Abstract
This study was designed to investigate the hepatoprotective effect of diffractaic acid isolated from a lichen species, Usnea longissima, at 3 doses, 50, 100 and 200 mg/kg, against carbon tetrachloride (CCl4)-induced hepatic damage. For this purpose, 40 Wistar albino rats were divided in 5 groups, including 3 experimental and 2 control ones, and 0.2 mL/kg of CCl4 in olive oil (1:5 v/v) was injected daily for 6 weeks intraperitoneally. After the liver injury, diffractaic acid was applied at doses of 50, 100 and 200 mg/kg for 7 days. The group given physiologic saline (0.2 mL/kg) was used as a control group. Alanine aminotransferase, aspartate aminotransferase, gamma-glutamyl transpeptidase, creatinine, urea, direct and total bilirubin and C-reactive protein levels were also evaluated in the serum samples obtained from the rat groups. Liver tissues were removed and were examined histopathologically following staining with hematoxylin–eosin. The results showed that 50 mg/kg daily dose of diffractaic acid could be considered to have hepatoprotective effect by ameliorating the studied biochemical parameters and tissue histological structures. However, 100 and 200 mg/kg of diffractaic acid acted as hepatotoxin and did not show any hepatoprotective effect. Thus, diffractaic acid could be potentially used as a hepatoprotective agent at a low dose (50 mg/kg) against acute liver toxicity induced by CCl4.
Introduction
Liver is a vital organ that is responsible for the detoxification of toxic chemicals, drugs and viruses in humans and vertebrate animals. Therefore, it can be easily affected from various chemicals and free radicals.[Citation1] Liver disease refers to any disorder of the liver resulting from various internal and external factors and includes steatosis, fibrosis, hepatitis, inflammation, cirrhosis and liver cancer. Carbon tetrachloride (CCl4) is a common hepatotoxin and causes acute injury as well as chronic injury ranging to cirrhosis at prolonged exposure. That is why CCl4-induced hepatic toxicity is widely used as an experimental method to study the protective effects of various agents.[Citation2–4]
It has been shown that plant-derived agents for medicinal uses are relatively non-toxic, safe and even free from some of the serious side effects of the synthetic analogues of the drugs. Hence, there is an increasing interest in the biological activities of plant extracts and pure metabolites isolated from plants.[Citation3,Citation5–7] Lichens have been widely used in folk remedies for the treatment of various diseases, such as eczema, pulmonary diseases, arthritis and cancer.[Citation8,Citation9] Lichens synthesize characteristic metabolites that differ from those in the plant kingdom and many of them are phenolic metabolites, including orcinol and β-orcinol derivatives, dibenzofurans, depsidones, depsides, lactones, quinines and pulvinic acid derivatives.[Citation10] Usnea is a genus of mostly pale greyish-green fruticose lichens that grow like leafless mini-shrubs or tassels anchored on bark or twigs.[Citation11] Usnea longissima is an important member of the Usnea genus and is usually distributed in moist and shady areas. It synthesizes characteristic metabolites that belong to the polyketide family. It has been shown that U. longissima has various biological activities, such as anti-ulcer,[Citation12] anti-tuberculosis,[Citation13] anti-inflammatory,[Citation14,Citation15] inhibitory effect in melanogenezis,[Citation16] anti-microbial,[Citation17] anti-platelet and anti-thrombotic,[Citation18] anti-oxidant,[Citation19] inhibitory effect on plant growth,[Citation20] healing of bone fractures and injuries,[Citation21] anti-tumour and cytotoxic activity.[Citation22] Usnic and diffractaic acids are the major metabolites of U. longissima.[Citation23–25] Previous studies indicated that diffractaic acid possesses anti-ulcer,[Citation24,Citation25] anti-proliferative,[Citation23,Citation26] apoptotic,[Citation27] analgesic and anti-pyretic effect.[Citation28]
There are, however, few scientific reports on the hepatoprotective effect of diffractaic acid. That is why the aim of this study was to evaluate the potential hepatoprotective effect of this lichen metabolite.
