?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The production of 2-keto-L-gulonic acid (2-KGA) during the conversion from L-sorbose to 2-KGA in the two-step fermentation of vitamin C can be improved by using an efficient companion strain Bacillus subtilis A9 to facilitate the growth of Ketogulonicigenium vulgare and the production of 2-KGA. Two optimization models, namely response surface methodology (RSM) and artificial neural network (ANN), were built to optimize the medium components for mixed-culture fermentation of 2-KGA. The root mean square error, R2 and the standard error of prediction given by the ANN model were 0.13%, 0.99% and 0.21%, respectively, while the RSM model gave 1.89%, 0.84% and 2.9%, respectively. This indicated that the fitness and the prediction accuracy of the ANN model were higher than those of the RSM model. Furthermore, using genetic algorithm (GA), the input space of the ANN model was optimized, predicting that the maximum 2-KGA production of 72.54 g·L−1 would be obtained at the GA-optimized concentrations of the medium components (L-sorbose, 92.5 g·L−1; urea, 10.2 g·L−1; corn steep liquor, 16 g·L−1; CaCO3, 3.96 g·L−1; MgSO4, 0.28 g·L−1). The 2-KGA production experimentally obtained using the ANN–GA-designed medium was 71.21 ± 1.53 g·L−1, which was in good agreement with the predicted value. The same optimization process may be used to improve the production during bacterial mixed-cultures fermentation by changing the fermentation parameters.
Introduction
The primary method of industrial vitamin C (Vc) production in China is via mixed-culture fermentation of L-sorbose for producing 2-keto-L-gulonic acid (2-KGA), the precursor of Vc.[Citation1–3] Mixed-culture fermentation is achieved by the cooperation of two microorganisms: Ketogulonicigenium vulgare (the acid-producing strain) and Bacillus megaterium (companion strain, or co-culture helper).[Citation4] Thus, in the light of current systems biology analysis, not only the individual physiological characteristics of the two strains, but also the interactions between them in this ecosystem have been the focus of research.[Citation5,Citation6] Studies have shown that the interactions between B. megaterium and K.vulgare are a synergistic combination of mutualism and antagonism.[Citation7] By reconstructing genome-scale metabolic models of K. vulgare [Citation8] and B. megaterium [Citation9] based on genome annotation and data from the literature and biochemical databases, we found that K. vulgare was deficient in nutrient biosynthetic pathways.[Citation10] To date, many studies on the construction of combinatorial expressions of key enzymes and the related co-factors in K. vulgare [Citation11] and using the proposed high-throughput method to screen target companions from large numbers of random mutants for the co-culture process of 2-KGA biosynthesis [Citation12] have become feasible. However, these studies remain at an exploratory level; they need time for practice, repetition and improvement. The major companion strain currently used in industry is B. megaterium. The conversion rates from L-sorbose to 2-KGA by current mixed-culture strains are difficult to enhance sufficiently. Therefore, scholars from different countries have focused on increasing technical innovations to screen for efficient strains with higher conversion rates to enhance the 2-KGA productivity [Citation13] and reduce industry costs. It is known that bacteria that have the potential to serve as companion strains include Bacillus megaterium, Bacillus thuringiensis, Bacillus cereus, Pseudomonas striata, Sporobolomyces roseus and others.[Citation14] In this study, B. subtilis A9 from fresh milk was studied. It exhibited good companion ability and was able to effectively improve the production of 2-KGA, which is a promising development in terms of application prospects.
A major goal in the modelling and optimization of biotechnological processes is to improve the system and increase the process efficiency without increasing the cost.[Citation15] One of the empirical modelling systems that are commonly used for development, improvement and optimization of complex processes is response surface methodology (RSM). By RSM, the experimental response(s) are fit to quadratic function(s) and the assessment is made of the effect of the independent variables, alone or in combination, on the process based on their relationships with the response(s). Despite the fact that RSM has many advantages and has been shown to be successful in a wide range of fermentation processes,[Citation16] there are some cases in which it may not prove applicable.[Citation17] Alternative options are approaches based on artificial intelligence, e.g. artificial neural network (ANN) and genetic algorithm (GA). They achieve data modelling based on mimicking different aspects of biological information processing and have been demonstrated to be useful in media optimization.[Citation18,Citation19] ANN offers a powerful solution for studying non-linear problems. In contrast to regression equations, which need to be given as the function, ANN is a mathematical model obtained through finite iterative computation reflecting the connections within the experimental data. GA is a global optimization algorithm based on natural selection and population evolution mechanisms. Through GA, the ANN model can be extended to global training to obtain an optimal algorithm.
