Abstract
Salinity is one of the major constraints to world agriculture production and the recent phenomenon of global climatic change has further exacerbated the problem. To cope with excess salinity, manipulation of transgenic technology in crop plants has been proven effective in recent years. Among all available strategies, the membrane and vacuolar Na+/H+ antiporters provide the best mechanism for ionic homeostasis in plants under salt stress. In recent years, a large number of transgenic plants with variable salt tolerance have been produced on the basis of antiporter genes. Most of these experiments were conducted under laboratory conditions and at the early plant developmental stages. Only a few studies conducted under field conditions revealed the true potential of antiporter genes in salt tolerance and effects on the overall plant growth and yield. More field trials are required to investigate the already claimed salt tolerance and whether this salt tolerance has any incremental advantage in terms of the ultimate yield potential. In addition, alternative strategies are needed to improve the performance of antiporter genes and the underlying processes by studying the halophyte salt tolerance mechanisms. This review focuses on the present status and progress in the development of salt-tolerant transgenic plants with Na+/H+ antiporter genes. More importantly, the potential future target areas for enhancing salt tolerance with the antiporter genes are discussed.
Introduction
Among abiotic stresses, salt stress is the most threatening one that adversely affects plant growth, development and overall productivity.[Citation1] Approximately 20% of the irrigated agricultural land is under salt stress and the agricultural practices are further hampered due to the use of poor quality irrigation water.[Citation2,Citation3] In response to salt stress, plants undergo molecular changes that result in production of stress responsive metabolites, transcription factors and membrane proteins.[Citation2,Citation4,Citation5] Specifically in the case of salt stress, the important and vital strategy adopted by plants is to orchestrate the movement of Na+ and K+ across the cellular and vacuolar membranes resulting ionic and osmotic homeostasis.[Citation6,Citation7] Plants are classified as glycophytes and halophytes based on the potential of Na+ mitigation strategies coupled with Na+ restriction from entry into the cytoplasm and its compartmentalization into the vacuoles.[Citation8–10] Plants have developed a specialized network of cation channels across the cellular and vacuolar membranes to regulate the movement of Na+ and K+ and their balanced availability for cellular functions.[Citation8,Citation11]
In the recent past, extensive research activities have been conducted on the physiological, biochemical and molecular basis of salt stress. The main emphasis was on the study of Na+/H+ antiporters, their isolation from target plants and dissection of their vital role in salt stress tolerance. In this respect, Arabidopsis as a model plant provided the pioneering breakthroughs. Several membrane genes encoding membrane proteins such as salt overly sensitive 1 (SOS1), vacuolar NHX proteins and HKT1s, members of the NHX family were isolated from Arabidopsis and studied in detail.[Citation11–13] Some other plants such as cotton, wheat, rice, Atriplex gmelini and Mesembryanthemum crystallinum were also used for antiporters isolation.[Citation14–16]
Following their isolation from target plants, these various antiporter genes have been introduced into important crop plants. An increasing number of transgenic plants with antiporter genes, developed in various laboratories, resulted in enhanced salt stress tolerance and growth improvement.[Citation17–19] These numbers will continue to increase in the future; however, the challenge is whether we have sufficiently achieved the goal of increasing the salt tolerance of our existing crop plants. And can we manipulate this genetically introduced salt tolerance in the existing breeding programmes for developing high salt-tolerant varieties?
Despite much progress and loud claims, the reality is that we are still lagging behind and further efforts are required to achieve the level of salt tolerance that may have sufficient positive effects on the overall yield under stress in realistic field conditions. So far, there have been limited numbers of field trials for transgenic plants engineered with antiporter genes. Most of these transgenic plants have been evaluated under controlled conditions, which may not accurately predict the true performance of antiporter genes and their effect on salt tolerance and overall plant growth and yield. A three-pronged approach must be emphasized in the future to target salt stress tolerance in transgenic plants with antiporter genes. Improving the efficiency of antiporter genes, combination of multiple strategies and improving the evaluation systems could overcome some of the obstacles in achieving enhanced salt tolerance and plant productivity under marginal lands. The present review is a continuation of our previous work [Citation20] to highlight the current status of research on abiotic stress tolerance, including salt tolerance in plants, and some future prospects and challenges.