Materials and methods
Experimental animals
A total of 40 Wistar albino rats, weighing 180–200 g on average (provided by Gaziantep University Medical Faculty, Department of Physiology) were used in this study. All experiments were carried out according to the local guidelines for the care and use of experimental animals and approved by the Local Animal Ethics Committee, Cumhuriyet University, Sivas, Turkey (number: B.30.2.CUM.0.01.00.00-50/31).
Lichen sample
U. longissima Ach. was collected from Giresun region (Northern Anatolia, Turkey) in 2013 and identified by a lichenologist, Dr Ali Aslan.[Citation29] A voucher specimen (KKEF-374) has been deposited in the herbarium of Kazım Karabekir Education Faculty, Ataturk University, Erzurum, Turkey.
General analytical procedures
Column chromatography (CC) was carried out using silica gel 60 (70–230 mesh). Thin-layer chromatography (TLC) was performed on silica gel 60 F254-coated aluminium plates (Merck). The spots on TLC belonging to metabolites were visualized with UV254 nm. Infrared (IR) spectra were recorded with KBr pellets on a Shimadzu Fourier transform (FT-IR) 8000 spectrophotometer. Nuclear magnetic resonance (NMR) spectra were obtained with Varian spectrometer (Cary® 50 Bio) at 400 MHz for 1H and 100 MHz for 13C (δ). Tetramethyl silane was used as an internal standard. Electron ionization mass spectroscopy was performed on a gas chromatography--mass spectrometry (GC–MS) apparatus (Agilent-Technologie 6890N). Ultraviolet–visible (UV–vis) spectra of diffractaic acid and biochemical assays were recorded on a UV–vis spectrophotometer (Thermo Electron Corporation, Spectronic HELIOS β). Melting point was determined on a Buchi 510 melting point apparatus.
Extraction of lichen sample and isolation of diffractaic acid
Dried and powdered lichen sample (250 g) was extracted with 500 mL of diethyl ether, using a Soxhlet apparatus at 40 °C. The crude extract of the lichen sample was filtered and then this solution was stored in refrigerator for 24 h. As a result, usnic acid, the other major compound in the diethyl ether extract of the lichen sample, crystallized as yellowish needles. After the crystals were removed by filtration, the solution was concentrated using an evaporator under reduced temperature and pressure. The extract (18.75 g) was subjected to silica gel (70–230 mesh) CC eluting with chloroform:n-hexane (7:3, 75:25, 90:10 and 100:0) and chloroform–methanol (9:1) solvent systems. Thus, 3.75 g of diffractaic acid (1.5% yield) was obtained. The chemical structure was confirmed by UV–vis, IR, 1H-NMR, 13C-NMR and two-dimensional (2D) NMR spectroscopy and previous data.[Citation24,Citation30]
Experimental design
The Wistar albino rats were allowed to adapt to the laboratory environment for a week. The animals were maintained at a 12 h light/dark cycle at a constant temperature (25 °C) with free access to standard pellet food and tap water. The animals (eight rats per group) were divided into five groups, including three experimental groups and two control ones (a positive and a negative control). Group 1 was kept as the negative control group administrated only with 0.2 mL/kg physiological saline (PS) intraperitoneally (i.p.), without diffractatic acid. All other rats were treated with a CCl4 solution (0.2 mL/kg, i.p.) prepared by dissolving in olive oil (1:5 v/v), once daily for 6 weeks to develop a model of hepatic injury. The rats in Group 2 were only administrated with 0.2 mL/kg of CCl4 i.p. dissolved in olive oil (1:5, v/v) for these 6 weeks and served as a positive control. Following the liver damage (CCl4 administration), the 3 experimental groups were given 50, 100 and 200 mg/kg of diffractaic acid solution dissolved in dimethyl sulfoxide for 7 days i.p. The groups and treatments are summarized in .
Table 1. Experimental design: applications of diffractaic acid for each group of Wistar rats.
