Abstract
Corrosion of implant components, including that of cover screws, has been reported as a possible etiological factor for implant failure. This study evaluated the presence of metallic particles in the soft tissues overlying two-stage implants after placing cover screws, by using a histological technique and energy-dispersive X-ray spectroscopy. The results showed large and small titanium particles in different layers of the soft tissue overlying the implant and cover screws in the majority of samples, with more particles near the cover screws. These particles might have contributed to the various degrees of inflammatory reactions detected in the different layers of the overlying soft tissues. Further studies on the corrosion of implants' metallic components are recommended, so that the implant failure rates could be minimized.
Introduction
Several factors contribute to the failure of implants, including occlusal overload, bacterial plaque, metal fracture due to fatigue and premature exposure. In addition, in the prognosis evaluation of implants, the response of the adjacent soft tissues is considered as an important determinant. Olmedo et al. [Citation1] evaluated the soft tissues surrounding the failed implants and fixtures and found macrophages laden with metallic particles, which indicated the role of corrosion in the implant failure process. In addition, it has been shown that the amount of metallic particles in macrophages at a short distance from the implants is higher than those at a greater distance from the implants.[Citation2] It has been reported that cover screws have a great role in tissue irritation. Macrophages, adjacent to cover screws, contain larger amounts of metallic particles,[Citation3] which might be attributed to differences in the alloys used in cover screws compared to implant fixtures, or the presence of different external colours used to colour-code the cover screws.
Clinicians should use cover screws with maximum care and accuracy, because the screws might get scratched during placement and removal, triggering corrosion. Furthermore, differences in the chemical composition between cover screws and fixtures necessitate more care during their handling.
Etiological factors, involved in reactive lesions associated with dental implants, have not been fully elucidated to date. A clinical study on reactive lesions in the mucosa around dental implants, i.e. inflammatory hyperplastic granuloma and peripheral giant cell granuloma, has been carried out.[Citation4] The results have shown the presence of metallic particles by using histopathological evaluations. Therefore, the etiology of these lesions might be the corrosion of the metallic structures and the subsequent reactions to the metallic particles.[Citation4] Histopathological evaluations are useful for detecting metallic particles in cells retrieved from the mucous tissues surrounding the implants; they are also useful screening tools for corrosion of dental implants.
Release of ions/particles might result in the production of pigments in the soft tissues surrounding the implant, referred to as metallosis, which finally leads to the precipitation and formation of metallic debris in the soft tissues of the body. A study evaluated the tissue reactions of the oral cavity mucosa in patients to submerged titanium implants with histological techniques and through biopsies from the oral mucosa overlying the implants in the vicinity of cover screws.[Citation5,Citation6] The results showed the presence of metallic particles in different sizes intercellularly and intracellularly, and phagocytes and macrophages in the connective and epithelial tissues.[Citation7]
The health of the gingival tissue and the absence of premature exposure of the fixture to the oral cavity are important factors in implants' maintenance. Inflammatory reactions to metals and corrosion products might induce a chain of reactions, leading to the exposure of the soft tissues overlying the cover screw, especially in individuals with a thin type of periodontium, finally resulting in the failure and loss of the implant. Given the ever-increasing use of dental implants, it is highly important to identify the risk factors and also the factors predicting the short-term and long-term successes of implant treatment. In this context, the present study was undertaken to determine the histological characteristics of the gingival tissue overlying two-stage dental implants after placing cover screws.
Materials and methods
Study design
The present in vitro study was carried out on biopsies taken from implant patients. Data were collected using histopathological evaluations.
Study samples
The samples in the present study consisted of biopsies collected from the gingival tissues overlying the cover screws. Histopathological findings were evaluated and reported at four levels: epithelium, the junction of the epithelium and the connective tissue, the connective tissue, and the junction of the connective tissue and the cover screw.
