Abstract
Cyclophosphamide (CTX) is a DNA-alkylating chemotherapeutic agent and leads to leucopenia and hepatocyte injury. Granulocyte colony-stimulating factor (G-CSF) promotes granulocyte counts and increases leukocytes count. Beta (β)-glucans affect bone marrow cellularity and stem cell mobilization. This study aimed to investigate the effect of β-glucan in combination with G-CSF on leucopenia and hepatocytes injury induced by CTX in mice. Animals were injected with CTX, G-CSF and/or β-glucan for five days, then leukocyte counts and biochemical assays were performed. Our results showed that CTX administration decreased splenocytes and total white blood cells counts. Furthermore, CTX administration led to hepatocyte oxidative stress which was characterized by increased pro-oxidants (thiobarbituric acid reactive substances and xanthine oxidase) and decreased anti-oxidant levels (glutathione peroxidase) and energy disturbance and cell mobilization arrest due to the partial inhibition of adenosine triphosphatase and mannosidase. As a result, hepatocyte injury was observed as indicated by serum aspartate aminotransferase and alanine aminotransferase elevation. β-glucan alone or combined with G-CSF successfully normalized the biochemical parameters and the alteration in cell counts arising from CTX administration. In conclusion, CTX causes leukopenia and liver necrosis through the stimulation of oxidative stress, which leads to inflammation. Both G-CSF and glucan counteract this action through their anti-oxidant and anti-inflammatory properties.
Introduction
Cyclophosphamide (CTX) is a DNA-alkylating chemotherapeutic agent that is widely used in the treatment of different types of malignancies and lymphoproliferative and autoimmune disorders.[Citation1] CTX is transformed via hepatic and intracellular enzymes to active alkylating metabolites. CTX cytotoxicity is due to the formation of phosphoramide mustard, which generates DNA cross-links and leads to apotosis.[Citation2] Cells deficient in aldehyde dehydrogenase (ALDH), the enzyme involved in CTX detoxification, produce active phosphoramid metabolite. Low levels of ALDH are expressed in lymphocytes and, therefore, these cells are sensitive to CTX. Conversely, hematopoietic stem cells (HSCs) express high levels of ALDH and resist CTX-mediated cytotoxicity.[Citation1] This selective cytotoxicity is considered a major advantage of using high-dose CTX. Low myelotoxicity allows rapid restoration of the lymphoid compartments by HSCs after chemotherapy.
CTX has been found to induce severe neutropenia in humans.[Citation3–5] Detailed analysis of this leukopenia in mice showed that CTX decreased the absolute number of leukocytes in the peripheral blood (PBL) at days 3–15, and in the spleen at days 3–6 and came back to normal range at the recovery phase (day 9).[Citation4] The rebound phase is accompanied by growth factors release and proliferation of bone marrow precursors, and as a consequence associates with the mobilization of bone marrow precursors for transplantation.[Citation1,Citation2]
Granulocyte colony-stimulating factor (G-CSF) is commonly used to mobilize stem cells into the blood and as a growth factor to promote granulocyte counts in immunocompromised patients. As such, it is frequently used as an adjunctive agent in tumour chemotherapy owing to its regulatory effect on the maturation, proliferation and differentiation of leukocyte precursor cells. It can effectively antagonize myelosuppression induced by chemotherapy agents through raising the leukocyte level of peripheral blood.[Citation6] Our previous results as well as those of others have shown that administration of G-CSF can increase the numbers of peripheral blood progenitor cells (PBPC) manyfold above basal levels. Maximal levels of PBPC were observed on days 5 and 6 after G-CSF treatment.[Citation7,Citation8] Currently, G-CSF alone or CTX followed by G-CSF are the most common agents used for PBPC mobilization schedules. Application of CTX followed by G-CSF increases the number of circulating hematopoietic progenitor cells.[Citation9] Even after G-CSF treatment, which corrects the leukopenia, CTX treatment also associates with adverse effects, including alteration in the biochemical indices in the liver. Therefore, the discovery of agents that can circumvent this dysfunction is of great clinical significance.