Sodium influx, efflux and vacuolar compartmentalization
Since the discovery of the SOS1 pathway in Arabidopsis,[Citation16] considerable progress has been made towards understanding plant responses to excessive Na+ concentration. The important genes working in the SOS pathway include SOS1, encoding a plasma membrane Na+/H+ antiporter,[Citation16] SOS2, a serine/threonine protein kinase,[Citation21] SOS3, a Ca2+ sensor,[Citation22] HKT1, a membrane protein that controls Na+ uptake into the cell and NHX1, a Na+/H+ antiporter on the vacuolar membrane for Na+ accumulation in the vacuole.[Citation6,Citation7,Citation12] Initially, the SOS1 gene was studied extensively for its role in Na+ outward movement and salinity tolerance in Arabidopsis. The plasma membrane Na+/H+ antiporter encoded by the SOS1 gene has 12 trans-membrane domains in the N-terminal half and a long hydrophilic C-terminal tail.[Citation16] Several sos mutants have been generated and categorized into five groups or five loci, i.e. SOS1, SOS2, SOS3, SOS4 and SOS5.[Citation23–26]
For normal physiological functions, cells maintain a high K+/Na+ ratio in the cytosol.[Citation27] In case of excess Na+ accumulation in the extracellular spaces, it generates a high electrical membrane potential difference that results in passive movement of Na+ into the cytosol. There are a number of transporters and channels to facilitate this passive movement of Na+ across the plasma membrane. Some important transporters are the high affinity K+ transporter HKT1, LCT1, and other non-selective cation channels such as cyclic nucleotide-gated channels (CNGCs) and glutamate-activated channels (GLRs).[Citation6,Citation28] These transporters mainly function in the uptake of Na+ into root cells; however, dependent on the growth conditions and the type of plant species. An important member of this group is the HKT1 transporter, which functions as a symporter for both Na+ and K+.[Citation29] The structural and functional attributes of the HKT1 transporters have been extensively studied in Arabidopsis and rice.[Citation30,Citation31] Recently, the tissue specificity activity of HKT1 has been studied in Arabidopsis that showed a correlation between Na+ movement from root to shoot and plant salt tolerance.[Citation32] The transgenic Arabidopsis showed lower shoot Na+ content due to the increased Na+ influx into the root stellar cells, resulting in a decline in Na+ flow from root to shoot and increased salt tolerance. When HKT1 was expressed under the constitutive promoter, transgenic plants accumulated a very high Na+ content on the whole-plant level both in the roots and the shoots. As a result, transgenic plants were found with stunted shoots and overall poor plant growth. In addition to the HKT1, some other low affinity cation transporters were studied such as the low affinity Na+ transporter LCT1 that was cloned from wheat.[Citation33] LCT1 mediates the uptake of other ions such as Ca2+, Na+, Li+, Rb+ and Cs+.[Citation34]
Research on non-selective cation channels has marked a progress in the recent past. These cation channels have been categorized into two families of non-selective cation channels, i.e. CNGCs and GLRs.[Citation6,Citation35,Citation36] In Arabidopsis, at least five members of these genes (CNGC1, 2, 3, 4 and 10) have been characterized.[Citation28] AtCNGCs 1, 3 and 4 have specificity to both Na+ and K+,[Citation35,Citation37,Citation38] whereas CNGC2 and CNGC10 have permeability to K+ only.[Citation39]
Excess Na+ in the cell is actively transported out of the cytoplasm due to the establishment of an electrochemical gradient across the cell membrane.[Citation8] In animals and microorganisms, adenosine triphosphate (ATP) hydrolysis facilitates this active transport, mediated by specific Na+-ATPases, present in the plasma membrane. In plants, however, no such types of Na+ pumps have been found.[Citation40] In plants, Na+/H+ antiporters are the only channels through which Na+ is transported out of the cell. The plant Na+/H+ antiporters have been classified into three families; CPA1, NhaD and CPA2.[Citation41] Two out of eight members of the CPA1 family, i.e. AtNHX7/SOS1 and AtNHX8 are localized on the plasma membrane,[Citation11,Citation42] while the other six members, i.e. AtNHX1–6, are vacuolar/endosomal antiporters.[Citation13] The AtNHX7/SOS1 and SOS2 and SOS3 genes were studied in Arabidopsis in relation to root growth under saline conditions.[Citation23,Citation43] Induced mutations in the Arabidopsis SOS1 gene resulted in loss of the Na+/H+ exchange activity across the plasma membrane.[Citation43] Later on, the transformation of various plant species with SOS1 genes revealed their specific role in salt tolerance of these transgenic plants. Transgenic Arabidopsis lines have been produced with SOS1, SOS2, SOS3 and AtNHX1, either individually or in combination. Transgenic plants with either SOS1 or SOS3 showed high salt tolerance, while transgenic lines with only AtNHX1 expression did not show any such improvement.
In addition to the above-mentioned strategies, the vacuolar compartmentalization of Na+ is another very important mechanism to cope with excess Na+ concentration in the cells.[Citation44] This mechanism is mediated by the Na+/H+ antiporters, localized on vacuolar membranes, which are driven by the electrochemical gradient of protons generated by the vacuolar H+-translocating enzymes such as H+-ATPase and H+-PPase.[Citation28] A number of these vacuolar antiporter genes have been cloned from Arabidopsis and some other plant species.[Citation13,Citation17,Citation45,Citation46] Following the isolation and functional characterization, these vacuolar antiporters were transformed in a number of plant species to increase their salt stress tolerance.
Transgenic approaches with Na+/H+ antiporters
Until now, a large number of transgenic plants have been produced which express the Na+/H+ antiporter genes, particularly the vacuolar antiporters.[Citation47] These transgenic plants show variable salt stress tolerance as claimed by various laboratories. However, most of these transgenic plants were tested under controlled laboratory conditions and early plant developmental stages. For example, the plasma membrane Na+/H+ antiporter encoded by the AtSOS1 gene under the CaMV35S constitutive promoter was expressed in Arabidopsis.[Citation11] Transgenic Arabidopsis plants showed enhanced salt tolerance mainly due to lower Na+ accumulation in root, xylem stream and shoot cells. Moreover, improved germination rate, chlorophyll content and protein level were reported in transgenic lines under salt stress.
For example, transgenic Arabidopsis with the wheat vacuolar Na+/H+ antiporter (TaNHX1) showed improved growth under 200 mmol/L NaCl as compared to wild control plants.[Citation48] Li et al. [Citation49] reported expression of the SsNHX1 gene from Suaeda salsa, in Arabidopsis. Transgenic plants showed salt tolerance under 200 mmol/L NaCl. In another study, transgenic Arabidopsis was produced with MsNHX1 from alfalfa and the transgenic plants showed improved germination and seedling growth under salt stress.[Citation50] Several transgenic plants were produced with the Arabidopsis vacuolar membrane Na+/H+ antiporters. These transgenic plants showed enhanced salt tolerance. Transgenic Brassica that overexpressed the AtNHX1 gene showed high salt stress tolerance and produced flowers and seeds in the presence of 200 mmol/L NaCl.[Citation51] Despite high sodium accumulation (6% of their dry weight) in transgenic plants, growth was only marginally affected at high salt stress. In addition, seed yield and oil quality traits were not affected in transgenic plants at high salt stress.
Transgenic sugar beet plants were produced with AtNHX3.[Citation52] The transgenic plants showed high salt tolerance with high accumulation of K+ in roots under 300–500 mmol/L NaCl. In addition to salt tolerance, the sugar beet plants also accumulated high sugar content in storage roots.
