Abstract
An efficient protocol for clonal multiplication of an important mangrove, Avicennia marina, was developed through in vitro culture of nodal segments obtained from a mature plant. The nodal explant induced multiple shoots when cultured on the Murashige and Skoog (MS) basal medium supplemented with varying concentrations and combinations of 6-benzyladenine (BA) and α-naphthalene acetic acid. The highest response in terms of per cent regeneration (73%), average number of shoots/explant (3.25 ± 0.25) and maximum shoot length (5.2 ± 0.27 cm) was obtained on the MS medium supplemented with BA 5.0 μmol/L + NAA 1.0 μmol/L + 3 g/L activated charcoal after 8 weeks of culturing. The regenerated shoots were rooted well in the MS medium supplemented with 1.0 μmol/L indole-3-butyric acid with an average of 2.9 ± 0.24 roots per microshoot. The rooted plantlets were successfully transferred to pots containing normal garden soil with 70% survival rate. The genetic stability of the regenerated plants was evaluated using single primer amplification reaction (SPAR) methods viz., random amplified polymorphic DNA, directed amplification of minisatellite DNA and intersimple sequence repeat polymorphism. The SPAR analysis revealed monomorphic banding patterns in all in vitro regenerated plantlets of A. marina and similar with that of the mother tree confirming their genetic uniformity and clonal fidelity.
Introduction
Mangroves, the characteristic intertidal plant formations of sheltered tropical and subtropical shorelines, are usually referred to as ‘coastal woodlands’, ‘mangals’, ‘tidal forests’ or ‘mangrove forests’.[Citation1,Citation2] Avicennia marina (Forssk.) Vierh. (family: Avicenniaceae), commonly known as grey mangrove or white mangrove, is the most widespread mangrove distributed throughout the tropical and subtropical regions of the world. It is one of mangroves found in the arid regions of the coastal Arabian Peninsula as well as on both sides of the Red Sea in Egypt, Eritrea, Yemen Sudan and Saudi Arabia. The mangrove habitat is threatened throughout its range mainly because of the increasing human activities at coastal areas, and there has been a 21% assessed decline in mangrove areas since 1980 (International Union for Conservation of Nature, IUCN).[Citation3] Nowadays, A. marina are more at risk from coastal development for tourism and other land use purposes and extraction at the extremes of their distribution as well as the climate changes due to global warming.
Biotechnology, offering new tools for sustainable development and utilization of natural resources, has become increasingly imperative worldwide. The application of biotechnological approaches to plants would also benefit from the development of tissue culture systems for in vitro development and regeneration of rare and endangered species.[Citation4,Citation5] Tissue culture techniques could be a cost-effective means of bulk production of elite planting material throughout the year without any seasonal constraints. It may also play an essential role in clonal mass multiplication and ex situ preservation of germplasm that is rare, endangered or on the edge of extinction. An important limitation of the in vitro technique is the development of somaclonal variation among regenerated plantlets, which may affect the quality and quantity of micropropagated plants and, in turn, obstruct the ex situ conservation programme.
Confirmation of genetic uniformity using polymerase chain reaction (PCR)-based profiling methods is a common practice nowadays and has also found application in studies of varietal identification, phylogenetic analysis and genetic diversity.[Citation4] A group of such methods using a single primer in the PCR is collectively described as single primer amplification reaction (SPAR) methods and includes random amplified polymorphic DNA (RAPD) developed by Williams et al. [Citation6] and Welsh and McClelland [Citation7], directed amplification of minisatellite DNA (DAMD) developed by Heath et al. [Citation8] and intersimple sequence repeat (ISSR) polymorphism developed by Zietkiewicz et al. [Citation9]. These methods are robust, rapid and have proven useful in the diversity studies permitting precise and versatile analysis of genetic stability. The SPAR techniques have been employed extensively in characterization of micropropagated plantlets.[Citation10–16]
Earlier, a study was conducted by Al-Bahrany and Al-Khayri [Citation17] to obtain in vitro plantlets of A. marina, but there is still no report on the molecular characterization of micropropagated plants. However, the developed method is meager and gave a low number of shoots. This study aimed to develop an efficient method for in vitro clonal propagation and ex situ conservation of A. marina. Furthermore, to the best of our knowledge, the genetic stability of the in vitro regenerated A. marina plants was assessed for the first time by SPAR methods (RAPD, DAMD and ISSR).
