurate sequenced genome at present, but this, itself, does not counter the theory that nanobacteria are the smallest self-replicating life forms on earth.
Detection and cultural methods
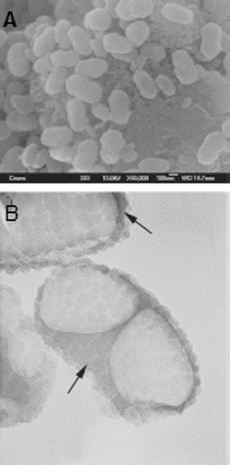
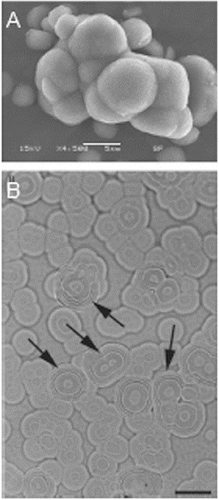
Nanobacteria, as the smallest known self-replicating bacteria, are classified as Gram-negative organisms. They grow best under aerobic conditions: 5% CO2 and 95% air.[Citation23] Nanobacteria grow slowly. Their doubling time is about three days with the metabolic rate, which is 10,000 times slower than that in Escherichia coli.[Citation24] Nanobacteria are found in biological systems, such as cells, tissues, blood and urine. The best methods to detect them include the immunodetection with nanobacteria-specific monoclonal antibodies, electron microscopy and culture techniques.[Citation25] Due to their very small size, 0.22 mm-pore size filters, which have the ability to disallow most common microbes, are often used to clean up fluid specimens, before the culturing of nanobacteria.[Citation26] Moreover, their replication rate can be measured by particle counting and specific optical density at 650 nm.[Citation14] Some other methods, capable of monitoring the growth of the nanobacteria, are turbidity, enzyme-linked immunosorbent assay (ELISA), sodium dodecyl sulfate-polyacrylamide gel electrophoresis or methionine and uridine incorporation ( and ).[Citation1,Citation10]
Bacterial defense and pathogenesis mechanisms
Under low nutrient conditions (like a serum-free region), nanobacteria are likely to form microscopic colonies in the liquid media, surrounded by a thick coat of calcium apatite. They can form a colony that may become even bigger than 1 mm in size. They also exhibit budding and fragmentation, social behaviour and communities, reminiscent of biofilms, but indicate unique characteristics, consistent with extremophilic organisms. For example, they can withstand 90 °C for 1 h, 15 kGy gamma irradiation and 5% NaCl. Nanobacteria hide themselves from the immune system and the antibodies (calcific semi-dormant). They can also live where other bacteria cannot survive (extremophilic defense), which is provided by their calcified shelter.[Citation23]
Findings show that nanobacteria bind as clusters on the cell surface for around 15 min and are then brought into the cell either by a receptor-mediated endocytosis or by a similar pathway. The bacterial cytotoxicity is mainly dependent on the concentration and the exposure time. Findings also show that dying cells always contain numerous ingested calcified nanoparticles.[Citation26]
Nanobacteria appear as self-propagating calcifying macromolecular complexes. They form active nidi and the biomineralization process is taking place out of the chemical equilibrium and under the subsaturation level of calcium and/or phosphate.[Citation29]
There are various pathogenesis mechanisms for nanobacteria, including the known effects of calcium on blood vessels, blood coagulation and thrombus formation and elevation of intracellular [Ca2+] levels. There may be also several consequences, such as stimulation to apoptotic cell death or to uncontrolled growth, which can potentially lead to tumor growth or malignancies, induction of autoimmune reactions, inflammation, arthritis and pathological calcification.[Citation29]
The role of CNPs in etiopathogenesis of diseases
Many studies show the significant role of CNPs, which may be associated with various organ diseases, particularly, disorders with pathological calcifying processes.