Wu et al. [Citation53] reported expression of the cotton GhNHX1 in tobacco plants. The transgenic tobacco plants revealed salt tolerance and showed about 100% increase in plant dry weight. In addition to the above study, transgenic tobacco plants transformed with the BnNHX1 gene showed improved plant growth and better seed production under 200 mmol/L NaCl stress.[Citation54] In other studies, transgenic tobacco transformed with HbNHX1, SynnhaP1 and AtNHX1 also showed salt stress tolerance.[Citation55–57] More importantly, Zhang et al. [Citation58] reported the expression of vacuolar Na+/H+ antiporter (AlNHX1) from Aeluropus littoralis in transgenic tobacco. Transgenic plants showed about 2.5-fold increase in plant dry weight on average under 400 mmol/L salt stress.
Rice is another important crop plant under extensive research for increasing its slat stress tolerance. In one study, transgenic rice plants with AgNHX1 showed salt tolerance and exhibited improved survival of seedlings under salt stress.[Citation59] In subsequent studies, transgenic rice plants with OsNHX1 and PgNHX1 showed higher shoot and root growth under salt stress.[Citation60–62] Moreover, the tissue-specific expression of antiporter gene was investigated in rice plants. Wu et al. [Citation63] reported expression of the TaNHX2 gene in rice under the root-specific promoter of the PR10 gene from Pinus griffithii. Transgenic plants were found with enhanced salt tolerance, which was attributed to the improved activities of vacuolar ATPase and H+-PPase only in roots cells. Such studies may be helpful in unraveling the salt tolerance mechanisms at the root level.
Wheat and maize are important cereal plants and are potential candidates for salt tolerance improvement. Transgenic wheat and maize plants with the Arabidopsis AtNHX1 gene showed salt stress tolerance.[Citation64,Citation65] Improved shoot and root dry weight were reported in the transgenic wheat; while the transgenic maize exhibited improved germination under salt stress. In another study, transgenic maize plants were produced with the OsNHX1 gene from rice.[Citation66] Transgenic rice plants were found to have high biomass under 200 mmol/L NaCl concentration.
In another study, transgenic tomato plants with improved salt stress tolerance were produced with the HAL1 gene, which encodes a K+ regulator from Saccharomyces cerevisiae.[Citation67] In addition to the vacuolar antiporter genes, the proton pumps localized in the cell and vacuolar membranes, such as the plasma membrane H+-ATPase, the vacuolar-type H+-ATPase and the vacuolar H-pumping pyrophosphatase, are important contributors to salt stress adaptation in plant species. These proton pumps play a crucial role in proton gradient generation and ion movement across the membranes.[Citation68] Transgenic Arabidopsis that expressed the vacuolar H+-PPase pump encoded by the AVP1 gene showed salt and drought tolerance.[Citation69] The authors reported better plant growth in transgenic plants than the non-transgenic control plants under limited water conditions. Similarly, transgenic tomato plants with the AVP1 gene showed improvement in root biomass under drought condition.[Citation70]
He et al. [Citation71] reported transformation of cotton with the AtNHX1 gene. The transgenic plants showed enhanced salt tolerance as compared to control plants under greenhouse and field conditions. More importantly, the transgenic plants demonstrated improved root growth, plant height and photosynthetic rate, which resulted in a visible increase (25%) in boll numbers and fibre yield in transgenic lines as compared to control lines. In some recent studies, transgenic Arabidopsis and cotton were transformed with the vacuolar AVP1 gene and the resultant plants showed salt and drought tolerance.[Citation48,Citation72] These studies revealed that the AVP1 gene has enormous potential to increase the tolerance of plants under cultivation in marginal lands affected with both salt and drought stresses. Furthermore, the expression of other antiporter genes has been shown to confer abiotic stress tolerance in transgenic Arabidopsis,[Citation73,Citation74] Brassica,[Citation75] jatropha,[Citation76] alfalfa,[Citation77] tobacco,[Citation78] petunia [Citation79] and tall fescue.[Citation80] summarizes some of the prominent studies on transgenic plants with antiporter genes.
Table 1. List of abiotic stress-tolerant transgenic plants with ion transporters.
In addition to the cellular and vacuolar membrane antiporters discussed above, the Na+ transporter HKT1 is another very important candidate gene for plant transformation to target salt stress tolerance. The importance of the HKT1 gene is evident from its role in reducing the excessive movement and accumulation of Na+ in shoot cells. Increased accumulation of Na+ in shoot cells exerts negative effects on plant growth and survival. Attempts to restrict excessive Na+ concentration at the root level and reduce its transport to the shoots have been based on cell-type-specific expression of the HKT1 gene. For example, Arabidopsis was transformed with cell-type-specific expression of HKT1 in mature root stele cells.[Citation32] The transgenic plants showed high Na+ accumulation in the root stele cells, which led to significant reduction in root-to-shoot Na+ transport. However, when the HKT1 gene was expressed under the constitutive CaMV35S promoter, high Na+ content was reported in shoots, which resulted in poor plant growth. This study revealed that the cell-type-specific gene expression of HKT1 is one of the important strategies that can be used to cope with excessive Na+ accumulation in shoots and subsequent growth and yield loss.
Future research areas
So far, the use of antiporter genes in transgenic development has been proven effective in conferring significant salt stress tolerance. An increasing number of plants have been produced and tested for their performance under salt stress conditions. Although most of these studies were conducted under controlled laboratory conditions and on early plant developmental stages, some transgenic plants showed not only enhanced salt tolerance, but also improved plant growth and yield under field conditions. These few examples suggest that the future of transgenic plants with antiporter genes is bright. However, despite these positive developments, several issues still need proper attention in order to fully exploit the technology for improvement of plant salt tolerance. Some of the important and key issues are discussed in the following section.
Genetic engineering of crop plants with antiporter genes aims to improve the plants' ability to survive and ultimately reduce the losses in yield under realistic saline conditions. Most of the transgenic plants developed so far have been tested under controlled laboratory conditions. These transgenic plants were evaluated on the early plant developmental stages. Such data may not predict the actual performance of antiporter genes, as the plant response to salt stress may considerably vary under natural field conditions and during various plant developmental stages from seedling growth to reproductive stage.