Materials and methods
Explants source and disinfection
The plant materials were collected from a healthy field plant at Al-Mawassam (16°25′6.66″N/42°45′55.52″E), Jazan, Saudi Arabia. Nodal segments were excised from collected materials and used as explants. The explants were thoroughly washed under running tap water for 30 min, followed by soaking with 5% (v/v) liquid detergent solution for 5 min. After repeated washes with sterile distilled water, the explants were surface sterilized with 0.1% (w/v) mercuric chloride solution for 5 min. After five washes with sterile distilled water, the explants were finally cut into 0.5–0.8 cm-sized pieces and aseptically cultured on the Murashige and Skoog (MS) medium.[Citation18]
Nutrient media and culture conditions
The basic nutrient medium consisted of MS salts and vitamins containing 3% (m/v) sucrose, 0.8% (m/v) agar and 3% (m/v) activated charcoal. Growth regulators and their combinations were added to the medium, as specified below. The pH of the medium was adjusted to 5.8 with 1 mol/L NaOH or HCl. The culture vials containing the media were steam sterilized in an autoclave at 121 °C at 1.06 kg/cm2 for 20 min. All the cultures were maintained at 24 ± 2 °C under a 16/8 h photoperiod with a photosynthetic photon flux density of 50 μmol/(m2 s2) provided by cool white fluorescent lamps (Philips, Poland).
Shoot initiation and multiplication
In this study, experiments were carried out on multiple shoot induction and proliferation from nodal explants of A. marina. Sterilized nodal segment explants were cultured on the MS medium supplemented with various concentrations and combinations of 6-benzyladenine (BA; 0.5, 2.5, 5.0 or 10.0 μmol/L) and α-naphthalene acetic acid (NAA; 1.0 μmol/L). Data for shoot formation and proliferation were recorded after eight weeks of culturing. MS medium without any growth regulators was used as a control.
In vitro rooting and establishment of plantlets
For rooting, elongated healthy shoots measuring about 4–5 cm in length were excised from shoot clumps and transferred into the MS medium supplemented with indole-3-butyric acid (IBA) at different concentrations (0.5, 1.0, 1.5 or 2 μmol/L) as described in . The percentage of root formation, root numbers and root length were recorded four weeks after transfer. Regenerated plantlets with well-developed shoots and roots were transferred to pots containing sterile potting soil (Planta Guard) after thorough washing in running tap water. The plants were covered with transparent plastic bags to ensure high humidity and maintained under diffuse light conditions (16/8 h photoperiod). The potted plants were watered every three days with half-strength MS salt solution for two weeks. After one month, the successfully acclimatized plantlets were transferred to pots containing normal garden soil and maintained in glasshouse under normal conditions.
Table 1. Effect of IBA on root formation from in vitro raised shoots of A. marina in the MS medium after 4 weeks of culturing.
Genomic DNA extraction
Genomic DNA was extracted from 500 mg of young leaves of micropropagated plantlets and the donor plant, using the modified cetyltrimethyl ammonium bromide method.[Citation19] Purified total DNA was quantified and its quality verified by spectrophotometry (6705 UV/vis spectrophotometer, Jenway, UK), and each sample was diluted to 25 ng/μL in sterile Milli-Q water and stored at 4 °C.
PCR amplification and analysis of SPAR
Regenerated plantlets were tested for genetic stability, using 20 RAPD (Kit-B), 4 DAMD and 12 ISSR primers (Gene Link, New York, USA) for their unambiguous and reproducible banding patterns. PCR amplification was performed in a volume of 20 μL reaction mixture containing 1 × PCR buffer, 25 ng sample DNA, 2.0 mmol/L MgCl2, 200 μmol/L of deoxyribonucleoside triphosphates (dNTPs) mix, 5 pmol primer (RAPD, DMAD or ISSR) and 2.5 U Taq DNA polymerase (Fermentas, GmbH, Germany). The amplifications were carried out in a thermocycler (T100™ Thermal Cycler, Bio-Rad, USA). PCR reactions were performed with initial DNA denaturation at 94 °C for 5 min followed by 35 cycles of denaturation at 94 °C (30 s), primer annealing at 35 °C –58 °C (–) for 45 s, primer extension at 72 °C for 90 s and final extension at 72 °C for 7 min. PCR products obtained from RAPD, DAMD and ISSR markers were resolved in a 1.5% agarose gel for 2 h in 1 × TBE (Tris–borate–EDTA) buffer, stained with ethidium bromide, and photographed using a Gel Documentation System (G-Box, Syngene, UK).
Table 2. RAPD primers used to evaluate the extent of genetic fidelity of micropropagated Avicennia marina plantlets.
Table 3. DAMD primers used to evaluate the extent of genetic fidelity of micropropagated Avicennia marina plantlets.
