?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The development of ‘green’ technologies in nanoparticle synthesis is of considerable importance to broaden their biological applications. Cadmium sulphide nanoparticles are considered very promising in applied chemistry, bioscience and medicine. The aim of this study was to develop an efficient, easily reproducible and environmentally friendly method for biosynthesis of cadmium sulphide quantum dots based on the usage of mycelium of the basidiomycete fungus Pleurotus ostreatus. By incubating P. ostreatus mycelium with inorganic cadmium sulphate and sodium sulphide, we synthesized stable luminescent CdS nanocrystals. They showed absorption peaks at 453 nm (ultraviolet–visible spectrometry) and a main luminescent peak at 462 nm. Transmission electron microscopy revealed that the obtained quantum dots were of a spherical shape and predominantly from 4 to 5 nm in size. The electron diffraction pattern confirmed the wurtzite crystalline structure of the synthesized cadmium sulphide quantum dots. The obtained results confirm for the first time that the system based on basiodiomycete fungi could be considered promising for synthesizing semiconductor quantum dots.
Introduction
Biosynthesis of nanoparticles has been gaining more and more attention in the last few years. Nanoparticles, due to their small size, can modify the physicochemical properties of materials.[Citation1] It is well known that many physical properties of nanostructured semiconductors strongly depend on their size, shape and crystal structure. That is why, one of the main trends in nanomaterials chemistry attempts to throw light upon the factors and mechanisms that determine the size and shape of nanocrystals with the aim to develop well-controlled synthetic methods for adjusting the shape of nanocrystals.[Citation2]
For example, nanocrystalline cadmium sulphide (CdS) belongs to group II–VI semiconductors. Its band gap varies between 2.1 and 2.45 eV. The CdS thin films demonstrate promising properties for potential use in field effect transistors, light-emitting diodes, photocatalysis and biological sensors.[Citation3] In chemical and biological research, CdS quantum dots (QDs) appear to be superior to traditional fluorescent organic dyes and green fluorescent proteins by circumventing the limitations resulting from photobleaching, low signal intensity and spectral overlapping.[Citation4,Citation5] QDs are highly photostable, with broad absorption, narrow and symmetric emission spectra, slow excited-state decay rates and broad absorption cross-sections. Their emission colour depends on their size, chemical composition and surface chemistry, and can be tuned from the ultraviolet to the visible or near-infrared wavelengths.[Citation6] Due to these properties, CdS QDs have recently begun to attract increasing interest as candidate luminescent probes and labels in molecular histopathology, disease diagnostics, biological imaging, etc.[Citation7,Citation8]
The preparation of CdS QDs has been carried out using various methods, such as microwave heating, microemulsion synthesis and ultrasonic irradiation.[Citation9,Citation10] However, the chemical methods are complicated, outdated, costly, inefficient and have low productivity, produce hazardous toxic wastes raising environmental safety issues and human health concerns. Therefore, an alternative approach suggests the use of biological systems for synthesis of nanomaterials in order to produce nanoparticles at ambient temperature and pressure without requiring hazardous agents and generating poisonous by-products.[Citation11] Our previous studies demonstrated that living organisms have a unique potential for environmentally friendly extracellular production of CdS nanoparticles of different shapes and sizes.[Citation12–14]
Recently, it has been found that the ascomycetous fungus Fusarium oxysporum has several advantages for nanoparticles production because it has sulphoxide reductases, which are active in the medium in the presence of appropriate metal salts known to mediate the reduction of sulphate ions and can thus generate CdS nanoparticles extracellularly.[Citation15] On the other hand, fungal biomass is easy to grow in vitro. Fungi are excellent secretors of proteins compared to bacteria and actinomycetes, with a resultant higher yield of nanoparticles.[Citation16,Citation17]
As part of a larger research project, the aim of this study was to describe a newly developed [Citation14] ‘green’, fast and easily reproducible approach to biological synthesis of water-soluble CdS nanoparticles by Pleurotus ostreatus mycelium, and make a comparative analysis of their structural, morphological and optical features. To the best of our knowledge, this report is the first to confirm the successful exploitation of mycelium of a basidiomycetous fungus as the template for effective extracellular biosynthesis of CdS QDs.