In a few examples, however, transgenic plants developed with antiporter genes not only conferred salt tolerance but also exerted positive effects on yields under greenhouse and field conditions. One of the prominent examples of such transgenic plants is the transgenic cotton transformed with the vacuolar AVP1 gene. Transgenic cotton plants showed enhanced salt tolerance and yield improvement under greenhouse and field conditions.[Citation71,Citation72] Similarly, transgenic wheat and barley were developed with several genes, including the AtAVP1 gene, to improve the salt tolerance of these plants. The transgenic wheat and barley plants have been under field trials for agronomic performance and environmental risk assessment studies in Australia.[Citation81] Moreover several transgenic events with the antiporter genes are under field trials for salt tolerance and risk assessment studies, conducted by the Arcadia Biosciences in the USA.[Citation82]
Despite these few examples, a large number of transgenic plants developed with the antiporter genes are yet to be tested under field conditions for salt tolerance and yield improvement. There is a possibility that these transgenic plants may not show appreciable salt tolerance and plant growth and yield improvement under natural filed conditions, as the controlled conditions are largely different from those in the natural field. In natural conditions, plants may interact with multiple environmental factors including biotic, abiotic and soil conditions and a combination of these factors. In natural field conditions, plants may experience multiple stresses other than the target stress and this may increase the severity of the overall stress on the plants. Multiple stresses may include extreme of temperatures, drought stress, light intensity, ultraviolet radiation, anoxia and soil nutrient imbalances. In addition, the severity of salt stress may increase in magnitude in the presence of other salts, such as CaCl2, CaSO4 and Na2SO4, in natural field conditions.[Citation19] Apart from that, evaluation of salinity tolerance in the early growth stages may not exactly predict the plant response to stress conditions in other advanced plant growth stages. Most of the experiments conducted so far have been based on salt tolerance evaluation at the early stages of plant growth and development. It is unclear whether the transgenic plants will reveal the same tolerance during vegetative and reproductive stages on the whole-plant level. These questions are still to be answered, since the number of transgenic plants under field trials is limited so far. With the increasing number of field trials in the near future, plant scientists will be able to fully explain the true potential of antiporter genes in salt tolerance of important crop plants. In addition to the efforts to develop transgenic plants with antiporter genes and evaluate their field performance, further research strategies are needed to improve the potential of antiporter genes in transgenic glycophyte crop plants. Some of these strategies are discussed below.
Na+/H+ exchange activity of antiporters
Further efforts are, in addition, required to improve the activity of Na+/H+ antiporters and stress tolerance in transgenic plants (). The relatively low exchange activity of vacuolar Na+/H+ antiporters isolated from glycophyte monocot and dicot species limits their use in future breeding programmes aimed at development of high salt-tolerant crop varieties. The potential of these antiporter genes depends on the Na+/H+ exchange activity, which should be improved through modern biotechnology tools. DNA-shuffling technology is a recently developed tool that may be used to improve the activity of antiporter genes. By using this technology, new versions of the genes with improved activities are produced through mutations and recombinations. In one study, the Arabidopsis NHX1 gene was mutated and a new version, designated as AtNHXS1, was produced with relatively high Na+/H+ exchange activity.[Citation83] The AtNHXS1 gene was introduced in yeast cells and the resultant transgenic cells exhibited improved antiporter activity and salt stress tolerance. Therefore, modern tools such as DNA shuffling, mutation and chimeric strategies should be used to enhance the effectiveness and Na+/H+ exchange activity of other antiporter genes of glycophyte origin.
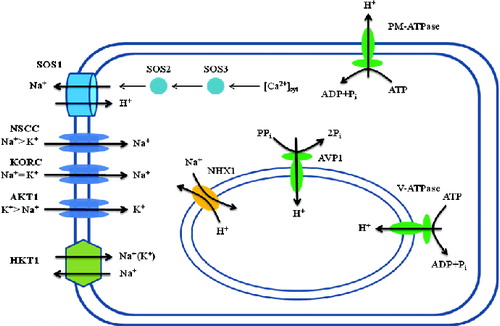
Figure 1. Schematic representation of Na+ transport across the cellular and vacuolar membranes. Na+/K+ homeostasis is established by various specific and non-specific cation channels. High Na+ content enters the cytoplasm through the non-selective cation channels (NSCC), the outward-rectifying K+ channels (KORC) and the high Na+ affinity HKT1 antiporter. Proton gradient across the plasma membrane is established by plasma membrane ATPase (PM-ATPase). Excess Na+ is taken inside the vacuole through vacuolar membrane NHX1. The proton gradient across the vacuolar membrane is established by H+-pyrophosphatase (AVP1) and the vacuolar H+-ATPase (V-ATPase).
Exploring regulatory mechanisms
So far, research on the regulation of these antiporter genes is limited. There is not enough literature available regarding how the activities of the antiporter genes are regulated at the transcriptional and post-transcriptional levels. In comparison to the glycophyte antiporters, the halophyte antiporter genes are tightly regulated in an orchestrated manner. Due to this tight control and regulation of the cell-type-specific and stress-dependent expression of halophyte antiporter genes, halophytes may tolerate very high salt concentrations. The halophyte antiporter regulation mechanisms should be studied in more detail and these studies should be extended towards glycophyte antiporters and transgenic development.
Gene-stacking approach
As salt stress adaptation is a highly complex process involving multiple stress response mechanisms, a multigenic approach should be adopted. The important stress response mechanisms are driven by genes such as those for transcription factors, and genes working in biosynthesis of important osmoprotectants such as glycine betaine.[Citation84,Citation85] In this regard, the DREB genes and the glycine betaine biosynthetic genes, such as the codA gene, should be stacked with Na+/H+ antiporter genes, specifically the halophyte vacuolar NHX1, AVP1 and the HKT1 genes, with simultaneously expressed in transgenic plants.