Table 4. ISSR primers used to evaluate the extent of genetic fidelity of micropropagated Avicennia marina plantlets.
Data analysis
All the experiments were repeated thrice with a minimum of 10 explants per treatment. The data were analysed statistically using SPSS Ver. 20 (SPSS Inc., Chicago, USA) and the significance of differences among the means was evaluated using Tukey's test at P < 0.05 probability level. The data presented in figures and tables are mean values from three repeated experiments, with standard error of the means (±SEM).
Results and discussion
Nodal segments containing axillary buds have the potential to develop into complete plantlets. In natural conditions, these buds remain inactive for a specific period depending on the growth pattern of the plant. However, by tissue culture, enhanced axillary branching can be achieved for clonal mass multiplication of plants by nodal segment culture on a nutrient medium augmented with suitable plant growth regulators.
Clonal in vitro multiplication of A. marina
In this study, a protocol for direct regeneration of A. marina from nodal segments explants was formulated by testing plant growth regulators either singly or in different combination on the MS medium. Nodal segments explants of A. marina cultured on the plant-growth-regulators-free MS medium or medium supplemented with cytokinin alone did not show any morphogenetic response and failed to develop shoots even after eight weeks of culture.
Multiple shoots formations were achieved from nodal explants on the MS medium supplemented with BA in combination with NAA ( and ). Among the various concentrations and combinations of BA and NAA, the highest shoot regeneration frequency (73%), number of shoots (3.25 ± 0.25) and maximum shoot length (5.2 ± 0.27 cm) were recorded on the MS medium supplemented 5.0 µmol/L BA, 1.0 µmol/L NAA and 3 g/L activated charcoal. A decrease in the number of shoots was observed by increasing the BA concentration beyond 5 µmol/L ().
Figure 1. Effect of 6-benzyladenine (BA) on shoot regeneration (%) of A. marina in the MS medium augmented with NAA (1.0 μmol/L) + AC (3.0 g/L). Data represent means ± SEM. Values followed by the same letter within response variables are not significantly different (P < 0.05) based on Tukey's test.
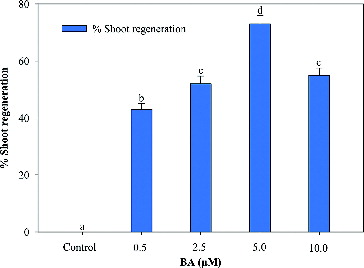
Figure 2. Effect of 6-benzyladenine (BA) on in vitro shoot multiplication and shoot length of A. marina in the MS medium augmented with NAA (1.0 μmol/L) + AC (3.0 g/L). Data represent means ± SEM. Values followed by the same letter within response variables are not significantly different (P < 0.05) based on Tukey's test.
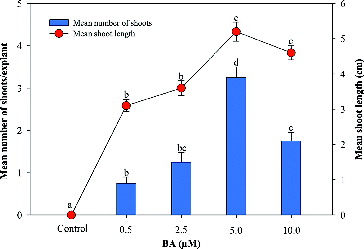
Similarly, the synergism of BA in combination with NAA has been well documented in several plant species, such as Rauvolfia tetraphylla,[Citation14] Tylophora indica,[Citation20] Enicostemma littorale [Citation21] and Metabriggsia ovalifolia [Citation22]. In agreement with these findings, the present report also demonstrates the positive modification of shoot induction efficiency achieved by using a low concentration of NAA in combination with a high concentration of BA. This differential morphogenetic response may be due to apical dominance. Apical dominance is governed by the ratio of auxin and cytokinin and is very well documented to be caused by the action of basipetally transported auxin from the apex and its consequent inhibition of axillary bud growth.[Citation23] It is well known that cytokinin regulates auxin levels and vice versa. Similarly, the effectiveness of BA and NAA on in vitro axillary shoot multiplication has also been observed in Excoecaria agallocha as described by Arumugam and Panneerselvam.[Citation24]
In vitro rooting and establishment of plants
For rooting, 4–5 cm long in vitro regenerated shoots were transferred to the MS medium augmented with different concentrations of IBA (). The frequency of rooting varied from 45% to 71%, depending on the concentration of IBA used. The highest frequency of root formation (71%), number of roots (2.9 ± 0.24) and maximum average root length (3.8 ± 0.30) was recorded at 1.0 µmol/L of IBA after 4 weeks of culturing. The efficacy of IBA in root induction has been reported in Rauvolfia serpentina,[Citation5] Mentha arvensis,[Citation16] Sansevieria cylindrica [Citation25] and in Cyamopsis tetragonoloba [Citation26]. Plantlets having 4–5 fully expanded leaves and well-developed roots were successfully hardened off in the growth room in pots containing sterile soilrite mixture and covered with transparent polybags to maintain high humidity. After four weeks, the plantlets were transferred to pots containing normal garden soil with a 70% survival rate.