Applications of ANN–GA technology in food science, environmental biotechnology and biochemical engineering have been reported,[Citation20] but to the best of our knowledge there are no reports on its application in the field of mixed-culture fermentation for the production of Vc. The aim of this work was to compare two optimization techniques, namely RSM and ANN coupled with GA, in optimizing the concentrations of medium components (L-sorbose, urea, corn steep liquor, CaCO3 and MgSO4) to maximize the production of 2-KGA, using B. subtilis A9 as the companion strain.
Materials and methods
Materials
The acid-producing strain of K. vulgare 25-B-1 and the companion strain of B. megaterium 2980 were provided by Northeast Pharmaceutical General Factory (Shenyang, China). The companion strain of B. subtilis A9 was screened and maintained in the Shenyang Agriculture University collection (Shenyang, China).
The seed culture medium contained the following: 20 g·L−1 L-sorbose; 2 g·L−1 glucose; 5 g·L−1 corn steep liquor; 1 g·L−1 urea and 1 g·L−1 CaCO3. The basic fermentation medium contained the following: 80 g·L−1 L-sorbose; 12 g·L−1 urea; 10 g·L−1 corn steep liquor; 5 g·L−1 CaCO3; 0.2 g·L−1 MgSO4 and 1 g·L−1 KH2PO4.
Methods
The acid-producing strain colonies and the companion strain colonies were selected, respectively, and put in sterile water. Then, acid-producing strain suspension was added into the companion strain suspension and was shaken to obtain a mixed-culture strain suspension. A sterile inoculation loop was used to streak the mixed-culture strain suspension onto blank slants. This step was repeated 15 to 20 times, until the surface of the slants became wet. Then the mixed-culture strain slants were cultivated upside down at 29 °C for 48–72 h. The seed culture inoculated from a mixed-culture strain slant was cultivated at 29 °C and 180 r min−1 for 24 h.
Fermentation medium (5 mL) was mixed with 10% (v/v) of seed culture in 50 mL flasks. The inoculated mixed culture was incubated at 29 °C for 48 h with orbital shaking aeration at 180 r min−1. The initial culture acidity was adjusted to pH 6.8–7.0.[Citation21] The concentration of 2-KGA was determined using the iodometric method as previously described.[Citation22] For medium optimization, concentrations of L-sorbose, urea, corn steep liquor, CaCO3 and MgSO4 were chosen according to an experimental design matrix (). The remaining medium components were kept constant.
Table 1. Design matrix and results obtained in the central composite design (CCD).
Design of CCD
The preliminary study [Citation23] indicated that L-sorbose, urea, corn steep liquor, CaCO3 and MgSO4 were the significant factors influencing the production of 2-KGA. The symbols and levels of these five variables are shown in . Using RSM and ANN models, the combined effect of these five factors was evaluated according to the experimental results of the central composite design (CCD).
RSM model
Design Expert 8.0 [Citation24,Citation25] was applied to conduct a regression analysis of the experimental data in . The least square method was used to fit the quadratic polynomial equation and establish a quadratic regression model as follows:where Y is the response value (production), β0, βi, βii and βij are the coefficients of the equation and Xi and Xj are the code values of the variables. The accuracy and general ability of the above polynomial model was evaluated by the regression coefficient (R2).
ANN model
A neural network is a computer program architecture for non-linear computations and it simulates the brain's learning process by mathematically modelling the network structure of interconnected node cells. The architecture of the ANN consisted of a feedforward network with three layers: one input layer with five inputs, which were the content of the L-sorbose, urea, corn steep liquor, CaCO3 and MgSO4 in the fermentation medium in , one hidden layer and an output layer that rendered the predicted production of 2-KGA. The back-propagation method was applied to establish the ANN model. The transfer function of the neurons in the hidden layer was the tansig, and the neurons in the output layer had a linear transfer function. At the same time, the trainbr method was used for training. When the mean squared error (MSE) reached 1 × 10−3, the network stopped training.
To evaluate the fitness and prediction accuracy of the RSM and ANN models, the root mean square error (RMSE), R2 and standard error of prediction (SEP) of the model were employed[Citation26]where Yi,e represents the experimental data, Yi,p is the predictive data and
is the mean value of the experimental data.