The first and most investigated is the role of CNPs in kidney stones formation. The connection of CNPs with the risk of nephrolithiasis was firstly proposed in 1998 by Kajander and Ciftçioğlu.[Citation1] They revealed specific CNP antigens in all of the 30 examined kidney stones. These data were further proved in the investigation of Ciftçioğlu et al.,[Citation30] who indicated CNPs in 97.2% of the examined kidney stones by using several methods (immunostaining, assessment of Ca and 85Sr incorporation and bacterioscopic and bacteriological methods). In their study, CNPs were identified, extracted and cultured from the vast majority of kidney stones. Nearly all kidney stones embodied apatite, a component of CNPs' mineral shells. Moreover, CNPs labelled by radioactive technetium (99mTc) and intravenously injected into rabbits were renotropic and could be found in the urine. Based on the following findings, they have theorized that CNPs may be an etiological agent of nephrolithiasis.[Citation30]
Furthermore, Kumar et al. [Citation11] suggested a probable role of CNPs in the etiopathogenesis of Randall's plaques that are precursors of calcium oxalate kidney stones. Subsequently, their results were confirmed by Ciftçioğlu et al.’s study [Citation30]; they detected CNPs in ∼70%–90% of kidney papillae samples with Randall's plaques. Similar results were obtained during the analysis of serum in their study.[Citation31] In another study, Chen et al. [Citation32] detected CNPs in the serum of 92% of patients with nephrolithiasis and reported their absence in the serum of the control subjects (24 cases and three controls). Further investigations demonstrated the ability of CNPs to cause nephrolithiasis in rats.[Citation12,Citation33] In these studies, tetracycline demonstrated a specific anti-bacterial activity (by the inhibition of the protein synthesis) and prevented the formation of kidney stones.
CNPs was also found in patients with polycystic kidney disease by using several methods.[Citation18] Although the role of CNPs in the etiology of this disorder is still enigmatic and unclear, it is known that nephrolithiasis is several times more prevalent in patients with polycystic kidney disease, when compared to the general population.[Citation34]
Moreover, Shoskes et al. [Citation35] investigated the role of CNPs in the etiopathogenesis of type III chronic prostatitis/chronic pelvic pain syndrome. Their findings showed that the comET therapy (tetracycline + ethylenediaminetetraacetic acid (EDTA) + nutrients) for three months leads to, at least, 25% improvement of the condition in 80% of the patients that were resistant to other therapeutic modalities. In 53% of the cases, this parameter was higher than 50%. The size of the stones decreased significantly in 50% of the patients. CNP antigens and anti-CNP antibodies were also detected in 60% of the serum samples and in 40% of the urine samples.[Citation24] Their findings were confirmed by a placebo-controlled trial of therapy against CNPs in 2008, carried out on a larger group of patients.[Citation36] Finally, in 2010, Shen et al. [Citation37] demonstrated the influence of CNPs on the disease in an animal model. Rats showed prostatic acute inflammatory changes after 1–2 weeks from the beginning of CNPs injections (transurethrally) and after 4 weeks, these inflammatory alterations became chronic. Moreover, the levels of interleukin-1β and tumor necrosis factor-α increased in the prostate of the model rats in their research.
Dealing with other diseases of the genitourinary tract, Zhang et al. [Citation38] suggested a role of CNPs in the etiology of the interstitial cystitis/painful bladder syndrome and revealed them in 47.8% (11 out of 23) of the bladder tissue samples by using several methods. They also noted an improvement of the symptoms after a tetracycline treatment (orally and intravesically).[Citation38] The same authors proposed a theory, in which CNPs had a significant role in the etiology of testicular microlithiasis,[Citation39] implying that CNPs play an important role in the development of genitourinary tract diseases.
