Abstract
Probiotics have been used for the treatment of various disorders or as alternative therapies. The stability of dual-coated probiotics is increased in the gastrointestinal environment. The aim of the present study was to evaluate the hepatoprotective effects of dual-coated and uncoated probiotic supplements, following liver injury. Albino Wistar rats were orally treated with probiotics daily and carbon tetrachloride (CCl4) was administered on the seventh and eighth days to induce acute liver damage. Hepatoprotective effects were determined by assessment of serum glutamic–oxaloacetic transaminase (SGOT) and serum glutamic–pyruvic transaminase (SGPT) activities, as well as by histopathological examination. The CCl4-treated control group showed increased SGOT and SGPT activities as compared with the normal control group. However, treatment with probiotics reduced SGOT and SGPT activities, following CCl4 administration. Animals treated with probiotics showed reduced liver weight than that in the standard CCl4 group which did not receive probiotics. Histopathological analysis showed that administration of probiotics minimized liver damage by reducing the level of morphological changes and necrosis. Therefore, probiotics may be effective hepatoprotective agents and should be considered useful for the treatment and prevention of hepatic disorders.
Introduction
The liver is a vitally important organ that protects the body from various harmful substances and toxic metabolic byproducts.[Citation1,Citation2] Exposure of the liver to xenobiotics and other therapeutic agents has been reported to cause hepatic damage,[Citation3] and the incidence of liver disease continues to increase worldwide.[Citation4]
Carbon tetrachloride (CCl4) is widely used to induce acute toxic liver injury in animal models.[Citation5] The toxic effects of CCl4 are caused by oxidative stress.[Citation4,Citation6,Citation7] Despite the advances in modern medicine, few reliable drugs for liver disorders are available.[Citation8] Therefore, there is a need to find new effective and safe drugs, without notable side effects.
The use of probiotics is considered an effective and safe alternative treatment for hepatotoxicity.[Citation9,Citation10] Probiotics confer general health benefits on the host. Moreover, they contribute to the reduction in the risk of diseases.[Citation11]. Probiotics play important roles in the body, such as inhibition of harmful bacteria by lowering the pH of the intestinal environment, improvement of diarrhoea, synthesis of vitamins and lowering of blood cholesterol levels.[Citation12] Several studies have found that probiotics have beneficial effects against intestinal diseases and liver diseases.[Citation12,Citation13]
A recent study reported that dual-coated probiotics show improved viability when exposed to gastrointestinal conditions as compared with non-coated probiotics. The dual-coating offers better protection from heat, pH conditions and moisture.[Citation14,Citation15] Thus, the stability of dual-coated probiotics is increased in the gastrointestinal environment. The purpose of the present study was to examine the hepatoprotective effects of dual-coated probiotics and uncoated probiotics on CCl4-induced acute hepatic injury in rats.
Materials and methods
Dual-coated and uncoated probiotics
Dual-coated and uncoated probiotic strains were manufactured and supplied by Cell Biotech Co. Ltd, Korea. The dual-coated and uncoated probiotics contained a combination of three types of live probiotic bacteria, Lactobacillus acidophilus (LA), L. plantarum (LP) and Streptococcus thermophilus (ST), mixed in a 1:1:1 ratio. These probiotics were suspended in phosphate-buffered saline (PBS) and were used for animal experiments.