Materials and methods
Preparation of Pleurotus ostreatus mycelium
The basidiomycete fungus Pleurotus ostreatus (Jacq.) P. Kumm. (strain 551) used in this study was obtained from the culture collection of fungi of the M.G. Kholodny Institute of Botany of the National Academy of Sciences of Ukraine. Cultivation of P. ostreatus was carried out in 100 mL Erlenmeyer flasks containing 50 mL of sterile (1 atm for 20 min) glucose yeast peptone (GYP) liquid medium (25.0 g/L glucose, 3.0 g/L peptone, 2.0 g/L yeast extract, 1.0 g/L KH2PO4, 1.0 g/L K2HPO4; 0.25 g/L MgSO4·7H2O; pH 5.6). Three discs (7 mm in diameter) of the mycelium were placed into each flask. Hereinafter, the mycelium was cultured superficially in a thermostat at 26 °С for 10 days. Then, the mycelium was washed in sterile distilled water (at least 10 times) to eliminate the residual culture medium. Subsequently, 50 mL of sterile deionized water was added to the purified mycelium and it was incubated at 26 °С for 4 days.
Synthesis of CdS quantum dots
In order to produce CdS QDs, 2 mL of 0.025 mol/L CdSO4 solution (Sigma–Aldrich, USA, 99.99% purity) was added into a flask containing fungal mycelium and incubated for 10 days at 26 °C. Hereafter, 500 μL of 0.5 mol/L Na2S (Sigma–Aldrich, USA, 98% purity) was added to the solution. After 1 week, the samples (2 mL) with colloidal solutions containing synthesized nanoparticles were centrifuged at 8000 r/min for 10 min (MiniSpin Eppendorf, USA). Then, the culture supernatants were carefully collected and filtered using nitrocellulose filter Millipore (USA) (pore diameter of 0.22 μm). The solution without adding cadmium sulphate and sodium sulphide was used as a control.
Ultraviolet–visible spectrophotometry
The ultraviolet (UV)–visible absorption spectra of CdS nanoparticles were measured using a Specord UV–VIS spectrophotometer (Analytik Jena AG; Germany). The absorption spectra of samples were recorded in standard 10 mm quartz cuvettes (transmission range 170–1000 nm). According to the protocol, the accuracy of recording the wave numbers was 20 cm−1. However, due to digital processing and random factors, the actual experimental accuracy was 80 cm−1. The optical density was determined with an accuracy of up to 1% of the length of the optical scale in the optical density range from 0 to 1.4. The spectrum, which was recorded by a chart recorder Specord UV VIS, was analysed by a computer scanner and converted into a jpeg file figure. Then, the resulting file was processed by the software package GetData converting the spectrum data into a numerical format of dat-file. The numerical data were processed by Origin Pro 8.0 software.
Evaluation of the size (d) of CdS nanoparticles was carried out by the following empirical formula [Citation18]:where λ (nm) is the position of the low-energy absorption band.
Luminescence of CdS quantum dots
Luminescence spectra were measured at room temperature, using a Cary Eclipse serial spectrophotometer (Varian Inc., Agilent Tech, USA). The highest resolution of this spectrophotometer was 1.5 nm and was determined by the apparatus function and the smallest gap width. The spectral gap width selected for the measurement was 5 nm. The accuracy of recording the wavelength was 0.05 nm and the inaccuracy of determining the intensity did not exceed 1%. The device software allowed to correct the spectra by taking into account the sensitivity curve and the spectral sensitivity of multiplier photocell used in a fluorimeter as well. Standard quartz cuvettes (1 cm × 1 cm × 3 cm) were used for spectral measurements. Excitation of the produced QDs was carried out by a mercury-vapour lamp at λ = 340 nm.
High-resolution transmission electron microscopy (HRTEM)
Characterization of CdS QDs was performed using a JEOL JEM-2100F electron microscope (Japan) with accelerating voltage of 200 kV. Each sample was dispersed ultrasonically for 1 min in order to separate individual particles, and some drops of the suspension were deposited onto carbon-coated copper grids. Experimental material was precipitated by evaporation and used for further studies. A total number of 450 nanoparticles per field of view were scored for the particle-size distribution histogram.