Optimization of GA
GA is a type of adaptive and global optimization probability search method, which is based on the principle of genetic variation and hybridization in biology. It is a new field which is emerging from the simulation of population evolutionary mechanisms in biological communities through a computer. The solution to a given problem can be considered as an individual coded by chromosome strings. The fitness function values of the individual are regarded as the evaluation index of individual quality. In the process of population evolution, three genetic and evolutionary operators are continuously applied, e.g. selection, crossover and mutation, in order to gradually reach optimal solutions until generating the global optimal solution. GA is especially suitable for complex and non-linear problems which cannot be solved by traditional search methods. The floating-point coding method was adopted to conduct the ANN simulation calculation of L-sorbose, urea, corn steep liquor, CaCO3 and MgSO4 during the fermentation process in their respective ranges. The model fitting data were considered as the fitness function of the GA for selection, variation and exchange. Higher production of 2-KGA corresponded to higher fitness.
The optimization of the neural network was done using the ‘ga’ function of MATLAB 8.0. The input parameters of the ga function were as follows: population type, double vector; population size, 20; crossover fraction, 0.8; elite count, 2; migration direction, forward; migration interval, 20; migration fraction, 0.2; generations, 100; stall generations, 50 and function tolerance, 1e-6.
Results and discussion
Impact of different companion strains on 2-KGA production
The experimental results showed that B. subtilis A9 performed better than B. megaterium 2980 in promoting 2-KGA production as the companion strain ().
Table 2. Comparison of 2-KGA-producing ability of K. vulgare with different companion strains.
RSM analysis
The regression fitting of the experimental data is shown in . According to the significance testing of analysis of variance (ANOVA) in , the regression model shows significant correlation (R2 = 0.8358), indicating that the regression equation can be used to fit the experimental data in . The ‘lack-of-fit’ value is 0.0030, which means the prediction value cannot give a thorough description of the process of 2-KGA fermentation. The ‘lack-of-fit F-value’ of 18.19 implies that the lack of fit is not significantly relative to the pure error. The value of ‘Model Prob>F’ is less than 0.05, which means that this item is significant at the probability level of 95%. The values χ1, χ3, ,
,
and
are the significant items in our study. The non-significant terms χ2 (urea), χ4 (CaCO3), χ5 (MgSO4), χ1χ2 (L-sorbose × urea), χ1χ3 (L-sorbose × corn steep liquor), χ1χ4 (L-sorbose × CaCO3), χ1χ5 (L-sorbose × MgSO4), χ2χ3 (urea × corn steep liquor), χ2χ4 (urea × CaCO3), χ2χ5 (urea × MgSO4), χ3χ4 (corn steep liquor × CaCO3), χ3χ5 (corn steep liquor × MgSO4), χ4χ5 (CaCO3 × MgSO4),
(L-sorbose)2 were excluded from the model. The regression equation is as follows:
Table 3. ANOVA of the fitted quadratic model.
Modelling of ANN
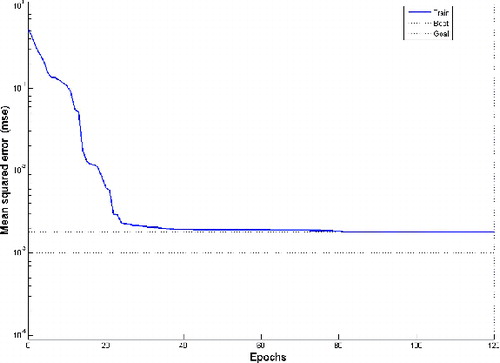
The experimental results used for training the network and respective experimental productions are given in . To avoid overfitting, the ANN architecture selected fewer neurons in the hidden layer on the sufficient condition of training accuracy. The network with 10 neurons in the hidden layer gave the lowest error. The performance of the network throughout the training until the final fitness is shown in . This figure illustrates the variation of the MSE of the fitness value with the number of generations.