The effect of CNPs on the etiopathogenesis of cardiovascular diseases has been examined in several studies. CNPs were found in calcified aneurysms, cardiac valves, plaques of carotid and femoral arteries in several studies.[Citation10,Citation40–42] Further investigations demonstrated that CNPs can increase the cytokine activity throughout the body, which may lead to harmful effects on the cardiovascular system, especially within the arteries of an animal model (rabbits). Moreover, Maniscalco and Taylor [Citation43] reported that the ‘comET therapy’ may have protective effect on coronary arteries and may decrease the calcific plaque formation. This remedy includes 1500 mg EDTA in a rectal suppository base, 500 mg tetracycline administered orally and a mixture of various nutrients in powder, administered orally for 4 months. As mentioned before, it is known that CNPs are as sensitive to tetracycline, as they are to EDTA. Findings also show that CNPs may lead to occlusion and calcification of damaged arteries, as well as to induction of pathological processes in blood vessels, even without the presence of mineral shells. It seems that arteries with healthy endothelium are resistant to CNPs.[Citation13] Finally, Kaya et al. [Citation44] used ELISA to show the association between the level of anti-CNP antibodies and the risk and severity of calcification of coronary arteries. The results of all these studies have indicated the connection between CNPs and pathological calcification in cardiac valves and blood vessels.[Citation24]
In a number of studies, the connection of CNPs with other diseases, related to the pathological calcification, has also been explored.[Citation24] CNP antigens were found in psammoma bodies of ovarian malignant tumor cells,[Citation45,Citation46] bile and gallstones of patients with cholecystolithiasis [Citation47,Citation48] and placental calcifications.[Citation49]
Finally, the prevalence of CNPs antigens and anti-CNP antibodies was investigated in human immunodeficiency virus (HIV)-infected women and healthy women in South Africa.[Citation50] According to their results, anti-CNP antibodies and CNP antigens were found in significantly higher levels in HIV-infected women. Interestingly, according to the ELISA results, 70% of the offspring, of the HIV-infected group of women, had CNP antigens. In addition, the antigen levels in their blood were higher, when compared with the mothers’ levels.[Citation50] Based on these results, it could be suggested that the CNP invasion may have an opportunistic nature and may have been transplacentally or perinatally transmitted ( and ).
Periodontal diseases and nanobacteria
Periodontal diseases have been described as an inflammation of the supporting tissues of the teeth and mainly include gingivitis and periodontitis. The main cause of the periodontal disease is the dental plaque, which is the community of microorganisms found on the tooth surface. It is a biofilm embedded in the matrix of polymers of the host and the bacterial origin.[Citation51]
Calculus, which is a mineralized dental plaque, occurs, if this accumulated plaque is not eliminated of the dental surface.[Citation52,Citation53] The mineralization process of calculus seems to be similar to other ectopic pathologic calcifying processes that occur in human organs, such as kidney stones and gallstones formation.
Based on epidemiological studies, dental calculus seems to have higher prevalence in men than in women.[Citation54,Citation55] Interestingly, this prevalence was similar to the results, obtained during a study, conducted on the distribution of nanobacteria by Hjelle et al.[Citation56] In this research, nanobacteria were randomly recovered from the urinary fluid of 30% male and 10% female young, healthy, control subjects. Nanobacteria, acting as self-propagating calcifying complexes, found in human blood and blood products, may have a significant role in the calculus formation process. More importantly, the presence of an alkali environment is essential for nanobacteria to cause calcification, as the calculus formation is facilitated in such environment.[Citation1,Citation26] All these evidences suggest that nanobacteria may be present in the macromolecular structure of the dental calculus.