Electron diffraction spectroscopy (EDS)
Electron diffraction patterns for the CdS nanocrystals deposited on the carbon-coated copper grid were obtained using a JEOL JEM-2100F electron microscope (Japan) at electron beam energy E = 200 keV (wavelength of electrons λ = 0.27 nm). The localization of the beam on the sample was 200 nm. For these experiments, 0.5 mL of the colloidal solution of CdS nanoparticles was deposited onto carbon-coated grids.
Results and discussion
This study was a part of a larger research project on biosynthesis of CdS QDs using different organisms, namely bacteria,[Citation12] plants [Citation13] and basidiomycete fungi.[Citation14] In our previous research on exploitation of bacteria Escherichia coli as the matrix for CdS luminescent nanoparticle production, it was demonstrated that CdS QDs have an absorption and luminescence spectrum typical for such types of nanosized particles (). Using an extract from plant hairy root culture, it was revealed that biosynthesized QDs are of a spherical shape and a predominant size of 5–7 nm.[Citation13] The resulting QDs did not form aggregates but were heterodispersed. To further elaborate an alternative procedure for aqueous-based extracellular biosynthesis of CdS QDs, the fungus Pleurotus ostreatus was chosen. This edible wide-spread fungus is a source of biologically active compounds with medicinal properties [Citation19] and is very convenient for cultivation in vitro. Also, the extracellular secretion of certain enzymes from the mycelium has significant advantage in nanoparticle biosynthesis.[Citation16]. Using the same low-cost precursors[Citation12,Citation13] in a set of preliminary experiments, we were successful in extracellular biosynthesis of luminescent CdS nanoparticles by Pleurotus ostreatus mycelium.[Citation14] Hereby, our results confirmed that the incubation of CdSO4 and Na2S salts in sterile water for fungal mycelium growth over 17 days caused a change in the colour of the solution from white to pale yellow, indicating the formation of water-soluble CdS nanoparticles in it. Conversely, the incubation of both inorganic salts in water free of mycelium resulted in the formation of an unsoluble orange yellow pellet only (). The obtained CdS nanoparticals were collected by centrifugation, purified through nitrocellulose filter and dispersed in ultrapure water. These colloidal solutions of CdS nanoparticals were used in the following steps of our study to determine their spectral properties.
Table 1. Comparative analysis of CdS QDs synthesized by bacterial, fungal and plant matrices.
Figure 1. Colloidal solution containing CdS nanoparticles biosynthesized using (a) the fungal matrix and (b) control sample containing deionized water with CdSO4 and Na2S salts. Scale bar = 1 cm.

UV–visible adsorption spectra
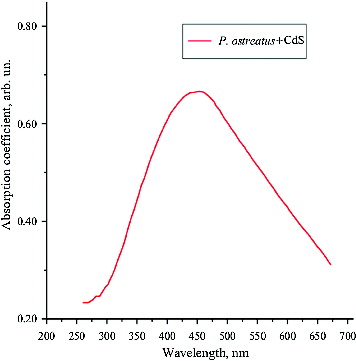
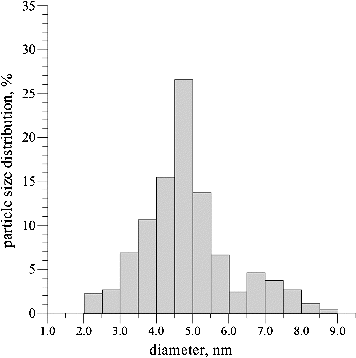
As a next step in our experiments, UV–visible adsorption spectrum analysis was performed, since semiconductor nanoparticles are known to have a characteristic energy absorption edge which is shifted toward the absorption band of the CdS macrocrystals in the shortwave region. This ‘blue’ shift is caused by quantum size effects and is indicative of the presence of semiconductor nanoparticles.[Citation19] The absorption spectra of the CdS QDs synthesized using P. ostreatus showed that the energy of the band gap for CdS macrocrystals is 2.42 eV, which corresponds to wavelength λ = 512 nm. The centre of the absorption peak was observed to correspond to wavelength λ = 453 nm (). The fact that the absorption band was rather wide suggests a considerable variation in the particle size. Based on the empirical formula used,[Citation18] the average size of the synthesized CdS nanoparticles was estimated to be 5.4 nm. It is noteworthy, however, that the highest per cent (about 27%) of biosynthesized particles had CdS nanocrystals with a size of 4.5 nm.