Genetic fidelity of regenerated plants
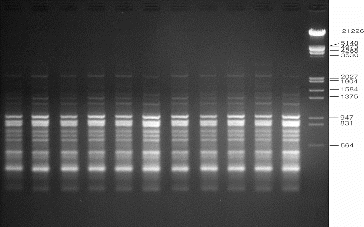
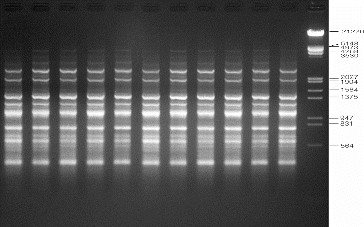
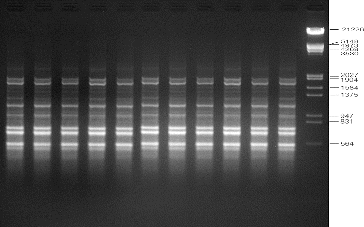
Genetic uniformity of regenerated plants is of key importance in the micropropagation of any plant species. The SPAR methods, viz. RAPD, DAMD and ISSR, were used in order to assess the genetic fidelity of micropropagated A. marina plantlets and compare them with the mother plants. Twenty RAPD primers were screened and yielded clear, reproducible bands. The number of bands varied from 3 to 11 with an average of 6.65 bands per primer (). Four DAMD primers were tested to evaluate the micropropagated plantlets of A. marina, and three of them yielded clear, reproducible bands with a total of 38 bands and an average of 9.5 bands per primer (). For ISSR analysis, 12 primers were used to screen the regenerated plantlets. Eleven primers gave clear and scorable bands and the number of bands for each primer ranged from 5 to 18, with an average of 10.25 bands per primer (). The results of this molecular study revealed that all the primers used in this study showed a monomorphic pattern in all the sample plants, confirming the genetic uniformity of the regenerated plantlets and that no variation was induced during in vitro propagation of A. marina (–). The findings of this investigation corroborate several earlier reports on genetic analysis of regenerated plantlets in Mentha arvensis,[Citation16] Aegle marmelos,[Citation27] Bambusa balcooa,[Citation28] Gerbera jamesonii [Citation29] and Gloriosa superba [Citation30], using SPAR analysis.
Figure 3. Representative RAPD profiles of in vitro raised and donor plants of A. marina generated using primer OPB-07. Lanes 1–10: randomly selected regenerated plants; lane D: donor plant; lane M: lambda DNA/EcoRI+HindIII marker (Cat no. SM0193; Fermentas, GmbH, Germany).
Conclusions
The results from this study demonstrated that the proposed protocol for clonal in vitro multiplication of A. marina is effective and showed a definite advantage over the earlier study. For the first time, to the best of our knowledge, the regenerated plants were genetically assessed by SPAR (RAPD, DAMD and ISSR) methods, and it was found that these plants were genetically stable under in vitro conditions. The developed protocol could be helpful to explore the possibility of preserving the genetic stability of the selections and ex situ conservation of this important plant.
Acknowledgements
The authors are thankful to the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdul Aziz City for Science and Technology, Kingdom of Saudi Arabia for financial assistance.
Disclosure statement
No potential conflict of interest is reported by the authors.
Additional information
Funding
References
- Duke NC. Mangrove floristics and biogeographys. In: Robertson AI, Alongi DM, editors. Tropical mangrove ecosystem. Vol. 41, Coastal and estuarine studies. Washington, DC: American Geophysical Union. 1992; p. 63–100.
- Saenger P. Mangrove ecology, silviculture and conservation. Dordrecht: Kluwer Academic Publishers; 2002.
- Duke N, Kathiresan K, Salmo SG 3rd, et al. Avicennia marina. The IUCN Red List of Threatened Species [Internet]. Version 2015.1. c. Cambridge (UK): International Union for Conservation of Nature; 2010 [cited 2015 June 13]. Available from: http://www.iucnredlist.org/details/178828/0.
- Waugh R, Powell W. Using RAPD markers for crop improvement. Trends Biotech. 1992;10:186–191.
- Alatar AA. Thidiazuron induced efficient in vitro multiplication and ex vitro conservation of Rauvolfia serpentina – a potent antihypertensive drug producing plant. Biotech Biotech Equip. 2015;29(3):489–497.