Biochemical analyses
At the end of the experimental protocol, the rats in all groups were starved overnight and all animals were anesthetized with 100 mg/kg ketamine/5 mg/kg diazepam and euthanized with 200 mg/kg thiopental sodium. Five millilitre cardiac blood samples were taken into heparinized tubes from the abdominal aorta. Blood samples for the biochemical analysis were taken from each treatment group at the beginning and on the 42nd day of the experiment. The blood samples taken from the animals were centrifuged at 5000 r/min for 10 min (Hettich Zentrifugen, Mikro 200). Erythrocytes and plasma components were separated by centrifugation of the blood. Erythrocytes were washed 3 times with 0.9% NaCl solution and then packed and stored at +4 °C until study and the plasma was collected in 1.5 µL Eppendorf® tubes. The collected plasma samples were stored at −80 °C for the biochemical analysis of urea, creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), C-reactive protein (CRP) and bilirubin.
Histopathological analysis
Liver tissues were removed from the killed animals and were fixed in 10% buffered formaldehyde. Then, tissue specimens were processed by the paraffin slice technique and sections were stained with haematoxylin–eosin (H&E stain), according to Guldur et al. [Citation31] and stained with reticulin for fibrosis evaluation, according to Tsukamoto et al. [Citation32] Paraffin-embedded tissues were examined morphologically with light microscopy by a pathologist to observe histopathological changes in the liver. A score system was used for histopathological evaluation of the paraffin-embedded tissues.[Citation32]
Necroinflammatory activity in the tissues was classified based on the Isaac score system.
Score 0 – no visible cell damage.
Score 1 – focal hepatocyte damage in less than 25% of the tissue.
Score 2 – focal hepatocyte damage in 25%–50% of the tissue.
Score 3 – extensive, but focal, hepatocyte lesions.
Score 4 – global hepatocyte necrosis.
Fibrosis was graded by the modified Knodell scoring system according to Knodell et al. [Citation33] as follows.
Grade 0 – no fibrosis.
Grade 1 – short fibrous septum or expansion without septum in some areas of portal.
Grade 2 – short fibrous septum or expansion without septum in most areas of portal.
Grade 3 – short fibrous expansion in most areas of portal, rare portal–portal bridging.
Grade 4 – both portal–portal and portal–central bridging with clear bridging in portal areas.
Grade 5 – clear bridging with rare nodules, incomplete cirrhosis.
Grade 6 – complete cirrhosis.
Statistical analysis
Statistical analysis was carried out using the one-way analysis of variance TUKEY-HSD (Post-Hoc) test for the parametric values. The Kruskal–Wallis test was used for the non-parametric values (SPSS 13.0 software). Values at p < 0.05 were considered statistically significant. All values were expressed as mean values with standard error of the means (SEM).
Results and discussion
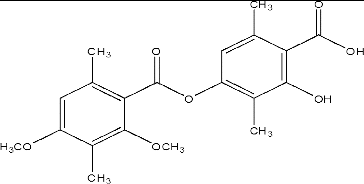
Diffractaic acid () is a lichen metabolite that belongs to the group of depsides and is an important metabolite of U. longissima.[Citation24] It was isolated from the diethyl ether extract of the lichen sample by column and TLC at a 1.5% yield along with another common lichen metabolite, usnic acid. Its chemical structure was characterized by UV–Vis, IR, 1H-NMR, 13C-NMR and 2D NMR spectroscopic methods and confirmed previous data.[Citation24,Citation26,Citation30] In our previous study, we studied the anti-tumoural effect of diffractaic acid against the experimental tumour model. The results revealed that the minimal concentration (50 mg/kg) is much more successful to minimize tumour growth than that of others.[Citation26] In this study, to evaluate the potential protective effect of diffractaic acid against CCl4-induced liver damage, standard biochemical assays (plasma AST, ALT, GGT, CRP, urea, creatinin and bilirubin levels) were performed and histological changes (hepatocellular necroinflammation and fibrosis) in all liver tissues were assessed.