Luminescence spectra
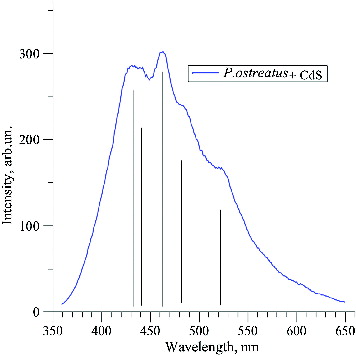
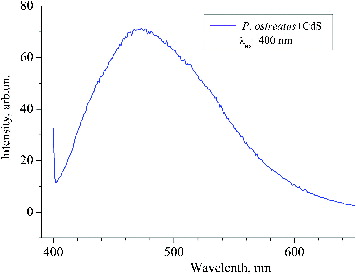
The luminescence spectrum of the synthesized CdS QDs () was typical for nanosized CdS.[Citation20] The luminescence maxima were found to correspond to wavelengths 431, 462 and 486 nm, which indicates excitonic bands of nanoparticles with different sizes. The maximum at λ = 524 nm indicates the presence of CdS crystals. It is established [Citation21] that, at excitation λ = 340 nm (3.65 eV), these luminescent peaks correspond to 1se–1sh transitions between dimensional quantization levels in CdS nanoparticles with different diameters. Based on the relationship between the energy of the optical transition 1se–1sh and the diameter of the CdS nanoparticles,[Citation21] the luminescence peaks at 431 (2.88 eV), 462 (2.68 eV) and 486 nm (2.55 eV) were determined to correspond to 1se–1sh transitions in cadmium sulphide nanoparticles with a diameter of 4.7, 5.2 and 6.8 nm, respectively. illustrates the photoluminescence of CdS QDs under 400 nm excitation. The observed maximum at 472 nm corresponded to radiative transitions that are caused by the presence of surface defects on CdS nanoparticles. The photoluminescence spectrum also gives information about the particle size distribution.
Figure 4. Photoluminescence of CdS quantum dots.Note: 400 nm excitation; clear luminescent band corresponding to 472 nm.
Based on spectral analysis, our previous research [Citation12] showed that the maximum luminescence peak of CdS nanocrystals was at 443 nm, which is typical for CdS nanoparticles synthesized using E. coli. It was observed that nanoparticles were aggregated but retained their luminescence ability for 10 days, 1 and 3 months after sample preparation.[Citation12] In addition, the results obtained in this study were consistent with our previous work [Citation13] in which the CdS QDs produced using a plant matrix showed several distinct luminescent peaks and a crystal lattice typical of nanoscale CdS.[Citation22]
High-resolution transmission electron microscopy
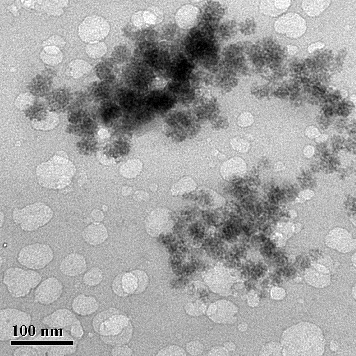
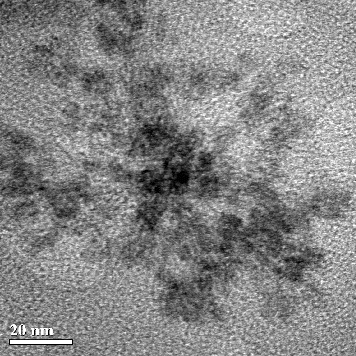
It was shown that CdS QDs form spherical conglomerates with a diameter of 40–70 nm over a one-month period. For further analysis of the obtained aggregates of CdS nanoparticles, their shape was characterized by HRTEM. A representative HRTEM image is given in . Within these clusters, individual CdS QDs were of a spherical shape, homogeneous morphology and a diameter in the range of 4–7 nm (). Interestingly, in our previous study with a bacterial system,[Citation12] we observed the presence of separate small CdS particles which formed larger aggregates over time, but they were spherical without any surface defects. When we used plant culture,[Citation13] there were generally no CdS conglomerates in the samples; only individual small nanoparticles remained in the solution for a long time. In addition, high-magnification HRTEM was used to reveal the crystal structure of synthesized CdS nanoparticles. shows the crystal lattice of CdS nanoparticles produced by P. ostreatus.