In addition to the above, other alternatives should also be utilized such as increasing the photosynthetic rate/efficiency in the plants under stress conditions. In this regard, the carbon-concentrating mechanisms (CCM) and HCO3− transporters of cyanobacteria and microalgae should be used in combination with Na+/H+ antiporter genes. Co-expression of the Na+/H+ antiporter genes with either CCM or HCO3− transporters may confer enhanced salt stress tolerance and yield increase by (1) protection of the photosynthetic machinery during salt stress and (2) increase of carbon assimilation under limited CO2 supply. Considering all of the factors described above, future research should be targeted towards genetic engineering of economically important crop plants for enhanced and durable tolerance to salt stress.
Antiporters role in developmental processes
Apart from their established role in Na+ sequestration in the vacuole, the vacuolar Na+/H+ antiporters have recently emerged as key players in plant development, cell growth and osmoregulation.[Citation86] Several recent research findings have established the important role of endosomal NHX antiporters in vesicular trafficking, protein processing, endosomal pH regulation and cellular cargo delivery.[Citation87,Citation88] However, it is still to be investigated (1) what the exact role of endosomal NHX antiporters on the regulation of the above activities is, (2) whether these activities are mediated by regulating the endosomal pH or by maintaining endosomal ion homeostasis and (3) how the functions of NHX antiporters are regulated at the transcriptional and post-transcriptional levels. In this regard, more knockout mutants for both vacuolar and endosomal NHX antiporters are required to dissect their exact role in salt stress tolerance and developmental processes.
Research on halophytes
So far, most of the genetic engineering studies on transgenic plants have focused on antiporter genes isolated from glycophyte species such as Arabidopsis, rice and others. However, research on the halophyte Na+/H+ antiporters is limited and restricted to only some examples where the halophyte antiporters have been used in genetic transformation of important crop plants. Halophytes are known to have highly developed physiological and molecular mechanisms to cope with excess salinity. It has been documented in several studies that the high salt tolerance of halophyte species may partly be contributed to the high efficiency of their vacuolar antiporters as compared to those of glycophyte species. Therefore, future research should be focused on isolation of vacuolar antiporters from potential halophyte species. In addition, the halophyte antiporters should be used in a cell-type-specific manner in glycophytes genetic engineering so that effective and stress based expression and salt tolerance could be achieved. Similarly, other factors such as monocot and dicot origin of the halophyte antiporters may also be taken into account. Antiporters from monocot halophytes may be highly effective in monocot glycophytes and vice versa. The relationship between the origin of the antiporter gene, its expression in the transgenic plant and the resultant stress tolerance has been partly studied. For instance, Liu et al. [Citation74] transformed Arabidopsis with various vacuolar antiporter genes isolated from diverse plant species such as Arabidopsis, cotton, wheat, yeast and the halophyte species Suaeda salsa. The magnitude of salt tolerance in the transgenic Arabidopsis lines varied depending on the origin of the antiporter gene, i.e. GhNHX1 > ScNHX1 > AtNHX1 > SsNHX1 > TaNHX1. In this study, the transgenic Arabidopsis lines transformed with the monocot wheat NHX1 gene did not show any salt tolerance. However, this may not be the case for antiporter genes isolated from halophytes. These may be equally effective in all glycophyte species irrespective of their origin. This is evident from one example where the vacuolar NHX gene from the monocot halophyte Aeluropus littoralis showed high salt tolerance in the dicot tobacco plants.[Citation58] In another study, expression of the SbNHX1 gene from halophyte Salicornia brachiata in tobacco plants conferred enhanced salt tolerance.[Citation76] Previously, the transcript level of SbNHX1 was shown to be up-regulated at very high salt concentration, i.e. 500 mmol/L NaCl. These studies indicate that the antiporter genes from the halophyte species can be considered the best candidates for salt tolerance improvement in important glycophyte species such as maize, rice and wheat, members of family Gramineae. Research on isolation of new vacuolar antiporter genes from diverse halophyte species should be progressed. A number of potential halophyte species, their monocot and dicot origin, uses and worldwide distribution are documented ().
Table 2. List of potential halophytes to be used for salt tolerance improvement in crop plants.
Focus on halophytes specialized mechanisms
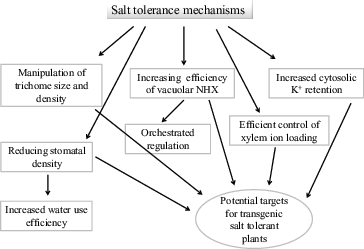
In addition to the use of antiporter genes from halophytes in transgenic crop plants, emphasis should be placed on the various components of halophytes involved in adaptation to high salt concentrations. There are several indicators or mechanisms through which salt tolerance is achieved in both glycophytes and halophytes. However, the mechanisms and related structures are highly efficient in halophytes compared to those of glycophytes (). For example, Shabala [Citation89] pointed out that the salt tolerance mechanisms and their physiological basis in halophytes should be studied in detail and should be utilized in the breeding programmes for production of high salt-tolerant transgenic crop plants. Over the course of their evolution, halophytes have developed several mechanisms which are highly efficient to cope with excessive salt concentrations. These mechanisms include trichome size and density, highly efficient vacuolar antiporters through orchestrated regulation, increased cytosolic K+ retention, efficient control of xylem ion loading, and reduced stomatal density to promote increased water use efficiency. The molecular determinants governing these mechanisms and structures have been partially revealed but some details are still to be worked out.
Conclusions
In recent years, the focus on plant genetic engineering for salt stress tolerance has been on expression of antiporter genes. However, many of the studies have resulted in transgenic plants with stress tolerance at early plant developmental stages and experiments were mainly conducted under controlled conditions. The time is ripe to combine multiple strategies in order to increase stress tolerance at various plant developmental stages and convert the stress tolerance into meaningful plant growth improvement and yield increment under realistic field conditions. The study of unraveling the diverse salt stress mechanisms in halophytes should be coupled with search for superior alleles of antiporters in these species. The incorporation of these superior alleles and other determinants of halophyte salt stress mechanisms would possibly improve the present status of salt stress tolerance and yield in crop plants.