Naturally occurring β-glucans are β-glycosidic polymers of D-glucose with varying molecular mass. The soluble forms of these compounds have been used for tumour immunotherapy.[Citation10]
They protect against myelotoxic injury from radiation and chemotherapy [Citation11] and enhance the recovery of bone marrow cellularity and stem cell mobilization [Citation12–16] through increasing the levels of stromal cell-derived factor alpha (SDF-1 alpha) in plasma.[Citation17] Moreover, glucan was found to directly enhance hematopoietic progenitor cells (HPC) expansion ex vivo and promoted homing and engraftment of CD34+ cord blood cells in a combined immune-deficient mouse model.[Citation15,Citation16]
Given these biological activities of β-glucan, this study aimed to investigate whether the administration of β-glucan in combination with G-CSF in mice can delay the leucopenia induced by treatment with high dose of the anti-cancer drug CTX and to test whether its effect is correlated with correction of the alteration in the biochemical readouts.
Subjects and methods
Mice
Adult female Swiss albino mice (CD1 strain) weighting 20 ± 25 g were purchased from National Research Center (NRC, Cairo, Egypt). Animals were housed (5 animals per cage) at the animal facility at Zoology Department, Faculty of Science (Tanta University, Egypt) in clean and dry plastic cages, at a 12 h/12 h dark/light cycle under laboratory condition of temperature and humidity. The mice were fed with rodent pellets and tap water ad libitum. This study was performed in accordance to the guidelines for the use of experimental animals in research at Zoology Department, Faculty of Science, Tanta University, Egypt.
Chemicals
CTX was purchased from Sigma (Sigma–Aldrich, CA, USA) and reconstituted in phosphate buffered saline (PBS) in a stock solution and kept at –80 °C until use. G-CSF (Neupogen®) was purchased from a local pharmacy. G-CSF was diluted in PBS to the required concentration before injection.
Animal design and treatment
For the induction of leucopenia, mice (n = 6/group) were treated once with intraperitoneal (i.p.) injection of 200 mg/kg (4 mg/mouse) CTX. For the amelioration of leucopenia that occurred 1 day after CTX injection, mice were treated with subcutaneous (s.c.) injection of 5 µg/mouse G-CSF (Neupogen®) daily for 5 consecutive days. To test the impact of β-glucan on leucopenia, mice were treated for 5 consecutive days with injection of 100 µg i.p.; each injection was administered 30 min after G-CSF injection.
Preparation and counting of peripheral blood mononuclear cells. Mice were anesthetized by inhalation of isoflurane (1-chloro-2,2,2-trifluoroethyl difluoromethyl ether; Hospira, Inc. Lake Forest, IL, USA) and blood samples were collected from the orbital sinus using heparinized microhematocrit tubes into 1.5 mL Eppendorf® tubes. Samples were analysed for the total number of leukocytes, using an automated instrument for complete blood counts (Vet-Scan HM2™ Hematology System, Abaxis, Union City, CA) to determine white blood cells (WBCs), platelets, relative and absolute number of neutrophils and lymphocytes.
Tissue sampling. Mice were sacrificed and blood and sera were collected from all groups. Spleen and liver samples were quickly removed and washed in cold saline, then cut into pieces. Tissue pieces were then homogenized with 9 volumes of phosphate buffer, 0.1 mol/L, pH 7.4, and then centrifuged at 3000 r/min for 15 min and the supernatant was collected to be used as a homogenate.
Biochemical analyses
The serum liver enzymes aspartate transaminase (AST; EC 2.6.1.1) and alanine transaminase (ALT; EC 2.6.1.2) as well as albumin and the liver total protein level were determined according to the manufacturer's instructions (Biosystem, Egypt).