Biochemical tests results
ALT is a hepato-specific enzyme that is generally located in the cytoplasm of hepatocytes.[Citation34] The serum ALT and AST levels are sensitive to the action of hepatotoxic agents and serve as markers of liver damage, which is accompanied by the release of such aminotransferases from hepatocytes into the blood stream.[Citation7,Citation34] Most causes of liver cell injury are associated with AST levels that are lower than ALT ones, which is reflected in the AST/ALT ratio in a tested blood sample. An AST to ALT ratio of 2:1 or greater is suggestive of liver disease, particularly in the setting of an elevated GGT.[Citation35]
The results from the biochemical analyses of the studied rat blood samples are summarized in . The values of the biochemical parameters indicated that liver damage was indeed induced in the positive control group in our experimental conditions (i.e. the group given CCl4 only). An increase in the level of serum urea was observed in the CCl4 group compared to that in the negative control (healthy) group (). Compared to the levels in the positive controls, the urea level slightly decreased in the group given 50 mg/kg of diffractaic acid, whereas it increased in the other two groups, especially in the one given 200 mg/kg of diffractaic acid (p < 0.05) (). The statistical analysis, however, showed no significant differences among the creatinine levels in the studied groups ().
Table 2. Biochemical parameters in CCl4-treated mice.
Bilirubin levels can be increased due to hepatocellular injury and cholestatic liver diseases.[Citation5] However, the obtained results showed that the administration of CCl4 did not significantly affect the bilirubin level (). Nevertheless, the bilirubin level was significantly reduced by all doses of diffractaic acid as compared to that in the CCl4 (positive control) group (p > 0.05).
Although the serum bilirubin levels did not increase in the positive control (CCl4) group, there was a considerable elevation in the serum AST, ALT and GGT values in this group as compared to those in the healthy (negative control) rats (). These changes are thought to be an indicative factor of severe liver damage, since these enzymes are well known to be released from hepatocytes into the blood at the site of hepatonecrosis. In contrast to CCl4 treatment only, the administration of 50 mg/kg of diffractaic acid led to a decrease in these parameters to the normal levels. At high doses of diffractaic acid, however, a protective effect was not observed. As shown in , the AST, ALT and GGT levels in these two groups were elevated, and especially in the blood samples from animals given the highest dose (200 mg/kg) of diffractaic acid as compared with the positive and negative control group (p > 0.05).
The animals in the group administered with CCl4 only showed lower levels of serum CRP as compared with those in the negative control group, which were administered only 0.2 mL/kg PS (). The CRP levels were observed to decrease in the groups administered with 50 and 100 mg/kg doses of diffractaic acid but increased significantly (p > 0.05) in the group that received 200 mg/kg of diffractaic acid (). All these results suggest that, at high doses, diffractaic acid may not act as a hepatoprotective agent and could be toxic at such doses. These results are in agreement with other reports on diffractaic acid and other hepatoprotective agents.[Citation2,Citation3,Citation8,Citation36]
Histopathological results
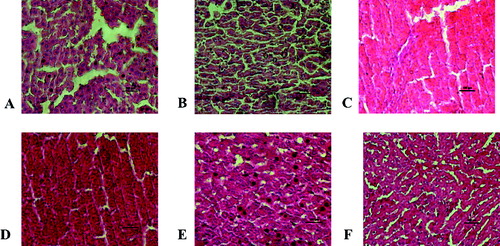
In liver tissue from healthy rats (negative controls), normal parencyhmal structure was observed, with no changes in vena centralis structures, portal areas, hepatocytes cords and sinusoids (Score 0, Grade 0). In the CCl4-treated group, there was global hepatocyte necrosis and both portal–portal and portal–central bridging fibrosis with clear bridging in portal areas (Score 4, Grade 4) ((A) and 2(B)). In the diffractaic acid-treated groups, generally, focal hepatocyte damage on less than 25% of the tissue (Score 1) and short fibrous septa or expansion without septa formation in some portal areas (Grade 2) were observed. In particular, administration of 50 mg/kg dose of diffractaic acid diminished the liver damage caused by CCl4 and decreased the inflammation, which was in agreement with the observed decrease in the CRP level. In this group, focal hepatocyte damage was found at low levels (Score 1) and fibrosis was not seen in most parts of the tissue (Grade 0) ((C) and 2(D)). However, further increase in the doses of diffractaic acid caused a marked increase in fibrosis area and hemorrhages ((E) and 2(F)), enlargement of the portal vein and also increased inflammation. Thus, these histological observations were in support of the biochemical evidence for damaging effects of high-dose diffractaic acid. Taken together, these results suggest that the hepatoprotective effect of diffractaic acid was not dose-dependent in the studied dose range.