Animal experiments
Thirty male albino Wistar rats (aged 4weeks) were purchased from Raon Bio (Raon Bio, Korea) and housed in a temperature-controlled animal facility (22 ± 2 °C/humidity 55% ± 5%) under a 12 h light/dark cycle. Food and water were provided ad libitum from the day of the rats' arrival until the completion of the experiment. The rats were randomly assigned to five groups (six rats per group); Group I served as normal controls and received only PBS; Group II served as CCl4 controls and received PBS and 1 mL/kg body weight (BW) of CCl4 per os (p.o.); Group III served as the standard group and received silymarin (100 mg/kg BW p.o.) and CCl4 (1 mL/kg BW p.o.); Group IV served as a test group and received dual-coated probiotic supplement (109 CFU/kg BW p.o.) and CCl4 (1 mL/kg BW p.o.); Group V served as another test group and received uncoated probiotic supplement (109 CFU/kg BW p.o.) and CCl4 (1 mL/kg BW p.o.). We carried out the experiment after a stabilization period of one day. All treatments were administered by oral gavage continuously for nine days. Animals in Groups II–V were administered with CCl4 on the 7th and 8th day. At the end of the experimental period, on the 10th day, they were sacrificed under mild ether anaesthesia and blood was collected from the animals by cardiac puncture. The serum was separated for determination of biochemical parameters. The liver, spleen and kidneys from each animal were carefully excised and washed in ice-cold normal saline solution and weighed. Liver tissues were then kept in 10% formalin solution for histopathological studies. Animal studies were performed according to the guidelines for the care and use of laboratory animals issued by Sahmyook University (SYUIACUC 2014-032).
Biochemical analysis
The blood samples were allowed to clot for 45 min at room temperature. The serum was separated by centrifugation at 2500 ×g for 15 min and utilized for the quantification of serum glutamic–oxaloacetic transaminase (SGOT, E.C. 2.6.1.1) and serum glutamic–pyruvic transaminase (SGPT, E.C. 2.6.1.2), using commercially available assay kits (BioVision Inc., USA), according to the manufacturer's protocol. Briefly, 100 µL of reaction mixture was added to the serum sample present in each well of a 96-well microplate (NunC, Denmark), and the mixture was incubated at 37 °C for 60 min. SGOT and SGPT were then quantified at 450 nm and 570 nm, respectively, by using an ELISA plate reader (Molecular Devices, USA). All measurements were calculated using a standard curve.
Histopathological studies
Processing of liver tissue for histopathological analysis was performed by following the modified method of Luna,[Citation16] and hematoxylin and eosin (H&E) staining was carried out using the standard protocol.
Statistical analysis
The results from the study were expressed as means with standard deviations (±SD). Data were analysed with one-way analysis of variance (ANOVA), followed by Dunnett's t-test for multiple comparisons. These statistical tests were performed with SPSS for Windows software V.15.0.2 (SPSS, Chicago, IL, USA). Values with p < 0.05 were considered statistically significant.
Results and discussion
Body weight and weight of the liver, spleen and kidneys
The body weight of Group II was significantly decreased from 161.1 g on day 7 to 149.8 g on day 8, but the body weights in Groups IV and V were almost comparable to those in Group III (). The drop in the body weight in Group IV was minimal and the final body weight in this group was higher than that in Group V. shows that in Groups IV and V the gain of body weight in rats was not significantly affected by CCl4 treatment. In particular, Groups IV and III showed very similar gain in body weight ().
Figure 1. Change in body weight in normal and CCl4-treated rats. Group I (CTL): normal control; Group II (PBS): CCl4 control; Group III (silymarin): silymarin standard and CCl4; Group IV (coated-PRO): dual-coated probiotics and CCl4; Group V (uncoated-PRO): uncoated probiotics and CCl4. Note: Values represent means from six determinations ± SD.
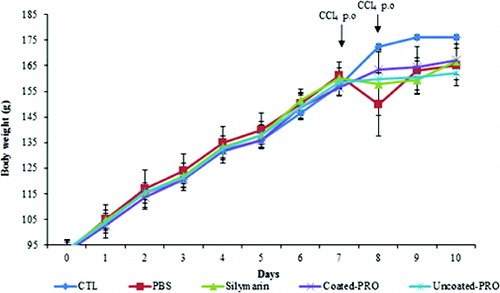
Table 1. Effect of dual-coated and uncoated probiotics on the gain of body weight and the organ weights in CCl4 treated rats.
In addition, the assessment of liver and kidney weights showed that the increase in weight in Group II was greater than that in all other groups. Moreover, there was no difference in body, liver and kidney weight gains between Groups III, IV and V. However, the spleen weight gain was less in Groups II–V compared to Group I, and probiotic treatment had no effects against CCl4 treatment ().