A histogram of the particle size distribution determined using the HRTEM images is presented in . The average particle size was 3.5–6 nm, but, as already mentioned, the predominant part of the synthesized CdS nanoparticles were 4.5 nm in size. The smallest clusters of QDs were 7.5–9 nm large. The size distribution determined based on the HRTEM data correlated well with the optical absorption spectra. In addition, according to electron microscopy data, it was determined that the synthesized nanoparticles were elliptic or spherical in shape.
Electron diffraction spectroscopy
The performed EDS analysis showed that the electron diffraction patterns of cadmium sulphide nanocrystals deposited on a carbon-coated copper grid demonstrated diffraction maxima corresponding to interplanar distances of d1 = 0.334 nm, d2 = 0.205 nm and d3 = 0.188 nm (). According to a study by Guinebretière,[Citation22] such data can indicate polycrystalline wurtzite CdS. In addition, EDS measurements indicated the presence of Cd and S at a level of about 30%. It should be noted that other elements were detected in the experiment samples as well, including О (47.02%), Si (10.5%), Fe (0.52%), P (3.62%), K (8.79%) (). In comparison, EDS data showed that the control samples, which were free from cadmium and sulphide ions, contained the same inorganic elements except Cd and S at the following concentrations: O (46.18%), Si (14/07%), Fe (1.74%), P (4.57%), K (7.71%). These samples contained water solution after fungus incubation. The high oxygen level can be explained by the incubation of the fungal mycelium in an aqueous medium and aerobic conditions for four days as mentioned above. The possibility for external penetration of other inorganic ions could largely be excluded, since the fungus biomass was cultivated aseptically and the GYP growth medium was removed, with the P. ostreatus mycelium being washed and incubated in sterile distilled water prior to experiments. Therefore, it could be considered that only products of fungal metabolism were a source of the above-mentioned inorganic elements.
Table 2. Elemental composition of control and experimental (with nanoparticles) samples determined by electron diffraction spectroscopy.
Comparative analysis
In a similar investigation on synthesis of CdS nanoparticles with the ascomycetous filamentous fungus Aspergillus versicolor, binding of cadmium with sulphur groups of the functionalized mycelia was confirmed by spectroscopic and microscopic techniques.[Citation23] Formation of 3 ± 0.2 nm-sized CdS nanoparticles was determined by HRTEM measurements.[Citation24] In another study,[Citation15] formation of extracellular CdS nanoparticles (5–20 nm in size) was achieved by the enzymatic reduction of sulphate ions by Fusarium biomass. Compared to our previous studies,[Citation12,Citation13] it could be concluded that all tested matrices (bacteria, plant culture and fungal mycelium) can be considered effective for extracellular, eco-friendly biosynthesis of CdS nanoparticles. The fastest rate of CdS nanoparticles production was achieved with the use of bacteria, which was associated with rapid growth rate of the bacterial culture in vitro.[Citation12] Spectral analysis showed that the nanoparticles are stabile, despite the enlargement of the small individual particles over time.
Although the plant-matrix-based approach for the production of CdS QDs described earlier proved successful [Citation13] and the method would be convenient in practice, the obtained nanocrystals were heterogeneous in size. Moreover, the hairy root culture is a biotechnologically engineered matrix developed through genetic transformation. Consequently, it is rather time-consuming to obtain and may not be reproducible in other laboratories. That is why, our focus of research shifted onto basidiomycetous fungi because they are common in nature, they are a source of secondary metabolites and enzymes and can be an effective matrix for CdS biosynthesis, as already discussed.[Citation14] Thus, in this study we confirmed that Pleurotus mycelium can be successfully used for extracellular biosynthesis of semiconductor nanoparticles, as shown, to the best of our knowledge, for the first time in our lab.[Citation14] It should be noted that extracellular production is preferable as it eliminates the requirement for cell lysis during harvesting, decreasing protein and other biomacromolecule contamination of the obtained nanocrystals.[Citation24] However, the exact extracellular mechanism of CdS formation still remains unclear and requires further detailed investigation.