Sample size
Based on a study by Olmedo et al.,[Citation8] the sample size was calculated at minimum 81 biopsy samples at α = 0.05, β = 0.2 and mean standard deviation (SD) = 3.3, in order to determine a mean difference of 2.5. However, 96 biopsy samples were included in the present study.
Sampling technique
The biopsy samples were taken using a non-random technique, based on inclusion criteria. The samples were taken from the gingival tissues adjacent to the cover screws of implant fixtures. All the cover screws used were new. The minimum width of the attached gingiva in eligible areas was 3 mm.
Inclusion criteria
The following criteria were used for the inclusion of subjects in the present study: systemically healthy, absence of any background medical conditions, good oral hygiene based on plaque index, no history of any medications usage and absence of allergies to any metallic and non-metallic substances.
Exclusion criteria
Subjects with the following criteria were excluded from the study: smokers, patients with amalgam restorations, porcelain fused to metal or full-metal crowns in the vicinity of the area to be evaluated and the presence of an amalgam tattoo in the gingival tissue at the time of taking biopsies.
Procedural steps
In each patient only one implant site underwent a sampling procedure. All the samples were taken from subjects, who had a two-stage treatment plan for implant placement. The samples were taken at least three months after treatment from the same location the implant was expected to be exposed to the oral cavity. All the implants had the clinical and radiographic criteria of a successful implant at the time of sampling during the second stage. In none of the patients the cover screw had been exposed to the oral cavity.
A punch was used to take samples measuring 0.6 cm in size, with adequate thickness for the histopathological evaluation of the epithelium and the connective tissue. All the samples were over 0.3 cm in size.[Citation8]
The gingival tissue sample was placed in 10% formalin for 48 h, followed by rinsing with water, trimming, dehydrating in ethyl alcohol and immersing in paraffin. The samples underwent a staining procedure with hematoxylin and eosin (H&E) to evaluate the presence of metallic particles. Four levels were assigned to the samples: epithelium, the epithelium‒connective tissue junction, within the connective tissue and the connective tissue‒cover screw junction by a blinded pathologist based on histomorphometric corrections made by the Pedrazzoli technique.[Citation9] After confirmation of the presence of metallic particles in the samples by H&E staining, the nature of these metallic particles was determined by an energy-dispersive X-ray spectroscopy (EDS) using the dispersive X-ray analysis (EDAX) 9900 analyzer (EDAX, Paoli, PA, USA).
Inflammation severity
The inflammation severity was evaluated as follows:
No inflammation: absence of any histopathological signs of inflammation.
Mild inflammation: mild infiltration of leukocytes into the connective tissue; the cells are predominantly lymphocytes and macrophages.
Moderate inflammation: increased infiltration of leukocytes and an increase in the number and diameter of blood vessels in the connective tissue; the cells are predominately lymphocytes, macrophages and plasma cells, with a small number of neutrophils.
Severe inflammation: severe infiltration of leukocytes and an increase in the number and diameter of blood vessels; the cells are predominantly lymphocytes, macrophages, plasma cells and neutrophils.
It should be pointed out that the particle sizes were determined in a conventional manner under a light microscope (BX51/BX52 Olympus, Tokyo, Japan) and the particles were categorized into two groups:
metallic particles with large size: >30 µm;
metallic particles with small size: <10 µm.
Statistical analyses
Statistical analyses were carried out with SPSS 22. The frequencies and percentages of samples with signs of metallic particles and different degrees of inflammation along with other variables were determined at different histological levels. The results were analysed with non-parametric Mann–Whitney U test. Spearman's correlation coefficient was used to evaluate the correlation between the presence of metallic particles and the inflammation severity. The type I error was set at <0.05.
Ethical considerations
The patients signed an informed consent form to undergo a sampling procedure from the gingival tissue overlying the implants. This sampling procedure did not interfere with the treatment procedures of the patients.