Disclosure statement
All authors declare that they have no conflict of interest.
Additional information
Funding
References
- Munns R. Genes and salt tolerance: bringing them together. New Phytol. 2005;167:645–663.
- Chinnusamy V, Jagendorf A, Zhu JK. Understanding and improving salt tolerance in plants. Crop Sci. 2005;45:437–448.
- Flowers TJ, Yeo AR. Breeding for salinity resistance in crop plants-where next? Aus J Plant Physiol. 1995;22:875–884.
- Vinocur B, Altman A. Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Biotechnol. 2005;16:123–132.
- Wang WX, Vinocur B, Shoseyov O, et al. Biotechnology of plant osmotic stress tolerance: physiological and molecular considerations. Acta Hortic. 2001;560:285–292.
- Tester M, Davenport R. Na+ tolerance and Na+ transport in higher plants. Ann Bot. 2003;91:503–527.
- Sun J, Chen SL, Dai SX, et al. Ion flux profiles and plant ion homeostasis control under salt stress. Plant Signal Behav. 2009;4:261–264.
- Blumwald E. Sodium transport and salt tolerance in plants. Curr Opin Cell Biol. 2000;12:431–434.
- Hasegawa PM, Bressan RA, Zhu JK, et al. Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:463–499.
- Khan MA, Duke NC. Halophytes – a resource for the future. Wetlands Ecol Manag. 2001;9:455–456.
- Shi H, Lee BH, Wu SJ, et al. Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nat Biotechnol. 2002;21:81–85.
- Rus A, Lee BH, Munoz-Mayor A, et al. AtHKT1 facilitates Na+ homeostasis and K+ nutrition in planta. Plant Physiol. 2004;136:2500–2511.
- Pardo JM, Cubero B, Leidi EO, et al. Alkali cation exchangers: roles in cellular homeostasis and stress tolerance. J Exp Bot. 2006;57:1181–1199.
- Fukuda A, Nakamura A, Tanaka Y. Molecular cloning and expression of the Na+/H+ exchanger gene in Oryza sativa. Biochim Biophys Acta. 1999;1446:149–155.
- Chauhan S, Forsthoefel N, Ran Y, et al. Na+/myo-inositol symporters and Na+/H+-antiport in Mesembryanthemum crystallinum. Plant J. 2000;24:511–522.
- Shi H, Ishitani M, Kim C, et al. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci USA. 2000;97:6896–6901.
- Yokoi S, Quintero FJ, Cubero B, et al. Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. Plant J. 2002;30:529–539.
- Yoshida K. Plant biotechnology – genetic engineering to enhance plant salt tolerance. J Biosci Bioeng. 2002;94:585–590.
- Ashraf M, Akram NA. Improving salinity tolerance of plants through conventional breeding and genetic engineering: an analytical comparison. Biotechnol Adv. 2009;27:744–752.
- Khan MS, Ahmad D, Khan MA. Utilization of genes encoding osmoprotectants in transgenic plants for enhanced abiotic stress tolerance. Electron J Biotechnol. Forthcoming 2015. Available from: http://dx.doi.org/10.1016/j.ejbt.2015.04.002.
- Liu J, Ishitani M, Halfter U, et al. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc Natl Acad Sci USA. 2000;97:3730–3734.
- Liu J, Zhu JK. A calcium sensor homolog required for plant salt tolerance. Science. 1998;280:1943–1945.
- Wu SJ, Ding L, Zhu JK. SOS1, a genetic locus essential for salt tolerance and potassium acquisition. Plant Cell. 1996;8:617–627.
- Liu JP, Zhu JK. An Arabidopsis mutant that requires increased calcium for potassium nutrition and salt tolerance. Proc Natl Acad Sci USA. 1997;94:14960–14964.
- Zhu JK, Liu J, Xiong L. Genetic analysis of salt tolerance in Arabidopsis: evidence for a critical role of potassium nutrition. Plant Cell. 1998;10:1181–1191.
- Zhu JK. Genetic analysis of plant salt tolerance using Arabidopsis. Plant Physiol. 2000;124:941–948.
- Higinbotham N. Electropotentials of plant cells. Annu Rev Plant Physiol Plant Mol Biol. 1973;24:25–46.
- Apse MP, Blumwald E. Na+ transport in plants. FEBS Lett. 2007;581:2247–2254.
- Garciadeblas B, Senn ME, Banuelos MA, et al. Sodium transport and HKT transporters: the rice model. Plant J. 2003;34:788–801.
- Essah PA, Davenport R, Tester M. Sodium influx and accumulation in Arabidopsis. Plant Physiol. 2003;133:307–318.
- Kader MA, Seidel T, Golldack D, et al. Expressions of OsHKT1, OsHKT2 and OsVHA are differently regulated under NaCl stress in salt-sensitive and salt-tolerant rice (Oryza sativa L.) cultivars. J Exp Bot. 2006;57:4257–4268.
- Møller IS, Gilliham M, Jha D, et al. Shoot Na+ exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na+ transport in Arabidopsis. Plant Cell. 2009;21:2163–2178.
- Schachtman DP, Kumar R, Schroeder JI, et al. Molecular and functional characterization of a novel low affinity cation transporter (LCT1) in higher plants. Proc Natl Acad Sci USA. 1997;94:11079–11084.
- Amtmann A, Fischer M, Marsh EL, et al. The wheat cDNA LCT1 generates hypersensitivity to sodium in a salt sensitive yeast strain. Plant Physiol. 2001;126:1061–1071.
- Leng Q, Mercier RW, Hua BG, et al. Electrophysiological analysis of cloned cyclic nucleotide-gated ion channels. Plant Physiol. 2002;128:400–410.