Figure 2. Histopathological lesions in the liver tissue. (A, B) Hepatocytic degeneration and multifocal areas of necrosis in the positive control (CCl4-treated) group; (C–F) groups treated with diffractaic acid: (C, D) 50 mg/kg, (E) 100 mg/kg and (F) 200 mg/kg.
According to the growing number of reports on the hepatoprotective properties of natural products,[Citation2,Citation3,Citation5–8,Citation31,Citation36] it is noteworthy that although some products have high potential activities, others have shown weak protective properties. In addition, some metabolites with high potential have proved cytotoxic. Our test molecule, diffractaic acid, showed protective effect on the liver at a low dose. As a result, diffractaic acid can be considered a promising agent in liver therapy for further research.
Conclusions
To the best of our knowledge, the results from this study demonstrated for the first time that diffractaic acid isolated from U. longissima lichen at a low dose has hepatoprotective effect on the biochemical and histological level against CCl4-induced hepatic fibrosis in Wistar rats. Overall, based on the obtained results, it could be suggested that diffractaic acid could be considered as a promising hepatoprotective agent. However, further in vitro and animal pre-clinical trials are required to gain deeper insight into the pathways involving diffractaic acid.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Johnston DE, Kroening C. Mechanism of early carbon tetrachloride toxicity in cultured rat hepatocytes. Pharmacol Toxicol. 1998;83:231–239.
- El-Halawany AM, El Dine RS, El Sayed NS, et al. Protective effect of Aframomum melegueta phenolics against CCl4-induced rat hepatocytes damage; role of apoptosis and pro-inflammatory cytokines inhibition. Sci Rep. 2014;4:5880.
- Maranhao HLM, Vasconcelos CFB, Rolim LA, et al. Hepatoprotective effect of the aqueous extract of Simarouba amara Aublet (Simaroubaceae) stem bark against carbon tetrachloride (CCl4)-induced hepatic damage in rats. Molecules. 2014;19:17735–17746.
- Wang D, Zhao Y, Sun Y, et al. Protective effects of Ziyang tea polysaccharides on CCl4-induced oxidative liver damage in mice. Food Chem. 2014;143:371–378.
- Akther N, Andrabi K, Nissar A, et al. Hepatoprotective activity of LC-ESI-MS standardized Iris spuria rhizome extract on its main bioactive constituents. Phytomedicine. 2014;21:1202–1207.
- Kalegari M, Bruel Gemin CA, Araujo-Silva G, et al. Chemical composition, antioxidant activity and hepatoprotective potential of Rourea induta Planch. (Connaraceae) against CCl4-induced liver injury in female rats. Nutrition. 2014;30:713–714.
- Parimoo HA, Sharma R, Patil RD, et al. Hepatoprotective effect of Ginkgo biloba leaf extract on lantadenes-induced hepatotoxicity in guinea pigs. Toxicon. 2014;81:1–12.
- Wang Y, Kim JA, Cheong YH, et al. Isolation and characterization of a non-reducing polyketide synthase gene from the lichen-forming fungus Usnea longissima. Mycol Prog. 2012;11:75–83.
- Jayanthi S, Priya P, Monica Devi D, et al. Lichens: origin, types, secondary metabolites and applications. J Acad Indus Res. 2012;1(1):45–49.
- Aslan A. Lichens from the regions of Artvin, Erzurum, and Kars (Turkey). Isr J Plant Sci. 2000;48:143–155.
- Wikipedia: the free encyclopedia [Internet]. St. Petersburg, FL: Wikimedia Foundation, Inc. 2001 [cited 2007 May 5]. Available from: http://en.wikipedia.org/.
- Halici M, Odabasoglu F, Suleyman H, et al. Effects of water extract of Usnea longissima on antioxidant enzyme activity and mucosal damage caused by indomethacin in rats. Phytomedicine. 2005;12:656–662.
- Yamamoto Y, Miura Y, Kinoshita Y, et al. Screening of tissue-cultures and thalli of lichens and some of their active constituents for inhibition of tumor promoter-induced epstein-barr-virus activation. Chem Pharm. 1995;43:1388–1390.
- Choudhary MI, Atta-ur-Rahman ASJ, Jalil S, et al. Bioactive phenolic compounds from a medical lichen, Usnea longissima. Phytochemistry. 2005;66:2346–2350.