As serum transudate, CNPs could be transported from the blood to the gingival crevice fluid. As mentioned, there is an evidence that CNPs can induce the calcification of mammalian cells.[Citation1] In CNP-infected human fibroblasts, electron microscopy revealed the presence of intra- and extracellular crystal deposits that were positive for the von Kossa staining and resembled calcospherites, found in the pathologic calcification.[Citation1,Citation26]
It was reported that CNPs could adhere to gingival epithelial cells and cause apoptosis and cell vacuolization.[Citation26,Citation57] It seems the certain cytotoxic pathways of CNPs could induce these morphological changes in cells.[Citation26] Gingival epithelial cells, exposed to CNPs, showed gross vacuolization. Electron microscopy demonstrated that CNPs entered the cells and the calcification appeared in the intracellular vacuoles. These findings have been confirmed by immunofluorescence and laser scanning confocal microscopy studies.[Citation57] It is suggested that CNPs might be transferred into cells via a receptor-mediated endocytotic mechanism. Based on the nephrolithiasis model, suggested by Kajanderet et al.,[Citation58] phosphatidylserine could act as a binding site for CNPs on the surface of injured or apoptotic cells. Then, the relocation of this molecule might also occur in CNPs-infected gingival epithelial cells, as a result of injury or apoptosis. The presence of phosphatidylserine on the cell's surface could recruit more CNPs aggravation and cause further cell injuries.[Citation57]
Exposed phosphatidylserine, as a crystal-binding molecule, also increases the attachment of calcium oxalate and calcium phosphate crystals to the surface of the cells and facilitates the calcification process.[Citation59,Citation60] This calcified cell matrix may contribute to the formation of dental calculus.[Citation61]
Besides, CNPs could also act as an active nidi, which could cause biomineralization out of the chemical equilibrium and form calcium phosphate crystals under the subsaturation level of calcium and/or phosphate. This apatite formation process was in direct association with the calcium level, halted only when the calcium level decreased by half and the phosphate levels subsided nearly to zero.[Citation23]
On the other hand, literature suggests that nanobacteria may contribute to the etiopathology of two other important dental diseases, including pulp stone and dental caries.
Further investigations support the probable involvement of CNPs in the dental stone formation by several methods. In these studies, 84.6% of the tissue samples were positive for CNPs antigen by immunohistochemical staining; the corresponding rate by indirect immunofluorescence staining was 92.3%. Besides, after incubation, concentric circles of apatite crystals were revealed by extracted CNPs, manifesting similar scanning electron microscopy (SEM)-scale morphological features, when compared to pulp stones.[Citation62,Citation63]
On the other hand, Jing et al. [Citation64] hypothesized a therapeutic use of CNPs in dental caries. The results of the study suggested that CNPs may be involved in enamel repair, just like the synthetic apatite. Moreover, an epidemiological study on 228 children showed that the caries prevalence is significantly lower in calculus-prone than in calculus-free subjects.[Citation65,Citation66] Others authors proposed that a gelatinous synthetic mix (free fluoride, calcium and phosphate ions and CNPs) could be applied on a cracked tooth surface, therapeutically, in order to limit the further propagation of the crack deeper into the dentin.[Citation2,Citation67]
Although nanobacteria were mainly detected in pathological calcifying processes in organ diseases, the literature revealed that nanobacteria infection could also be present in healthy adults.[Citation7,Citation68] All these data lead to the assumption that nanobacteria may be included in the resident microflora of the host, conjecturing that they may be opportunistic organisms and may not always be harmful agents to the human body.
Anti-nanobacterial mouthwash/toothpaste
As mentioned, nanobacteria are also incredibly resistant to heat and other methods that would normally kill bacteria, which makes some scientists wonder if they might be an unusual form of crystals, rather than organisms.[Citation69] Findings show that nanobacteria cannot be killed by penicillin, cephalosporin, macrolides and most of the other antibiotics, as well as heat under 91 °C (196 F), freezing, dehydration, gamma radiation under 0.0015 Gy (150 mrd) other bacteria or viruses, alcohol, peroxides, colloidal silver, lactoferrin, immune boosters, immunoglobulins or herbs. Present data suggest that nanobacteria are only killed by EDTA and tetracycline.[Citation69]
It seems the first step to the anti-nanobacterial therapy is to weaken the calcified shells by using substances like liquid zeolites and fulvic acid, which loosen the molecular bond. This step could be followed by adding EDTA and / or dimethyl sulfoxide in order to cause further weakening.[Citation70]
Tetracycline, already used in the treatment of the periodontal disease and the dental stone formation, is reported to inhibit the apatite-binding protein synthesis, chelate calcium and inhibit metalloproteinase. Besides, doxycycline is more highly protein-binding and approximately 10 times more lipophilic than tetracycline, which correlated with their comparative levels of calcium binding. Also, data show that gentamycin can cause a reduction in the amount of the putative biofilm surrounding nanobacteria, although it could not block the multiplication of organisms.