- Demidchik V, Essah PA, Tester M. Glutamate activates cation currents in the plasma membrane of Arabidopsis root cells. Planta. 2004;219:167–175.
- Balague C, Lin B, Alcon C, et al. HLM1, an essential signaling component in the hypersensitive response, is a member of the cyclic nucleotide-gated channel family. Plant Cell. 2003;15:365–379.
- Gobert A, Park G, Amtmann A, et al. Arabidopsis thaliana cyclic nucleotide gated channel 3 forms a non-selective ion transporter involved in germination and cation transport. J Exp Bot. 2006;57:791–800.
- Li X, Borsics T, Harrington HM, et al. Arabidopsis AtCNGC10 rescues potassium channel mutants of E. coli, yeast and Arabidopsis and is regulated by calcium/calmodulin and cyclic GMP in E. coli. Funct Plant Biol. 2005;32:643–653.
- Horie T, Schroeder JI. Sodium transporters in plants. Diverse genes and physiological functions. Plant Physiol. 2004;136:2457–2462.
- Sze H, Padmanaban S, Cellier F, et al. Expression patterns of a novel AtCHX gene family highlight potential roles in osmotic adjustment and K+ homeostasis in pollen development. Plant Physiol. 2004;136:2532–2547.
- An R, Chen QJ, Chai MF, et al. AtNHX8, a member of the monovalent cation: proton antiporter-1 family in Arabidopsis thaliana, encodes a putative Li+/H+ antiporter. Plant J. 2007;49:718–728.
- Qiu QS, Barkla BJ, Vera-Estrella R, et al. Na+/H+ exchange activity in the plasma membrane of Arabidopsis. Plant Physiol. 2003;132:1041–1052.
- Apse MP, Blumwald E. Engineering salt tolerance in plants. Curr Opin Biotechnol. 2002;13:146–150.
- Gaxiola RA, Rao R, Sherman A, et al. The Arabidopsis thaliana proton transporters, AtNhx1 and Avp1, can function in cation detoxification in yeast. Proc Natl Acad Sci USA. 1999;96:1480–1485.
- Li HT, Liu H, Gao XH, et al. Knock-out of Arabidopsis AtNHX4 gene enhances tolerance to salt stress. Biochem Biophys Res Commun. 2009;382:637–641.
- Khan MS. Role of sodium and hydrogen (Na+/H+) antiporters in salt tolerance of plants: present and future challenges. Afr J Biotechnol. 2011;10:13693–13704.
- Brini F, Hanin M, Mezghani I, et al. Overexpression of wheat Na+/H+ antiporter TNHX1 and H+-pyrophosphatase TVP1 improve salt and drought stress tolerance in Arabidopsis thaliana plants. J Exp Bot. 2006;58:301–308.
- Li J, Jiang G, Huang P, et al. Overexpression of the Na+/H+ antiporter gene from Suaeda salsa confers cold and salt tolerance to transgenic Arabidopsis thaliana. Plant Cell Tissue Organ Cult. 2007;90:41–48.
- An BY, Luo Y, Li JR, et al. Expression of a vacuolar Na+/H+ antiporter gene of alfalfa enhances salinity tolerance in transgenic Arabidopsis. Acta Agron Sin. 2008;34:557–564.
- Zhang HX, Hodson JN, Williams JP, et al. Engineering salt-tolerant Brassica plants: characterization of yield and seed oil quality in transgenic plants with increased vacuolar sodium accumulation. Proc Natl Acad Sci USA. 2001;98:12832–12836.
- Liu H, Wang Q, Yu M, et al. Transgenic salt-tolerant sugar beet (Beta vulgaris L.) constitutively expressing an Arabidopsis thaliana vacuolar Na+/H+ antiporter gene, AtNHX3, accumulates more soluble sugar but less salt in storage roots. Plant Cell Environ. 2008;31:1325–1334.
- Wu CA, Yang GD, Meng QW, et al. The cotton GhNHX1 gene encoding a novel putative tonoplast Na+/H+ antiporter plays an important role in salt stress. Plant Cell Physiol. 2004;45:600–607.
- Wang J, Zuo K, Wu W, et al. Expression of a novel antiporter gene from Brassica napus resulted in enhanced salt tolerance in transgenic tobacco plants. Biol Plant. 2004;48:509–515.
- Lu SY, Jing YX, Shen SH, et al. Antiporter gene from Hordeum brevisubulatum (Trin.) link and its overexpression in transgenic tobaccos. J Integr Plant Biol. 2005;47:343–349.
- Hossain GS, Waditee R, Hibino T, et al. Root specific expression of Na+/H+ antiporter gene from Synechocystis sp. PCC 6803 confers salt tolerance of tobacco plant. Plant Biotechnol. 2006;23:275–281.
- Soliman MH, Omar HS, El-Awady MA, et al. Transformation and expression of Na+/H+ antiporter vacuolar (AtNHX1) gene in tobacco plants under salt stress. Arab J Biotechnol. 2009;12:99–108.
- Zhang GH, Su Q, An LJ, et al. Characterization and expression of a vacuolar Na+/H+ antiporter gene from the monocot halophyte Aeluropus littoralis. Plant Physiol Biochem. 2008;46:117–126.
- Ohta M, Hayashi Y, Nakashima A, et al. Introduction of a Na+/H+ antiporter gene from Atriplex gmelini confers salt tolerance to rice. FEBS Lett. 2002;532:279–282.
- Fukuda A, Nakamura A, Tagiri A, et al. Function, intracellular localization and the importance in salt tolerance of a vacuolar Na+/H+ antiporter from rice. Plant Cell Physiol. 2004;45:146–159.
- Chen H, An R, Tang JH, et al. Over-expression of a vacuolar Na+/H+ antiporter gene improves salt tolerance in an upland rice. Mol Breed. 2007;19:215–225.
- Verma D, Singla-Pareek SL, Rajagopal D, et al. Functional validation of a novel isoform of Na+/H+ antiporter from Pennisetum glaucum for enhancing salinity tolerance in rice. J Biosci. 2007;32:621–628.