The level of thiobarbituric acid reactive substances (TBARS) and xanthine oxidase (XO; EC 1.17.3.2) as pro-oxidants indicator were measured according to Tappel and Zalkin [Citation18] and Litwack et al. [Citation19], respectively. The level of liver TBARS was calculated with the following equation (nmol/g wet tissue) = (At/0.156) × 10, where At is the absorbance of the test sample and ϵ = 0.156 is the extinction coefficient. The liver XO activity (μmole/h/g tissue) was estimated as follows: (C) ×10/(0.284 × xanthine M. Wt), where 0.284 is a constant and C is the concentration in the test sample. The activity of the anti-oxidant enzyme glutathione peroxidase (GPx; EC 1.11.1.9) was measured according to Paglia and Valentine [Citation20]. The enzyme activity was calculated by using the following equation; GPx activity (U/g wet tissue) = (At × 6.2 × 10 × 10)/(13.1 × 0.05 × 10), where ϵ1 = 6.2 and ϵ2 = 13.1 are extinction coefficients for H2O2 and DTNB (5,5′-dithiobis-(2-nitrobenzoic acid)).
For mannosidase (EC 3.2.1.24) activity estimation; 100 μL of sample and 100 μL of substrate ((p-nitrophenyl-α-D-mannoside, 4 mmol/L) were mixed with 300 μL buffer (sodium acetate buffer, pH 4.4, 0.1 mol/L) and incubated for 30 min at 37 °C. The reaction was stopped by adding alkaline buffer (0.133 mol/L glycine, 0.067 mol/L NaCl, 0.083 mol/L Na2CO3 adjusted to pH 10.7 with NaOH). The amount of p-nitrophenol released was estimated by measuring the optical density at 400 nm. One unit of enzyme is defined as the amount catalysing the release of 1 nmol of product/h at 37 °C. For adenosine triphosphatase (ATPase) (EC 3.6.3.9) assessment, 200 μL buffer (5 mmol/L MgCl2, 80 mmol/L NaCl, 20 mmol/L KCl, 40 mmol/L Tris-HCl buffer, pH 7.4) was added to 20 μL homogenate and pre-incubated for 5 min at 37 °C; then 20 μL of 10 mmol/L adenosine triphosphate (ATP) were added and the reaction mixture was incubated for 30 min. Then, 200 μL of tricholoroacetic acid (10%) were added and the samples were centrifuged at 3000 r/min for 10 min at room temperature. Fifty microliters of incubation mixtures were added to 5 mL ammonium molybdate–methyl green mixture (5.8 mL molybdate (1.2% w/v in 0.73 mol/L H2SO4), 1.7 mL methyl green (0.16 mmol/L in 0.34% triton X-305), and 1.3 triton X-305/NaOH (0.2% w/v / 0.1 mol/L) and 1 mL water). The samples were mixed well and incubated for 10 min at room temperature; then the absorbance of samples and standards was read at 630 nm against a blank sample. Enzyme activity was expressed as µmol Pi released per min.
Statistical analysis
Data obtained from each experiment were analysed using Microsoft Excel (Seattle, WA). The significance of the difference between the parameters of different experimental groups and their corresponding controls were assessed using the Mann–Whitney U-Test. The Z score, p-value and U value were calculated and the results were considered significantly different at p < 0.05 by using the free Mann–Whitney U-Test calculator online (http://www.socscistatistics.com). Values in figures and tables represent means with standard deviation (±S.D.).
Results and discussion
Although CTX is a drug widely applied in the treatment of malignant and nonmalignant tumours, the clinical outcomes of treatments with these agents are severely limited, mostly due to its toxicity to normal tissues.[Citation21] The predominant toxicity of CTX includes bone marrow suppression. The resultant granulocytopenia greatly increases the risk of serious infection in patients undergoing cancer treatment. Therefore, it is necessary to develop adjuvant therapy which may be used in combination with CTX to improve the efficacy of the treatment or reduce the associated undesirable side effects.[Citation21,Citation22] The main objective of this study was to evaluate the effect of administration of β-glucan in combination with G-CSF on leucopenia and alteration in biochemical parameters induced in mice by high dose of the anti-cancer drug CTX.
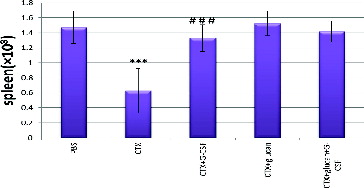
It is well known that hepatocytes and HSCs produce a high amount of ALDH, which makes them relatively resistant to CTX, while CTX is cytotoxic to mature hematopoietic progenitors and lymphocytes.[Citation23] In agreement with this, our results illustrated in showed that CTX administration for 5 days decreased the splenocyte numbers by 57.1% (p < 0.05) when compared to the control PBS group.