Thus, the present study showed that CCl4 caused body weight loss but organ weight gain in the rat model. On the other hand, probiotics (dual-coated probiotics and uncoated probiotics) maintained the body weight and organ weights at almost normal values. These results are similar with other reports on the effect of other probiotics against CCl4-induced hepatotoxicity.[Citation3,Citation15,Citation17,Citation18]
Biochemical analysis
SGOT and SGPT are important hepatic metabolic enzymes that indicate liver damage by xenobiotics or other causes; these enzymes are released from the liver into the blood serum when liver damage occurs. Therefore, the activities of SGOT and SGPT are considered to reflect the degree of liver damage.[Citation19]
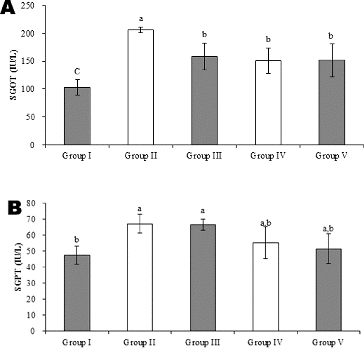
The experiments showed statistically significant differences in the levels of SGOT in all groups; however, the levels of SGPT in Groups II–V were not statistically different (). The levels of SGOT and SGPT in Group IV were 151 UI/L and 55 UI/L, respectively. The levels of SGOT and SGPT in Group V were 151.8 UI/L and 51.5 UI/L, respectively, and those in Group II were 206.5 UI/L and 67 UI/L, respectively. Whereas, Group II showed an increase in the activities of SGOT and SGPT as compared to those observed in Group I, Groups IV and V showed a significant reduction in SGOT and SGPT activities compared to those observed in Group II. These results demonstrate that the marker enzymes were released in much lesser quantities in the probiotic groups than in the group treated with CCl4 alone.
Figure 2. Effects of dual-coated and uncoated probiotics on SGOT (A) and SGPT (B) levels against CCl4-induced hepatotoxicity in rats. Group I: normal control; Group II: CCl4 control, Group III: silymarin standard and CCl4; Group IV: dual-coated probiotics and CCl4; Group V: uncoated probiotics and CCl4. Note: Values represent means from six determinations ± SD. a–cMeans with different superscripts differ (p < 0.05) by ANOVA.
Light microscopy findings
CCl4 is a hepatotoxin that induces liver injury in rats. The metabolisms of CCl4 by cytochrome P450 can generate trichloromethyl radicals (CCl3 and/or CCl3OO),[Citation20] which can lead to membrane lipid peroxidation and cell necrosis.
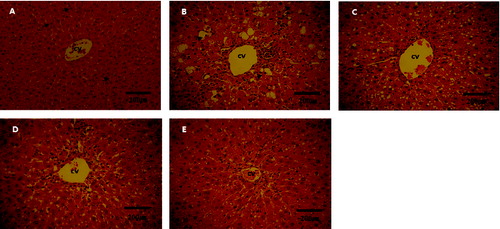
The histopathological analysis showed that the livers of rats from Group I had no noticeable histological changes (). In contrast, in the livers of all rats from the CCl4-treated groups, there were consistently observed liver morphological changes such as necrosis and cytoplasmic vacuolization. However, in Groups IV and V, there was a marked reduction in these types of liver morphological changes. That is, the histopathological status of the liver was improved in all animals from the probiotic Groups IV and V. Therefore, it could be suggested that the studied probiotics could have an antioxidant and anti-necrotic effect. In , the dual-coated probiotic and the uncoated probiotic demonstrated equally effective hepatoprotective potential. However, according to a study on dual-coated probiotics, double coating increases the survival of probiotic bacteria in the intestine.[Citation21] Therefore, dual-coated probiotics may be expected to be more health beneficial and economically viable than uncoated probiotics.