Further research shows that the inhibitory outcome of bisphosphonates on atherosclerosis formation could be the result of its bactericidal effect on nanobacteria.[Citation71] Also, gallium, as a multipotent element, has manifested antibiotic properties to iron-dependent bacteria, besides its intrinsic anti-inflammatory and anti-hypercalcemic properties. It readily reverses osteoporosis and has anti-nanobacterial effects, illustrated with observations from the kidney disease treatment.[Citation72]
Anti-nanobacterial mouthwashes or toothpastes should contain bisphosphonates, specifically etidronate and clodronate (1 mg/mL), gallium nitrate 14% (3.4% w/w gallium at 99.995% purity) and EDTA (1%) [Citation73] with neutral pH (7.0) that will be effective for the interruption of the calculus formation and will result in the prevention of periodontal diseases.[Citation74]
The wide spectrum of anti-bacterial activities of tetracycline could affect the normal oral flora and would possibly lead to suprainfection, therefore it has been removed from the formulation, despite of its excellent anti-nanobacterial activity.[Citation74]
Conclusions
As it is known, nanobacteria are a fairly new field of study in medicine. This study can bridge a gap between the etiological factors in some systemic cardio vascular and renal diseases with periodontal diseases. Also, due to their unique system of replication and metabolic nature, only specific agents can affect them. More studies need to be conducted to further understand the biological characteristics of these microorganisms.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Kajander EO, Ciftcioglu N. Nanobacteria: an alternative mechanism for pathogenic intra-and extracellular calcification and stone formation. Proc Natl Acad Sci. 1998;95(14):8274–8279.
- Alenazy MS, Mosadomi HA. Clinical implications of calcifying nanoparticles in dental diseases: a critical review. Int J Nanomed. 2014;9:27–31.
- Joshipura KJ, Hung HC, Rimm EB, et al. Periodontal disease, tooth loss, and incidence of ischemic stroke. Stroke. 2003;34(1):47–52.
- Joshipura KJ, Rimm E, Douglass C, et al. Poor oral health and coronary heart disease. J Dental Res. 1996;75(9):1631–1636.
- Hung HC, Willett W, Merchant A, et al. Oral health and peripheral arterial disease. Circulation. 2003;107(8):1152–1157.
- Çiftçioğlu N, McKay DS, Kajander EO. Association between nanobacteria and periodontal disease. Circulation. 2003;108(8):e58–e59.
- Kajander EO, Kuronen I, Åkerman K, et al. Nanobacteria from blood: the smallest culturable autonomously replicating agent on earth. In: Hoover RB, editor. Instruments, methods, and missions for the investigation of extraterrestrial microorganisms. Vol. 3111. San Diego (CA): The International Society for Optical Engineering; 1997. p. 420–428.
- Mathew G, McKay DS, Çiftçioglu N. Do blood-borne calcifying nanoparticles self-propagate? Int J Nanomed. 2008;3(2):265–275.
- Khullar M, Sharma S, Singh S, et al. Morphological and immunological characteristics of nanobacteria from human renal stones of a north Indian population. Urol Res. 2004;32(3):190–195.
- Miller VM, Rodgers G, Charlesworth JA, et al. Evidence of nanobacterial-like structures in calcified human arteries and cardiac valves. Am J Physiol Heart Circ Physiol. 2004;56(3):H1115–1124.