There are contradictory opinions about the toxicity of QDs. Some data suggest that QDs have very low toxicity, whereas other reports demonstrate that QDs may exert negative influence on cell viability, growth and development.[Citation25] It was also found that biosynthesized CdS possess lower toxicity on plant, insect (our unpublished data) and non-human primate [Citation26] cells. However, the toxicity of CdS semiconductor nanoparticles is associated with their physicochemical properties. Systematic cytotoxicity assessment of QDs is of critical importance for their biological and biomedical applications.[Citation27] It has also been suggested that the cytotoxic effects are much smaller when QDs are only present in the external medium than if the QDs become ingested by cells.[Citation28] To solve the problem of toxicity, some authors have attempted to employ different methods for reduction of the particle size.[Citation29,Citation30]
It is also known that most commercial QDs have hydrophobic surface and therefore cannot be applied in vivo unless their surface is modified. Surface modification can increase the hydrophilicity of QDs and at the same time, reduce their toxicity.[Citation25] It is the extracellular biosynthesis of nanoparticles by means of living organisms that can result in the production of water-soluble, stable and low-toxic luminescent nanoscale crystals because of their additional surface coating with biomolecules. Hence, the growing opinion that extracellular biosynthesis of nanoparticles could prove the most promising approach in nanobiotechnology. Moreover, different fungi could be considered one of the most promising systems for such technological developments, since they excrete a high amount of protein and can accumulate metal ions through extracellular binding by metabolites and polymers, e.g. specific polypeptides, and by metabolism-dependent accumulation.[Citation17,Citation31]
Conclusions
The results from this study confirm that P. ostreatus could be considered an effective biological matrix for reproducible and low-cost nanotechnological transformations. The CdS nanoparticles synthesized by us had optical properties typical for QDs, such as specific absorption and luminescent spectra, an ellipsoid or a spherical shape as well as particle size distribution ranging predominantly from 4–5 nm. CdS nanoparticles produced by the ‘green’ approach described here could potentially find vast application in biological and biomedical research. In particular, they could be a powerful tool for fluorescent microscopy studies as well as for investigation of signal transduction pathways and biomolecular interaction within cells.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Mazumdar H, Haloi N. A study on biosynthesis of iron nanoparticles by Pleurotus sp. J Microbiol Biotech. Res. 2011;1:39–49.
- Yong KT, Sahoo Y, Swihart MT, et al. Shape сontrol of CdS nanocrystals in one-pot synthesis. J Phys Chem. 2007;111:12266–12274.
- Jandhav UM, Shinde MS, Patel SN, et al. Structural, optical and electrical properties of nanocrystalline cadmium sulphide thin films deposited by novel chemical route. Indian J Pure Appl Phys. 2014;52:39–43.
- Alivisatos AP. Semiconductor сlusters, nanocrystals, and quantum dots. Science. 1996;271:933–937.
- Konkar A, Lu SY, Madhukar A, et al. Semiconductor nanocrystal quantum dots on single crystal semiconductor substrates: high resolution transmission electron microscopy. Nano Lett. 2005;5:969–973.
- Rizvi SB, Keshtgar M, Seifalian AM. Quantum dots: basics to biological applications. In: Sattler KD, editor. Handbook of nanophysics: nanomedicine and nanorobotics. Boca Raton, FL: CRC Press; 2010. p. 1–11.
- Rizvi SB, Ghaderi S, Keshtgar M, et al. Semiconductor quantum dots as fluorescent probes for in vitro and in vivo bio-molecular and cellular imaging. Nano Rev. 2010;1:1–15.
- Jin S, Hu Y, Gu Z, et al. Application of quantum dots in biological imaging. J Nanomater. 2011;2011:1–13. doi.org/10.1155/2011/834139.