Figure 3. Histopathological changes in the liver tissue of normal and CCl4-treated rats. (A) Group I: normal control rats; centrally located and round nuclei and homogeneous cytoplasm (arrows). (B) Group II: CCl4-treated rats, cell necrosis (arrows). (C) Group III: rats treated with silymarin plus CCl4. (D) Group IV: rats treated with dual-coated probiotics plus CCl4. (E) Group V: rats treated with uncoated probiotics plus CCl4. Note: H&E staining; magnification ×400; bar = 200 μm.
Final remarks
A recent in vitro study showed that a dual-coated probiotic supplement was highly resistant to acidic environment in the stomach and was more heat stable compared to the non-coated probiotic supplement.[Citation14] Another recent in vivo and in vitro study showed that dual-coated LAB (lactic-acid bacteria) have stronger probiotic effects compared to uncoated LAB.[Citation21] In in vivo experiments, double-coated live L. plantarum KCTC3928 has demonstrated hypocholesterolaemic effects in mice.[Citation22] In the present study, we showed that dual-coated and uncoated probiotic supplements have hepatoprotective effects.
Although the hepatoprotective mechanisms of probiotics were not addressed in this study, potential mechanisms were identified. Probiotics and their products have antioxidant activities and free-radical scavenging properties in vitro and in vivo.[Citation23,Citation24] For example, LcZ (L. casei Zhang) may be related to down-regulation of toll-like receptor 4 (TLR4), which may cause inhibition of oxidative stress and tumour necrosis factor alpha (TNF-α). In addition, probiotic products are important mediators of cell growth and differentiation and prevent lipid peroxidation in liver microsomes.[Citation25,Citation26] Our findings suggest that dual-coated and uncoated probiotic supplements may exhibit hepatoprotective effects by affecting the oxidative stress levels and TNF-α.
Conclusions
Our findings in Wistar rats as a model demonstrated the possible use of dual-coated and uncoated probiotic supplements (containing probiotic bacteria: L. acidophilus, L. plantarum and S. thermophilus) for the prevention of liver injury. However, the mechanism of probiotic action in relation to hepatoprotective effects needs to be investigated further under various preclinical conditions.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ashoush IS, El-Batawy OI, El-ShourbagyGehan A. Antioxidant activity and hepatoprotective effect of pomegranate peel and whey powders in rats. Ann Agri Sci. 2013;58:27–32.
- Eidi A, Mortazavi P, Tehrani ME, Rohani AH, Safi SB. Hepatoprotective effects of pantothenic acid on carbon tetrachloride-induced toxicity in rats. EXCLI J. 2012;11:748–759.
- Huo HZ, Wang B, Liang YK, Bao YY, Gu Y. Hepatoprotective and antioxidant effects of licorice extract against CCl4-induced oxidative damage in rats. Int J Mol Sci. 2011;12:6529–6543.
- Huang YT, Hsu YC, Chen CJ, Liu CT, Wei YH. Oxidative-stress-related changes in the livers of bile-duct-ligated rats. J Biomed Sci. 2003;10:170–178.
- Neubauer K, Eichorst ST, Wilfling T, Buchenau M, Xia L, Ramadori G. Sinusoidal intercellular adhesion molecule-1 up-regulation precedes the accumulation of leukocyte function antigen-1-positive cells and tissue necrosis in a model of carbontetrachloride-induced acute rat liver injury. Lab Invest. 1998;78:185–194.
- Poli G. Pathogenesis of liver fibrosis: Role of oxidative stress. Mol Aspects Med. 2011;21:49–98.
- Reeves HL, Friedman SL. Activation of hepatic stellate cells: a key issue in liver fibrosis. Front Biosci. 2002;7:808–826.
- Nallamilli BR, Kumar CP, Reddyc KV, Prasannac ML, Maruthic V, Sucharitac P. Hepatoprotective activity of Cichoriumintybus (Linn.) root extract against carbon tetrachloride induced hepatotoxicity in albino Wistar rats. Drug Invent Today. 2013;5:311.
- Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441–1449.
- Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease). ProcSocExpBiol Med. 1994;205:243–247.
- Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, Bazhukova TA, Soloviev AG, Barve SS, McClain CJ, Cave M. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42:675–682.