- Kumar V, Farell G, Yu S, et al. Cell biology of pathologic renal calcification: contribution of crystal transcytosis, cell‐mediated calcification, and nanoparticles. J Investig Med. 2006;54(7):412–424.
- Shiekh FA, Charlesworth JE, Kim SH, et al. Proteomic evaluation of biological nanoparticles isolated from human kidney stones and calcified arteries. Acta Biomater. 2010;6(10):4065–4072.
- Schwartz MA, Lieske JC, Kumar V, et al. Human-derived nanoparticles and vascular response to injury in rabbit carotid arteries: proof of principle. Int J Nanomed. 2008;3(2):243–248.
- Ciftcioglu N, Miller-Hjelle M, Hjelle J, et al. Inhibition of nanobacteria by antimicrobial drugs as measured by a modified microdilution method. Antimicrob Agents Chemother. 2002;46(7):2077–2086.
- Raoult D, Drancourt M, Azza S, et al. Nanobacteria are mineralo fetuin complexes. PLOS Pathog. 2008;4(2):e41.
- Martel J, Young JDE. Purported nanobacteria in human blood as calcium carbonate nanoparticles. Proc Natl Acad Sci . 2008;105(14):5549–5554.
- Martel J, Wu CY, Young JD. Critical evaluation of gamma-irradiated serum used as feeder in the culture and demonstration of putative nanobacteria and calcifying nanoparticles. PLOS One. 2010;5(4):e10343.
- Wu CY, Martel J, Young D, et al. Fetuin-A/albumin-mineral complexes resembling serum calcium granules and putative nanobacteria: demonstration of a dual inhibition-seeding concept. PloS One. 2009;4(11):e8058.
- Young JD, Martel J, Young D, et al. Characterization of granulations of calcium and apatite in serum as pleomorphic mineralo-protein complexes and as precursors of putative nanobacteria. PLOS One. 2009;4(5):e5421.
- Young JD, Martel J, Young L, et al. Putative nanobacteria represent physiological remnants and culture by-products of normal calcium homeostasis. PLOS One. 2009;4(2):e4417.
- Sommer AP, Hassinen HI, Kajander EO. Light-induced replication of nanobacteria: a preliminary report. J Clin Laser Med Surg. 2002;20(5):241–244.
- Bruckner M. Nanobacteria and nanobes – are they alive? [ Internet]. Bozeman, MT: Montana State University. [cited 2015 Feb 27]. Available from: http://serc.carleton.edu/microbelife/topics/nanobes/index.html
- Kajander EO, Ciftcioglu N, Aho K, et al. Characteristics of nanobacteria and their possible role in stone formation. Urol Res. 2003;31(2):47–54.
- Kutikhin AG, Brusina EB, Yuzhalin AE. The role of calcifying nanoparticles in biology and medicine. Int J Nanomed. 2012;7(10):339–350.
- Miller-Hjelle MA, Hjelle JT, Ciftcioglu N, et al. Nanobacteria: methods for growth and identification of this recently discovered calciferous agent. In: Olson WP, editor. Rapid analytical microbiology. Surrey: Davis Horwood International Publishing, Ltd., 2003. p. 297–312
- Çiftçioglu N, Kajander EO. Interaction of nanobacteria with cultured mammalian cells. Pathophysiology. 1998;4(4):259–270.
- Ciftcioglu N, McKay DS, Mathew G, et al. Nanobacteria: fact or fiction? characteristics, detection, and medical importance of novel self‐replicating, calcifying nanoparticles. J Investig Med. 2006;54(7):385–394.
- Dong Xia. Terror of the nanobacteria. [ Internet]. [cited 2013 Jun 14]. Available from: http://zhan.renren.com/h5/entry/3602888498040307841
- Kajander E. Nanobacteria–propagating calcifying nanoparticles. Lett Appl Microbiol. 2006;42(6):549–552.
- Çiftçioglu N, Björklund M, Kuorikoski K, et al. Nanobacteria: an infectious cause for kidney stone formation. Kidney Int. 1999;56(5):1893–1898.