- Talapin DV, Poznyak SK, Gaponik NP, et al. Synthesis of surface-modified semiconductor nanocrystals and study of photoinduced charge separation and transport in nanocrystal-polymer composites. Physica E. 2002;14:237–241.
- Wang GZ, Chen W, Liang CH, et al. Preparation and characterization of CdS nanoparticles by ultrasonic irradiation. Inorg Chem Commun. 2001;4:208–210.
- Marchiol L. Synthesis of metal nanoparticles in living plants. Ital J Agron. 2012;7:274–282.
- Borovaya MN, Naumenko AP, Yemets AI, et al. Stability of the CdS quantum dots, synthesized by the bacteria Escherichia coli. Trans Natl Acad Sci Ukraine. 2014;7:145–151.
- Borovaya MN, Naumenko AP, Matvieieva NA, et al. Biosynthesis of luminescent CdS quantum dots using plant hairy root culture. Nanocsale Res Lett. 2014;9:1–7.
- Borovaya MN, Naumenko AP, Pirko Y, et al. Production of CdS quantum dots with the use of the fungus Pleurotus ostreatus. Trans Natl Acad Sci Ukraine. 2014;2:153–159.
- Ahmad A, Mukherjee P, Mandal D, et al. Enzyme-mediated extracellular synthesis of CdS nanoparticles by the fungus, Fusarium oxysporum. J Am Chem Soc. 2002;124:12108–12109.
- Sastry M, Ahmad A, Khan MI, et al. Biosynthesis of metal nanoparticles using fungi and actinomycetes. Curr Sci. 2003;85:162–170.
- Rai M, Yadav A, Gade A. Mycofabrication, mechanistic aspect and multifunctionality of metal nanoparticles – where are we? and where should we go? In: Mendez-Vilas A., editor. Current research, technology and education topics in applied microbiology and microbial biotechnology. Badajoz: Formatex Research Center; 2010. p. 1343–1354.
- Morozov PV, Grigor'ev EI, Zav'yalov SA. Rectification effect in poly-p-xylylene-cadmium sulphide graded nanocomposites. Phys Solid State. 2012;54:2291–2295.
- Synytsya A, Mícková K, Synytsya A, et al. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohydr Polym. 2009;76:548–556.
- Mirnaya TA, Asaula VN, Volkov SV, et al. Synthesis and optical properties of liquid crystalline nanocomposites of cadmium octanoate with CdS quantum dots. Phys Chem Solid State. 2012;13(1):131–135.
- Radchenko MV, Lashkarev GV, Sichkovskyi VI, et al. Nanomaterials based on CdS nanoparticles in polyethylene matrix. Inorg Mater. 2009;45(5):468–473.
- Guinebretière R. X-ray diffraction by polycrystalline materials. London: ISTE Ltd; 2007.
- Das SK, Shome I, Guha AK. Surface functionalization of Aspergillus versicolor mycelia: in situ fabrication of cadmium sulphide nanoparticles and removal of cadmium ions from aqueous solution. R Soc Chem Adv. 2012;2:3000–3007.
- Yang Z, Lu L, Berard VF, et al. Biomanufacturing of CdS quantum dots. Green Chem. 2015;17:3775–3782.
- Valizadeh A, Mikaeili H, Samiei M, et al. Quantum dots: synthesis, bioapplications, and toxicity. Nanoscale Res Lett. 2012;7:1–14.
- Chou LYT, Chan WCW. Nanotoxicology: no signs of illness. Nature Nanotechnol. 2012;7:416–417.
- Chen N, He Y, Su Y, et al. The cytotoxicity of cadmium-based quantum dots. Biomaterials. 2012;33:1238–1244.
- Parak WJ, Pellegrino T, Plank C. Labelling of cells with quantum dots. Nanotechnology. 2005;16(2):R9–R25.
- Hardman RA. Toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ Health Perspect. 2006;114:165–172.
- Gomes SAO, Vieira CS, Almeida DB, et al. CdTe and CdSe quantum dots cytotoxicity: a comparative study on microorganisms. Sensors. 2011;11:11664–11678.
- Holan ZR, Volesky B. Accumulation of cadmium, lead and nickel by fungal and wood biosorbents. Appl Biochem Biotechnol. 1995;53:133–146.