CTX treatment significantly decreased the total numbers of WBCs when compared to the untreated group (). This reflected a decrease in lymphocyte, monocyte and neutrophil counts.
Table 1. Effect of different treatments on blood cell counts.
Despite the presence of ALDH in hepatocytes, CTX damages hepatocytes and nephrocytes due to the production of both reactive oxygen and nitrogen species, which triggers cell necrosis and stimulation of the immune system which, in turn, evokes inflammation as a self-defence mechanism.[Citation24–26] Furthermore, several studies indicate that CTX leads to oxidative stress, as it is recognized that the strong depletion of anti-oxidant enzymes is associated with the high production of pro-oxidant molecules.[Citation27–29] In line with these reports, our data shown in demonstrated that CTX increased the level of TBARS and XO activity in hepatocytes, which was associated with a decrease in the GPx activity comparing to that in the control group (p < 0.05).
Table 2. Effect of different treatments on hepatic pro-oxidant/anti-oxidant status.
It is reported that CTX can alter liver and kidney functions by modulating all liver enzymes. The liver and the kidneys are the richest source of both AST and ALT enzymes [Citation30] and, thus, the levels of both these enzymes are expected to increase as a result of damage to the liver and kidney cells.[Citation31] Increased tissue alkaline phosphatase (ALP) is indicative of chemical induced tissue injury along with hepatocellular necrosis.[Citation32] The elevation of ALP observed in our study is consistent with the data reported by Senthilkumar et al. [Citation33]. ALP is now frequently detected in order to estimate the degree of liver dysfunction due to CTX or advanced liver cirrhosis as well as expectation of heart failure development.[Citation34] In support of these reports, our data shown in indicate that CTX treatment resulted in a significant increase in the activities of AST and ALT in sera with no effect on the serum albumin level as compared to the control group (p < 0.05). Moreover, CTX treatment increased the hepatic protein and albumin contents but decreased the specific activities of ATPase and mannosidase (p < 0.05) when compared to normal control levels (). ATPase and monnosidase have been reported to be inhibited in the case of inflammation due to accumulation of nicotinamide adenine dinucleotide phosphate (NADPH). It is well known that CTX is catabolized by ALDH with utilization of NADP and production of NADPH. In immune cells, the NADPH system is also responsible for generating free radicals as part of the respiratory burst against pathogens.[Citation35–37]
Table 3. Effect of different treatments on serum liver function parameters.
Table 4. Effect of different treatments on liver albumin and protein levels, ATPase and mannosidase activity.
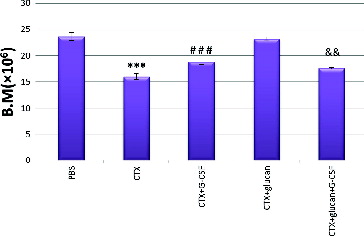
Our results showed that G-CSF alone or combined with β-glucan improved leucopenia and returned it to the normal level. This was most probably due to increasing the anti-oxidant enzymatic activities and prevention of oxidative stress. Furthermore, G-CSF alone or combined with β-glucan can prevent the incidence of inflammation by decreasing the NADPH level and stimulating the activities of both ATPase and mannosidase. shows that the combination of CTX with G-CSF, CTX with β-glucan or CTX with G-CSF plus β-glucan significantly normalized the CTX-induced decrease in the splenocyte counts. Interestingly, all the combined treatments showed similar effects, with no significant difference as compared with CTX treatment alone (p < 0.05). Moreover, CTX treatment reduced the total numbers of bone marrow cells by 32.3% as compared to the control group (). Treatment with CTX and β-glucan plus G-CSF slightly increased the cell number but did not return it to the normal level, whereas the treatment with β-glucan normalized the bone marrow cell counts.