Comparison of RSM and ANN
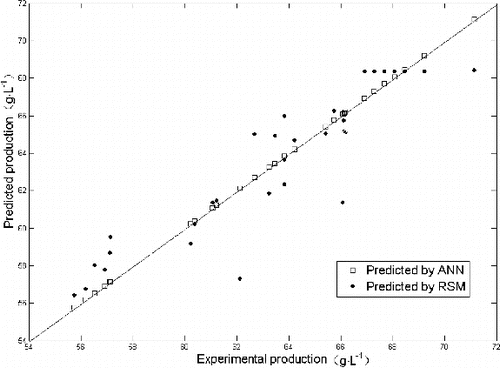
According to the results of the RSM and ANN, the RMSE, R2 and SEP of the ANN model were 0.13%, 0.99% and 0.21%, respectively, while the RMSE, R2 and SEP of the RSM model were 1.89%, 0.84% and 2.9%, respectively. In general, higher RMSE and R2 indicate better fitness between the model and experimental results, while lower SEP indicates better predictability and extrapolation of a model. The comparison results indicated that the fitness and the prediction accuracy of the ANN model were higher compared to those of the RSM model. As the mathematical regression equation which the RSM model established was a quadratic polynomial and had limited fitness ability, it was difficult to reflect the non-linear relationships among all factors of the 2-KGA fermentation process. shows the comparative parity plot for ANN and RSM predictions for the design. The ANN model needs no functions, unlike the regression equation, as it is a mathematical model obtained by using experimental results for limited iterative computations and reflects the inner relationship among experimental results. It has an extremely strong ability to perform non-linear processing. Thus, this experiment ultimately used the ANN model as the fitness function of GA to obtain the best combination of all factors.
GA optimization
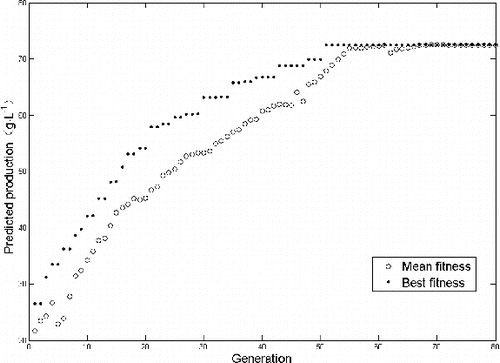
GA was implemented to optimize the fermentation medium to maximize the 2-KGA production by using the ANN as the fitness function. A population of individuals was randomly generated, and a fitness value was assigned to each individual by the ANN fitness function. The evolution process of GA is shown in . After 66 times of optimization calculation through GA, the maximum theoretical production of 2-KGA was 72.54 g·L−1. The optimal medium components were L-sorbose, 92.5 g·L−1; urea, 10.2 g·L−1; corn steep liquor, 16 g·L−1; CaCO3, 3.96 g·L−1 and MgSO4, 0.28 g·L−1. Under such cultivation conditions and after three repeated experiments, the average production of 2-KGA was 71.21 ± 1.53 g·L−1. These results indicated that the models of ANN were good for mixed-culture fermentation of Vc. Based on the obtained results, B. subtilis A9 could be recommended as a suitable companion strain in the production of Vc in the pharmaceutical and food industry.
Conclusions
This study demonstrated that B. subtilis A9 could serve as a companion strain in the two-step fermentation of Vc. ANN was successfully employed to model the complex fermentation process of 2-KGA which could inherently capture almost any form of non-linearity. A maximum 2-KGA production of 72.54 g·L−1 was predicted at the ANN–GA-optimized concentrations of medium components (L-sorbose, 92.5 g·L−1; urea, 10.2 g·L−1; corn steep liquor, 16 g·L−1; CaCO3, 3.96 g·L−1 and MgSO4, 0.28 g·L−1). The 2-KGA production experimentally obtained under the above conditions was 71.21 ± 1.53 g·L−1, which was in agreement with the predicted value. ANN–GA can not only effectively improve the 2-KGA production and substantially lower the culture medium cost, but can also dramatically decrease the experimental workload and shorten the study period. Further studies are needed by analysing complicated mixed-culture fermentation processes of bacteria.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Zhang J, Liu LM, Liu J, et al. Progress in biotechnological production of vitamin C. J Food Sci Biotechnol. 2008;27(5):1–7.
- Zhang J, Liu J, Shi Z, et al. Manipulation of B. megaterium growth for efficient 2-KLG production by K. vulgare. Process Biochem. 2010;45:602–606.
- Ai BL, Li JZ, Song JL, et al. Butyric acid fermentation from rice straw with undefined mixed culture: enrichment and selection of cellulolytic butyrate-producing microbial community. Int J Agric Biol. 2013;15(6):1075–1082.
- Feng S, Sun CB, Zhang ZZ, et al. Effects of Bacillus megaterium on growth and 2-KGA synthesizing of Gluconobacter oxydans in vitamin C two-step fermentation process. Chin J Microbiol. 1998;18(1):6–9.