- Çiftçioğlu N, Vejdani K, Lee O, et al. Association between Randall's plaque and calcifying nanoparticles. Int J Nanomed. 2008;3(1):105–115.
- Chen L, Huang X, Xu Q, et al. Cultivation and morphology of nanobacteria in sera of patients with kidney calculi. J Peking Univ Health Sci. 2010;42(4):443–446.
- Hu W, Wang X, Xu T, et al. Establishment nephrolithiasis rat model induced by nanobacteria and analysis of stone formation. J Peking Univ Health Sci. 2010;42(4):433–435.
- Torres VE, Wilson DM, Hattery RR, et al. Renal stone disease in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1993;22(4):513–519.
- Shoskes DA, Thomas KD, Gomez E. Anti-nanobacterial therapy for men with chronic prostatitis/chronic pelvic pain syndrome and prostatic stones: preliminary experience. J Urol. 2005;173(2):474–477.
- Zhou Z, Hong L, Shen X, et al. Detection of nanobacteria infection in type III prostatitis. Urology. 2008;71(6):1091–1095.
- Shen X, Ming A, Li X, et al. Nanobacteria: a possible etiology for type III prostatitis. J Urol. 2010;184(1):364–369.
- Zhang QH, Shen XC, Zhou ZS, et al. Decreased nanobacteria levels and symptoms of nanobacteria-associated interstitial cystitis/painful bladder syndrome after tetracycline treatment. Int Urogynecol J. 2010;21(1):103–109.
- Zhang QH, Lu GS, Shen XC, et al. Nanobacteria may be linked to testicular microlithiasis in infertility. J Androl. 2010;31(2):121–125.
- Bratos-Pérez MA, Sánchez PL, de Cruz SG, et al. Association between self-replicating calcifying nanoparticles and aortic stenosis: a possible link to valve calcification. Eur Heart J. 2008;29(3):371–376.
- Candemir B, Ertas FS, Kaya CT, et al. Association between antibodies against calcifying nanoparticles and mitral annular calcification. J Heart Valve Dis. 2010;19(6):745–752.
- Puskas L, Tiszlavicz L, Rázga Z, et al. Detection of nanobacteria-like particles in human atherosclerotic plaques. Acta Biol Hung. 2005;56(3):233–245.
- Maniscalco BS, Taylor KA. Calcification in coronary artery disease can be reversed by EDTA–tetracycline long-term chemotherapy. Pathophysiology. 2004;11(2):95–101.
- Kaya CT, Ertas FS, Hasan T, et al. Anticalcifying nanoparticle antibody titer is an independent risk factor for coronary artery calcification. Coron Artery Dis. 2011;22(6):394–400.
- Sedivy R, Battistutti WB. Nanobacteria promote crystallization of psammoma bodies in ovarian cancer. APMIS. 2003;111(10):951–954.
- Hudelist G, Singer C, Kubista E, et al. Presence of nanobacteria in psammoma bodies of ovarian cancer: evidence for pathogenetic role in intratumoral biomineralization. Histopathology. 2004;45(6):633–637.
- Wang L, Shen W, Wen J, et al. An animal model of black pigment gallstones caused by nanobacteria. Dig Dis Sci. 2006;51(6):1126–1132.
- Sheng-fu H, Jing E, Ciftciogiu N. Detection of nanobacteria in serum, bile and gallbladder mucosa of patients with cholecystolithiasis. Chin Med J. 2005;118(5):421–424.
- Agababov R, Abashina T, Suzina N, et al. Link between the early calcium deposition in placenta and nanobacterial-like infection. J Biosci. 2007;32(2):1163–1168.
- Pretorius AM, Sommer A, Aho K, et al. HIV and nanobacteria. HIV Med. 2004;5(6):391–393.
- Marsh PD. Dental plaque as a biofilm and a microbial community – implications for health and disease. BMC Oral Health. 2006;6(Suppl 1):S14
- Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontology. 2002;28(1):12–55.