The application of G-CSF returned the total WBC to pre-treatment values (PBS group) at the expense of relatively higher numbers of neutrophils. Co-treatment with β-glucan increased the numbers of WBC, monocytes and neutrophils as compared to PBS and CTX groups. Co-treatment with a combination of G-CSF and β-glucan significantly decreased the numbers of WBC, monocytes and neutrophils as compared to those in the β-glucan plus CTX group ().
Co-administration of G-CSF with CTX decreased XO and increased GPx activities with no effect on the TBARS level as compared to the CTX group. However, treatment with β-glucan alone or with β-glucan plus G-CSF post CTX treatment decreased the pro-oxidant parameters (TBARS and XO) and increased the anti-oxidant ones to their normal level (). Co-administration of G-CSF, β-glucan or a combination of both post CTX significantly decreased AST and ALT activities as compared to those in the group treated with CTX alone or the control group. The same trend was observed in the case of albumin, except for the combination of G-CSF plus β-glucan, which elevated the albumin level (p < 0.05) (). Treatment with G-CSF, β-glucan or their combination post CTX normalized the abnormalities in protein and albumin hepatic content and increased the ATPase and mannosidase activities to a level higher than the normal levels in the PBS group (p < 0.05) ().
It has been shown that β-glucans activate cellular and humoral components of the host immune system and increase the functional activity of macrophages, suggesting that they can serve as immune stimulants.[Citation38,Citation39] It has been reported that β-glucan induces the production of reactive oxygen species (ROS) [Citation40], nitric oxide and TNF-α (Tumor necrosis factor alpha) and other cytokines and its action is similar to the action of lipo-polysaccharides (LPS) [Citation32]. However, β-glucan did not modify the release of NO and cytokines, when macrophages were activated with LPS.[Citation41] In agreement with these reports, our results showed that β-glucan inhibited the lipid peroxidation and it can protect cells against oxidative stress by scavenging hydroxyl and superoxide radicals. β-glucan induced a reduction in the polymorphonuclear cell migration. Therefore, β-glucan has anti-oxidant and anti-inflammatory properties and they, as well as its anti-inflammatory effect, are mediated by the inhibition of both nitric oxide synthase and cyclooxygenase.[Citation42,Citation43] Altogether, it can be suggested that β-glucan acts as an anti-oxidant agent.
Conclusions
The results from this study demonstrated that CTX causes leucopenia and liver necrosis in rats through the stimulation of oxidative stress, leading to inflammation that decreases the stem cell mobilization by reducing ATPase and mannosidase activities. Both G-CSF and β-glucan could be suggested to counteract this action through their anti-oxidant and anti-inflammatory properties. This suggests that the usage of anti-oxidants combined with growth factor during chemotherapy would be helpful in stem cell mobilization. However, further research is needed to understand the exact mechanism of action of glucans in stem cell mobilization.
Acknowledgements
The authors would like to thank Ms Esraa Eid (Biochemistry Department, Faculty of Science, Alexandria University) for her effort in editing parts of this paper and in the experimental work.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Brode S, Cooke A. Immune-potentiating effects of the chemotherapeutic drug cyclophosphamide. Crit Rev Immunol. 2008;28(2):109–126.
- Colvin OM. An overview of cyclophosphamide development and clinical applications. Curr Pharm Des. 1999;5(8):555–560.
- Salem ML, Diaz-Montero CM, Al-Khami AA, et al. Recovery from cyclophosphamide-induced lymphopenia results in expansion of immature dendritic cells which can mediate enhanced prime–boost vaccination antitumor responses in vivo when stimulated with the TLR3 agonist poly(I:C). J Immunol. 2009;182:2030–2040.
- Salem ML, Al-Khami AA, El-Naggar SA, et al. Cyclophosphamide induces dynamic alterations in the host microenvironments resulting in a Flt3 ligand-dependent expansion of dendritic cells. J Immunol. 2010;184 1737–1747.
- Salem ML, Al-Khami AA, El-Nagaar SA, et al. Kinetics of rebounding of lymphoid and myeloid cells in mouse peripheral blood, spleen and bone marrow after treatment with cyclophosphamide. Cell Immunol. 2012;276(1–2):67–74.