- Zou W, Zhou MD, Liu LM, et al. Reconstruction and analysis of the industrial strain Bacillus megaterium WSH002 genome-scale in silico metabolic model. J Biotechnol. 2013;164(4):503–509.
- Ye C, Zou W, Xu N, et al. Metabolic model reconstruction and analysis of an artificial microbial ecosystem for vitamin C production. J Biotechnol. 2014;20(10):61–67.
- Zhou J, Ma Q, Yi H, et al. Metabolome profiling reveals metabolic cooperation between Bacillus megaterium and Ketogulonicigenium vulgare during induced swarm motility. Appl Environ Microbiol. 2011;77(19):7023–7030.
- Zou W, Liu LM, Zhang J, et al. Reconstruction and analysis of a genome-scale metabolic model of the vitamin C producing industrial strain Ketogulonicigenium vulgare WSH-001. J Biotechnol. 2012;161(1):42–48.
- Zou W, Liu LM, Chen J. Structure mechanism and regulation of an artificial microbial ecosystem for vitamin C production. Crit Rev Microbiol. 2013;39(3):247–255.
- Fan SC, Zhang ZY, Zou W, et al. Development of a minimal chemically defined medium for Ketogulonicigenium vulgare WSH001 based on its genome-scale metabolic model. J Biotechnol. 2014;169(10):15–22.
- Du J, Zhou J, Xue J, et al. Metabolomic profiling elucidates community dynamics of the Ketogulonicigenium vulgare–Bacillus megaterium consortium. Metabolomics. 2012;8(5):960–973.
- Zhu YB, Liu J, Du GC, et al. A high throughput method to screen companion bacterium for 2-keto-L-gulonic acid biosynthesis by co-culturing Ketogulonicigenium vulgare. Process Biochem. 2012;47(9):1428–1432.
- Takagi Y, Sugisawa T, Hoshino T. Continuous 2-keto-L-gulonic acid fermentation from L-sorbose by Ketogulonigenium vulgare DSM 4025. Appl Microbiol Biotechnol. 2009;82:1049–1056.
- Li Y, Zhou B, Liu YP, et al. Study on new strains in fermentation of vitamin C. J Microbiol. 2002;22(2):26–32.
- Baş D, Boyaci İH. Modeling and optimization I: usability of response surface methodology. J Food Eng. 2007;78:836–845.
- Banik RM, Santhiagu A, Upadhyay SN. Optimization of nutrients for gellan gum production by Sphingomonas paucimobilis ATCC-31461 in molasses based medium using response surface methodology. Bioresour Technol. 2007;98(4):792–797.
- Baş D, Boyaci İH. Modeling and optimization II: comparison of estimation capabilities of response surface methodology with artificial neural networks in a biochemical reaction. J Food Eng. 2007;78:846–854.
- Singh A, Majumder A, Goyal A. Artifical intelligence based optimization of exocellular glucansucrase production from Leuconostoc dextranicum NRRL B-1146. Bioresour Technol. 2008;99:8201–8206.
- Singh V, Khan M, Khan S, et al. Optimization of actinomycin V production by Streptomyces triostinicus using artificial neural network and genetic algorithm. Appl Microbiol Biotechnol. 2009;82(2):379–385.
- Almeida JS. Predictive non-linear modeling of complex data by artificial neural networks. Curr Opin Biotechnol. 2002;13(1):72–76.
- Lyu SX, Guo ZY, Pan J, et al. Effect of rare earth elements on vitamin C fermentation by mixed cultures. Int J Agric Bio. 2014;16(6):1135–1140.
- Jiang YY, Guo ZY, Zhang CG. Study on the purification of 2-keto-L-gulonate reductase and its physical, chemical and enzymic properties. Chin J Biotechnol. 1997;13(4):400–405.
- Gao M, Lv SX, Jin YN, et al. Optimization of fermentation conditions for two-step fermentation of vitamin C. Sci Tech Food Ind. 2012;14(33):235–238.
- Fang YY, Feng ZB, Zhang YX, et al. Optimization of fermentation conditions of tempeh by response surface analysis. Sci Tech Food Ind. 2009;4:168–170.
- Zhu T, Heo H, Row K. Optimization of crude polysaccharides extraction from Hizikia fusiformis using response surface methodology. Carbohydr Polymers. 2010;82(1):106–110.
- Wang X, Xu P, Yuan Y, et al. Modeling for gellan gum production by Sphingomonas paucimobilis ATCC 31461 in a simplified medium. Appl Environ Microbiol. 2006;72(5):3367–3374.