- Engel K, Schmidt U, Reuter J, et al. Usnea barbata extract prevents ultraviolet-B induced prostaglandin E-2 synthesis and COX-2 expression in HaCaT keratinocytes. J Photoch Photobiol B. 2007;89:9–14.
- Kim MS, Choi HB. Melanogenesis inhibitory effects of methanolic extracts of Umbilicaria esculenta and Usnea longissima. J Microbiol. 2007;45:578–582.
- Cansaran D, Kahya D, Yurdakulol E, et al. Identification and quantitation of usnic acid from the lichen Usnea species of Anatolia and antimicrobial activity. Z Naturforsch C. 2006;61:773–776.
- Lee KA, Kim MS. Antiplatelet and antithrombotic activities of methanol extract of Usnea longissima. Phytother Res. 2005;19:1061–1064.
- Odabasoglu F, Aslan A, Cakir A, et al. Comparison of antioxidant activity and phenolic content of three lichen species. Phytother Res. 2004;18:938–941.
- Nishitoba Y, Nishimura H, Nishiyama T, et al. Lichen acids, plant-growth inhibitors from Usnea longissima. Phytochemistry. 1987;26:3181–3185.
- Brij L, Upreti DK. Ethnobotanical notes on three Indian lichens. Lichenologist. 1995;27:77–79.
- Einarsdottir E, Groeneweg J, Bjornsdottir GG, et al. Cellular mechanisms of the anticancer effects of the lichen compound usnic acid. Planta Med. 2010;76:969–974.
- Kumar S, Muller K. Lichen metabolites. 2. Antiproliferative and cytotoxic activity of gyrophoric, usnic, and diffractaic acid on human keratinocyte growth. J Nat Prod. 1999;6:821–823.
- Bayir Y, Odabasoglu F, Cakir A, et al. The inhibition of gastric mucosal lesion, oxidative stress and neutrophil-infiltration in rats by the lichen constituent diffractaic acid. Phytomedicine. 2006;13:584–590.
- Odabasoglu F, Cakir A, Suleyman H, et al. Gastroprotective and antioxidant effects of usnic acid on indomethacin-induced gastric ulcer in rats. J Ethnopharmacol. 2006;103:59–65.
- Karagoz ID, Ozaslan M, Guler I, et al. In vivo antitumoral effect of diffractaic acid from lichen metabolites on Swiss albino mice with Ehrlich ascites carcinoma: an experimental study. Int J Pharmacol. 2014;10:307–314.
- Odabasoglu F, Yildirim OS, Aygun H, et al. Diffractaic acid, a novel proapoptotic agent, induces with olive oil both apoptosis and antioxidative systems in Ti-implanted rabbits. Eur J Pharmacol. 2012;674:171–178.
- Okuyama E, Umeyama K, Yamazaki M, et al. Usnic acid and diffractaic acid as analgesic and antipyretic components of Usnea diffracta. Planta Med. 1995;61:113–115.
- Yazici K, Aslan A. Lichens from the regions of Gümüşhane, Erzincan and Bayburt (Turkey). Cryptogamie Mycol. 2003; 24: 287–300.
- Huneck S, Yoshimura I. Identification of lichen substances. Berlin: Springer; 1996. p. 251.
- Guldur ME, Ozaslan M, Aytekin T, et al. Toxicity effect of zinc supplementation on the liver tissue. Pak J Biol Sci. 2006;9(6):1139–1142.
- Tsukamoto H, Horne W, Kamimura S, et al. Experimental liver cirrhosis induced by alcohol and iron. J Clin Investig. 1995;96:620–630.
- Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–435.
- Nyblom H, Bjornsson E, Simren E, et al. The AST/ALT ratio as an indicator of cirrhosis in patients with PBC. Liver Int. 2006;26:840–845.
- Drotman RB, Lawhorn GT. Serum enzymes as indicators of chemical induced liver damage. Drug Chem Toxicol. 1978;1:163–171.
- Eidi A, Mortazavi P, Behzadi K, et al. Hepatoprotective effect of manganese chloride against CCl4-induced liver injury in rats. Biol Trace Elem Res. 2013;155:267–275.