- Zhu L, Yin Y, Xing J, et al. Therapeutic efficacy of Bifidobacterium longum-mediated human granulocyte colony-stimulating factor and/or endostatin combined with cyclophosphamide in mouse-transplanted tumors. Cancer Sci. 2009;100(10):1986–1990.
- Kasymjanova G, Kreisman H, Dajczman E, et al. Utilization of granulocyte colony-stimulating factor in non-small cell lung cancer patients receiving carboplatin-based chemotherapy. J Support Oncol. 2004;2:56S–57S.
- Rubinstein MP, Salem ML, Doedens AL, et al. G-CSF/anti-G-CSF antibody complexes drive the potent recovery and expansion of CD11b+Gr-1+ myeloid cells without compromising CD8+ T cell immune responses. J Hematol Oncol. 2013;6:75–85.
- Cesana CC, Carlo-Stella, Regazzi E, et al. CD34+ cells mobilized by cyclophosphamide and granulocyte colony-stimulating factor (G-CSF) are functionally different from CD34+ cells mobilized by G-CSF. Bone Marrow Transplant. 1998;21(6):561–568.
- Tari KI, Satake, Nakagomi K, et al. Effect of lentinan for advanced prostate carcinoma. Hinyokika Kiyo. 1994;40(2):119–123.
- Patchen ML, MacVittie TJ, Solberg BD, et al. Survival enhancement and hemopoietic regeneration following radiation exposure: therapeutic approach using glucan and granulocyte colony-stimulating factor. Exp Hematol. 1990;18(9):1042–1048.
- Patchen ML, Vaudrain T, Correira H, et al. In vitro and in vivo hematopoietic activities of betafectin PGG-glucan. Exp Hematol. 1998;26(13):1247–1254.
- Lin H, She YH, Cassileth BR, et al. Maitake beta-glucan MD-fraction enhances bone marrow colony formation and reduces doxorubicin toxicity in vitro. Int Immunopharmacol. 2004;4(1):91–99.
- Lin H, Cheung SW, Nesin M, et al. Enhancement of umbilical cord blood cell hematopoiesis by maitake beta-glucan is mediated by granulocyte colony-stimulating factor production. Clin Vaccine Immunol. 2007;14(1):21–27.
- Lin H, De Stanchina E, Zhou XK, et al. Maitake beta-glucan enhances umbilical cord blood stem cell transplantation in the NOD/SCID mouse. Exp Biol Med (Maywood). 2009;234(3):342–353.
- Lin H, de Stanchina E, Zhou XK, et al. Maitake beta-glucan promotes recovery of leukocytes and myeloid cell function in peripheral blood from paclitaxel hematotoxicity. Cancer Immunol Immunother. 2010;59(6):885–897.
- Cramer D, Wagner ES, Li B, et al. Mobilization of hematopoietic progenitor cells by yeast-derived beta-glucan requires activation of matrix metalloproteinase-9. Stem Cells. 2008;26(5):1231–1240.
- Tappel L, Zalkin H. Inhibition of lipid peroxidation in mitochondria by vitamin E. Arch Biochem Biophys. 1959;80:333–336.
- Litwack G, Bothwell JW, Williams JN, et al. A colorimetric assay for xanthine oxidase in rat liver homogenates. J Biol Chem. 1953;200:303–310.
- Paglia E, Valentine N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169.
- Lin G, Yu X, Wang J, et al. Beneficial effects of 20(S)-protopanaxadiol on antitumor activity and toxicity of cyclophosphamide in tumor-bearing mice. Exp Ther Med. 2013;5(2):443–447.
- Robak T, Lech-Maranda E, Robak P. Rituximab plus fludarabine and cyclophosphamide or other agents in chronic lymphocytic leukemia. Expert Rev Anticancer Ther. 2010;10:1529–1543.
- Emadi A, Jones RJ, Brodsky RA. Cyclophosphamide and cancer: golden anniversary. Nature Rev Clin Oncol. 2009;6:638–647.