- Williams JGK, Kubelik AR, Livak KJ, et al. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990;18:6531–6535.
- Welsh J, McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990;18:7213–7218.
- Heath DD, Iwana GK, Delvin RH. PCR primed with VNTR core sequences yield species specific patterns and hypervariable probes. Nucleic Acids Res. 1993;21:5782–5785.
- Zietkiewicz E, Rafalski A. Labuda D. Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics. 1994;20:176–183.
- Ashmore SE. Current in vitro conservation techniques. In: Engelmann F, editor. Status reports on the development and application of in vitro techniques for the conservation and use of plant genetic resources. Rome: IPGRI; 1997. p. 5–18.
- Aronen TS, Krajnakova J, Haggman HM, et al. Genetic fidelity of cryopreserved embryogenic cultures of openpollinated Abis cephalonica. Plant Sci. 1999;142:163–172.2
- Silva LM, Montes de Oca H, Diniz CR, et al. Fingerprinting of cell lines by directed amplification of minisatellite-region DNA (DAMD). Braz J Med Biol Res. 2001;34:1405–1410.
- Joshi P, Dhawan V. Assessment of genetic fidelity of micropropagated Swertia chirayita plantlets by ISSR marker assay. Biol Plant. 2007;51:22–26.
- Faisal M, Alatar A, Ahmad N, et al. An efficient and reproducible method for in vitro clonal multiplication of Rauvolfia tetraphylla L. and evaluation of genetic stability using DNA-based markers. Appl Biochem Biotech. 2012;168:1739–1752.
- Faisal M, Alatar AA, Ahmad N, et al. Assessment of genetic fidelity in Rauvolfia serpentina plantlets grown from synthetic (encapsulated) seeds following in vitro storage at 4°C. Molecules. 2012;17:5050–5061.
- Faisal M, Alatar AA, Hegazy AK, et al. Thidiazuron induced in vitro multiplication of Mentha arvensis and evaluation of genetic stability by flow cytometry and molecular markers. Ind Crops Prod. 2014;62:100–106.
- Al-Bahrany A, Al-Khayri J. Micropropagation of grey mangrove Avicennia marina. Plant Cell Tissue Organ Cult. 2003;72:87–93.
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–477.
- Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987;19:11–15.
- Faisal M, Ahmad N, Anis M. An efficient micropropagation system for Tylophora indica: an endangered, medicinally important plant. Plant Biotech Rep. 2007;1:155–161.
- Nagarathnamma M, Sudarshana MS, Nirajan MH, et al. Rapid regeneration of Enicostemma littorale Blume from leaf and stem cultures. J Plant Interact. 2010;5:69–73.
- Ma G, Teixeira da Silva JA, Lu J, et al. Shoot organogenesis and plant regeneration in Metabriggsia ovalifolia. Plant Cell Tissue Organ Cult. 2011;105:355–361.
- Samir CD. Clonal propagation of dwarf raspberry (Rubus pubescens Raf.) through in vitro axillary shoot proliferation. Plant Growth Regul. 2004;4:179–186.
- Arumugam M, Panneerselvam R. Micropropagation and phenolic exudation protocol for Excoecaria agallocha-an important mangrove. Asian Pac J Tropical Biomed. 2012;2:S1096–S1101.
- Shahzad A, Ahmad N, Rather MA, et al. Improved shoot regeneration system through leaf derived callus and nodule culture of Sansevieria cylindrica. Biol Plant. 2009;53:745–749.
- Ahmad N, Faisal M, Anis M. Role of PGR on in vitro shoot propagation in Cyamopsis tetragonoloba L. (Taub.): a drought tolerant grain legume. Rend Fis Acc Lincei. 2013;24:7–12.
- Pati R, Chandra R, Chauhan UK, et al. In vitro clonal propagation of bael (Aegle marmelos Corr.) cv. CISHB1 through enhanced axillary branching. Physiol Mol Biol Plants. 2008;14:337–346.
- Negi D, Saxena S. Ascertaining clonal fidelity of tissue culture raised plants Bambusa balcooa Roxb. using inter simple sequence repeat markers. New Forests. 2010;40:1–8.
- Rathore MS, Chikara J, Shaik G, et al. Assessment of genetic stability and instability of tissue culture-propagated plantlets of Aloe vera L. by RAPD and ISSR markers. Biotech Appl Biochem. 2011;165:1356–1365.
- Yadav K, Aggarwal A, Singh N. Evaluation of genetic fidelity among micropropagated plants of Gloriosa superba L. using DNA-based markers–a potential medicinal plant. Fitoterapia. 2013;89:265–270.