- Bang CS, Hong SH, Suk KT, Kim JB, Han SH, Sung H, Kim EJ, Kim MJ, Kim MY, Baik SK, Kim DJ. Effects of Korean Red Ginseng (Panax ginseng), urushiol (Rhusvernicifera Stokes), and probiotics (Lactobacillus rhamnosus R0011 and Lactobacillus acidophilus R0052) on the gut-liver axis of alcoholic liver disease. J Ginseng Res. 2014;38:167–172.
- Wang Y, Li Y, Xie J, Zhang Y, Wang J, Sun X, Zhang H. Protective effects of probiotic Lactobacillus casei Zhang against endotoxin- and d-galactosamine-induced liver injury in rats via anti-oxidative and anti-inflammatory capacities. Int Immunopharmacol. 2013;15:30–37.
- Kang JY, Lee DY, Park JE, Kim MJ, Lee JS, Seo JG, Chung MJ, Shin HS, Ha NJ. Dual coating improves the survival of probiotic Bifidobacteriumstrains during exposure to simulated gastro-intestinal conditions. Kor J Microbiol. 2013;49:275–281.
- Yun JH, Kim YA, Chung MJ, Kang BY, Ha NJ. Hepatoprotective and anti-fatigue effects of lactic acid bacteria (Lactobacillus acidophilus, Bifidobacteriumbifidum and Streptococcus thermophiles). J Toxicol Pub Heath. 2007;1:11–17.
- Luna LG. Manual histology staining methods of armed forces institute of pathology. 3rd ed. New York (NY): McGraw-Hill; 1968.
- Liu Y, Liu Q, Ye G, Khan A, Liu J, Gan F, Zhang X, Kumbhar S, Huang K. Protective effects of selenium-enriched probiotics on carbon tetrachloride-induced liver fibrosis in rats. J Agric Food Chem. 2015;63(1):242–249.
- Onyesom I, Mordi J, Opajobi AO, Esume CO. Hepatoprotective potentials of Hibiscus rosasinensis petal anthocyanin extracts against carbon tetrachloride-induced acute liver damage in Wistar rats. Sudan JMS. 2008;3:33–36.
- Zhang J, Wang H, Yan X, Zhang L. Comparison of short-term toxicity between Nano-Se and selenite in mice. Life Sci. 2005;76:1099–1109.
- Reinke LA, Lai EK, McCay PB. Ethanol feeding stimulates trichloromethyl radical formation from carbon tetrachloride in liver. Xenobiotica. 1998;18:1311–1318.
- Cha MK, Chung MJ, Kim JE, Lee KO, Ha NJ. Comparison of dual coated (Duolac™) and uncoated lactic acid bacteria from potential probiotics. Biotechnol Biotechnol Equip. 2011;25:2489–2493.
- Jeun J, Kim S, Cho SY, Jun HJ, Park HJ, Seo JG, Chung MJ, Lee SJ. Hypocholesterolemic effects of Lactobacillus plantarum KCTC3928 by increased bile acid excretion in C57BL/6 mice. Nutrition. 2010;26:321–330.
- Nurrochmad A, Margono SA, Sardjiman, Hakim AR, Ernawati, Kurniawati E, Fatmawati E. Hepatoprotective and antioxidant activity of pentagamavunon-0 against carbon tetrachloride-induced hepatic injury in rats. Asian Pac J Trop Biomed. 2013;6:438–442.
- Osman N, Adawi D, Ahrné S, Jeppsson B, Molin G. Endotoxin- and d-galactosamine induced liver injury improved by the administration of Lactobacillus, Bifidobacterium and blueberry. Dig Liver Dis. 2007;39:849–856.
- Kitada M, Igarashi K, Hirose S, Kitagawa H. Inhibition by polyamines of lipid peroxide formation in rat liver microsomes. Biochem Biophys Res Commun. 1997;87:388–394.
- McCormack SA, Johnson LR. Role of polyamines in gastrointestinal mucosal growth. Am J Physiol. 1991;260:795–806.