- De Leve LD. Cellular target of cyclophosphamide toxicity in the murine liver: Role of glutathione and site of metabolic activation. Hepatology. 1996;24:830–837.
- Abraham P, Rabi S. Nitrosative stress protein tyrosine nitration PARP activation and NAD depletion in the kidneys of rats after single dose of cyclophosphamide. Clin Exp Nephrol. 2009;13:281–87.
- Ayhanci A, Nes Gu, Sahinturk S, et al. Seleno L-methionine acts on cyclophosphamide-induced kidney toxicity. Biol Trace Elem Res. 2009;136(Suppl. 2):171–179.
- Mathew S, Kuttan G. Antioxidant activity of Tinospora cordifolia and its usefulness in the amelioration of cyclophosphamide induced toxicity. J Exp Clin Cancer Res. 1997;16:407–411.
- Kaya H, Oral B, Ozguner F, et al. The effect of melatonin application on lipid peroxidation during cyclophosphamide therapy in female rats. Zentralbl Gynakol. 1999;121:499–502.
- Premkumar K, Pachiappan A, Abraham SK, et al. Effect of Spirulina fusiformis on cyclophosphamide and mitomycin-C induced genotoxicity and oxidative stress in mice. Fitoterapia. 2001;72:906–911.
- Soni S, Shrivastava V. Carbendazim induced histopathological changes and some enzyme activities (GOT, GPT, ACP and ALP) in liver and kidneys of male Rattus rattus. Int J Pharm Sci Health Care. 2013;5(3):19–35.
- Davila JC, Lenherr A, Acosta D. Protective effect of flavonoid on drug-induced hepatotoxicity in vitro. Toxicology. 1989;57(3):267–286
- Cole GW, Bradley. Hospital admission laboratory profile interpretation. The SGOT and SLDH-SGOT ratio used for the diagnosis of hepatic disease. Hum Pathol. 1973;4:85–88.
- Subramanian V, Shenoy S, Joseph AJ. Dengue hemorrhagic fever and fulminant hepatic failure. Diag Dis Sci. 2005;50(6):1146–1147.
- Senthilkumar S, Ebenezar KK Sathish V, et al. Modulation of the tissue defense system by squalene in cyclophosphamide induced toxicity in rats. Arch Med Sci. 2006;2:94–100.
- Shrivastav V. Cyclophosphamide induced changes in certain enzymological (GOT GPT ACP and ALP) parameters of adult male Rattus norvegicus. Int J Res Rev Pharmacy App Sci. 2013;3(1):155–163.
- Eisenhut M, Sidaras D, Barton P, et al. Elevated sweat sodium associated with pulmonary oedema in meningococcal sepsis. Eur J Clin Investig. 2004;34:576–579.
- Eisenhut M, Wallace H, Barton P, et al. Pulmonary edema in meningococcal septicemia associated with reduced epithelial chloride transport. Pediatr Crit Care Med. 2006;7:119–124.
- Eisenhut M. Changes in ion transport in inflammatory disease. J Inflam. 2006;3:5–20.
- Chang ZQ, Lee JS., Hwang MH, et al. A novel _-glucan produced by Paenibacillus polymyxa JB115 induces nitric oxide production in RAW264.7 macrophages. J Vet Sci. 2009;10:165–167.
- Liu C, Lin Q, Gao Y, et al. Characterization and antitumor activity of a polysaccharide from Strongylocentrotus nudus eggs. Carbohydr Polym. 2007;67:313–318.
- Pires A, Ruthes A, Cadena S, et al. Cytotoxic effect of Agaricus bisporus and Lactarius rufus β-D-glucans on HepG2 cells. Int J Biol Macromol. 2013;58:95–103.
- Carbonero E, Ruthes A, Freitas C, et al. Chemical and biological properties of a highly branched B-glucan from edible mushroom Pleurotus sajor-caju. Carbohydr Polym. 2012;90:814–819.
- Celina M, Dore C, Tarciana C, et al. Anti-inflammatory, antioxidant and cytotoxic actions of β-glucan-rich extract from Geastrum saccatum mushroom. Int Immunopharmacol. 2007;7:1160–1169.