- Wu LM, Chen W, Zhao Y, et al. Salt tolerance enhancement of transgenic rice with Na+/H+ antiporter gene driven by root specific promoter PmPgPR10. Res Papers. 2012;26:643–650.
- Xue ZY, Zhi DY, Xue GP, et al. Enhanced salt tolerance of transgenic wheat (Tritivum aestivum L.) expressing a vacuolar Na+/H+ antiporter gene with improved grain yields in saline soils in the field and a reduced level of leaf Na+. Plant Sci. 2004;167:849–859.
- Yin XY, Yang AF, Zhang KW, et al. Production and analysis of transgenic maize with improved salt tolerance by the introduction of AtNHX1 gene. Acta Bot Sin. 2004;7:12–20.
- Chen M, Chen QJ, Niu XG, et al. Expression of OsNHX1 gene in maize confers salt tolerance and promotes plant growth in the field. Plant Soil Environ. 2007;53:490–498.
- Gisbert C, Rus AM, Boların MC, et al. The yeast HAL1 gene improves salt tolerance of transgenic tomato. Plant Physiol. 2000;123:393–402.
- Sondergaard TE, Schulz A, Palmgren MG. Energization of transport processes in plants. Roles of the plasma membrane H+-ATPase. Plant Physiol. 2004;136:2475–2482.
- Gaxiola RA, Li S, Undurraga S, et al. Drought- and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc Natl Acad Sci USA. 2001;98:11444–11449.
- Park S, Li J, Pittman JK, et al. Up-regulation of a H+-pyrophosphatase (H+-PPase) as a strategy to engineer drought-resistant crop plants. Proc Natl Acad Sci USA. 2005;102:18830–18835.
- He C, Yan J, Shen G, et al. Expression of an Arabidopsis vacuolar sodium/proton antiporter gene in cotton improves photosynthetic performance under salt conditions and increases fiber yield in the field. Plant Cell Physiol. 2005;46:1848–1854.
- Pasapula V, Shen G, Kuppu S, et al. Expression of an Arabidopsis vacuolar H+-pyrophosphatase gene (AVP1) in cotton improves drought- and salt tolerance and increases fibre yield in the field conditions. Plant Biotechnol J. 2011;9:88–99.
- Rodríguez-Rosales MP, Jiang X, Gálvez FJ, et al. Overexpression of the tomato K+/H+ antiporter LeNHX2 confers salt tolerance by improving potassium compartmentalization. New Phytol. 2008;179:366–377.
- Liu P, Yang GD, Li H, et al. Overexpression of NHX1s in transgenic Arabidopsis enhances photoprotection capacity in high salinity and drought conditions. Acta Physiol Plant. 2010;32:81–90.
- Rajagopal D, Agarwal P, Tyagi W, et al. Pennisetum glaucum Na+/H+ antiporter confers high level of salinity tolerance in transgenic Brassica juncea. Mol Breed. 2007;19:137–151.
- Jha B, Mishra A, Jha A, et al. Developing transgenic jatropha using the SbNHX1 gene from an extreme halophyte for cultivation in saline wasteland. PLoS One. 2013;8:e71136.
- Zhang YM, Liu ZH, Wen ZY, et al. The vacuolar Na+/H+ antiport gene TaNHX2 confers salt tolerance on transgenic alfalfa (Medicago sativa). Funct Plant Biol. 2012;39:708–716.
- Yadav NS, Shukla PS, Jha A, et al. The SbSOS1 gene from the extreme halophyte Salicornia brachiata enhances Na+ loading in xylem and confers salt tolerance in transgenic tobacco. BMC Plant Biol. 2012;12:188.
- Xu K, Hong P, Luo L, et al. Overexpression of AtNHX1, a vacuolar Na+/H+ antiporter from Arabidopsis thaliana, in Petunia hybrida enhances salt and drought tolerance. J Plant Biol. 2009;52:453–461.
- Zhao J, Zhi D, Xue Z, et al. Enhanced salt tolerance of transgenic progeny of tall fescue (Festuca arundinacea) expressing a vacuolar Na+/H+ antiporter gene from Arabidopsis. J Plant Physiol. 2007;164:1377–1383.
- OGTR: risk assessment and risk management plan for DIR 102/2010: limited and controlled release of wheat and barley genetically modified for abiotic stress tolerance. The University of Adelaide. 2010 Apr. Available from: http://www.ogtr.gov.au.
- Rudelsheim PLJ, Smets G. Anticipating the environmental risk assessment of crops modified to enhance or preserve yield. COGEM Report CGM 2010-05. Available from: http://www.cogem.net/index.cfm/en/publications/categorie/research-reports.
- Xu K, Zhang H, Blumwald E, et al. A novel plant vacuolar Na+/H+ antiporter gene evolved by DNA shuffling confers improved salt tolerance in yeast. J Biol Chem. 2010;285:22999–23006.
- Khan MS. The role of DREB transcription factors in abiotic stress tolerance of plants. Biotechnol Biotechnol Equip. 2011;25(3):2433–2442.
- Khan MS, Yu X, Kikuchi A, et al. Genetic engineering of glycine betaine biosynthesis to enhance abiotic stress tolerance in plants. Plant Biotechnol. 2009;26:125–134.
- Bassil E, Coku A, Blumwald E. Cellular ion homeostasis: emerging roles of intracellular NHX Na+/H+ antiportes in plant growth and development. J Exp Bot. 2012;63:5727–5740.
- Bassil E, Ohto MA, Esumi T, et al. The Arabidopsis intracellular Na+/H+ antiporters NHX5 and NHX6 are endosome associated and necessary for plant growth and development. Plant Cell. 2011;23:224–239.
- Bassil E, Tajima H, Liang YC, et al. The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell. 2011;23:3482–3497.
- Shabala S. Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops. Ann Bot. 2013;112:1209–1221.