- Marsh P. Dental plaque as a microbial biofilm. Caries Res. 2004;38(3):204–211.
- Mandel ID, Gaffar A. Calculus revisited. J Clin Periodontol. 1986;13(4):249–257.
- Beiswanger B, Segreto V, Mallatt M, et al. The prevalence and incidence of dental calculus in adults. J Clin Dent. 1988;1(3):55–58.
- Hjelle JT, Miller-Hjelle MA, Poxton IR, et al. Endotoxin and nanobacteria in polycystic kidney disease. Kidney Int. 2000;57(6):2360–2374.
- Shiekh FA, Khullar M, Singh S. Lithogenesis: induction of renal calcifications by nanobacteria. Urol Res. 2006;34(1):53–57.
- Kajander EO, Ciftcioglu N, Miller-Hjelle MA, et al. Nanobacteria: controversial pathogens in nephrolithiasis and polycystic kidney disease. Curr Opin Nephrol Hypertens. 2001;10(3):445–452.
- Bigelow MW, Wiessner JH, Kleinman JG, et al. Surface exposure of phosphatidylserine increases calcium oxalate crystal attachment to IMCD cells. Am J Physiol Renal Physiol. 1997;272(1):F55–F62.
- Aihara K, Byer KJ, Khan SR. Calcium phosphate–induced renal epithelial injury and stone formation: Involvement of reactive oxygen species. Kidney Int. 2003;64(4):1283–1291.
- Zhang SM, Tian F, Jiang XQ, et al. Evidence for calcifying nanoparticles in gingival crevicular fluid and dental calculus in periodontitis. J Clin Periodontol. 2009;80(9):1462–1470.
- Yang F, Zeng J, Zhang W, et al. Evaluation of the interaction between calcifying nanoparticles and human dental pulp cells: a preliminary investigation. Int J Nanomed. 2011;15(6):13–18.
- Zeng J, Yang F, Zhang W, et al. Association between dental pulp stones and calcifying nanoparticles. Int J Nanomed. 2011;6:109–118.
- Jing J, Lu J, Hao Y, et al. Nanobacteria's potential involvement in enamel repair in caries. Med Hypotheses. 2009;73(3):359–360.
- Duckworth RM, Huntington E. Evidence for putting the calculus: caries inverse relationship to work. Community Dent Oral Epidemiol. 2005;33(5):349–356.
- Kolahi J, Shahmoradi M, Sadreshkavary M. Nanobacteria and dental practice. Raleigh: Lulu Press, Inc; 2012
- Lin Y, Zheng R, He H, et al. Application of biomimetic mineralization: a prophylactic therapy for cracked teeth? Med Hypotheses. 2009;73(4):493–494.
- Wang X, Liu W, Yang Z, et al. The detection of nanobacteria infection in serum of healthy Chinese people. Chin J Epidemiol. 2004;25(6):492–494. Chin.
- Demir T. Is there any relation of nanobacteria with periodontal diseases? Med Hypotheses. 2008;70(1):36–39.
- Abo-El-Sooud K, Hashem M, Ramadan A, et al. Research strategies for treatment of nanobacteria. Insight Nanotechnol. 2011;1:1–8.
- Silay YS, Altundag K, Altundag O, et al. Bisphosphonates may inhibit development of atherosclerosis formation through its bactericidal effect on nanobacteria. Med Hypotheses. 2005;64(6):1239–1240
- Eby GA. A hypothesis for anti-nanobacteria effects of gallium with observations from treating kidney disease. Med Hypotheses. 2008;71(4):584–590.
- Kumar CA, Bagga MB, Mohan V, et al. An overview on clinical implications of nanobacteria. J Indian Acad Oral Med Radiol. 2011;23(3):S354–359
- Kolahi J. Anti-nanobacterial therapy for prevention and control of periodontal diseases. Dent Hypotheses. 2011;2(1):4–8.