Results and discussion
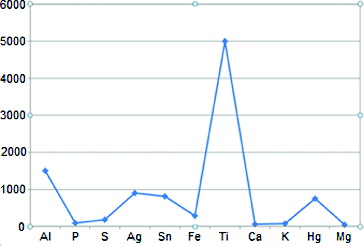
In the present study, the histopathological status of the gingival tissue overlying two-stage implants was evaluated subsequent to the placement of cover screws. The presence of metallic particles with different sizes was confirmed in all the soft tissues overlying the cover screw by histopathological evaluations under a light microscope. These metallic particles were confirmed to be titanium by the EDS technique ().
Figure 1. The result of the EDS test on a histological plate for the determination of the metallic particles' nature.
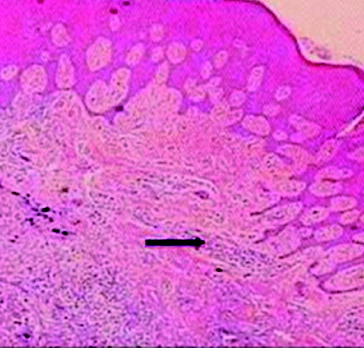
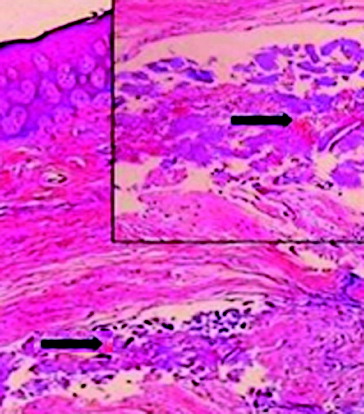
The maximum number of these metallic particles was detected at tissue layers near the cover screw, indicating a corrosion of the metallic components of the cover screw into the soft tissue overlying it. In addition, this conclusion was confirmed by more severe inflammatory reactions at tissue layers near the cover screw surface (–).
Figure 4. The presence of small metallic particles in the subepithelial connective tissue (upper arrow) and haemorrhagic areas in the connective tissue (lower arrow).
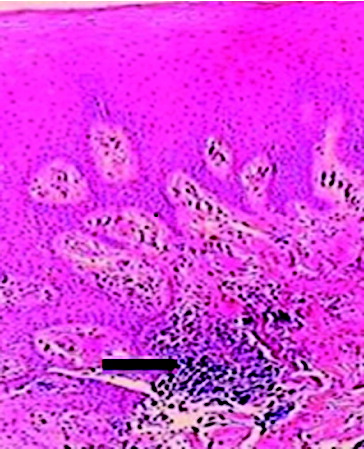
and present the inflammatory statuses of the samples in terms of the presence of metallic particles at the epithelium‒connective tissue junction and connective tissue‒cover screw surface junction, respectively.
Table 1. Evaluation of the inflammatory statuses of the samples in terms of the metallic particles detected at the epithelium‒connective tissue junction.
Table 2. Evaluation of the inflammatory statuses of the samples in terms of the metallic particles detected at the connective tissue‒cover screw surface junction.
In the majority of cases, there was a direct and significant relationship between the presence of metallic particles and the level of inflammation at the epithelium‒connective tissue junction, within the connective tissue and connective tissue‒cover screw surface junction (). For example, a significant relationship was detected between the presence of metallic particles and inflammation within the connective tissue (r = 0.472, P < 0.0001), and the presence of metallic particles and inflammation at the epithelium‒connective tissue junction (r = 0.386, P < 0.0001).
Table 3. Relationship between the presence of metallic particles in the samples and severity of inflammation at epithelium‒connective tissue junction, within the connective tissue and at connective tissue‒cover screw surface junction.
Based on the results of non-parametric Mann–Whitney U test, inflammation was significantly more severe in samples with metallic particles at epithelium‒connective tissue junction (p < 0.0001), within the connective tissue (p < 0.001) and at connective tissue‒cover screw surface junction (p < 0.0001).
Under normal conditions, the gingival tissue is composed of healthy oral epithelium and connective tissue along with varying percentages of inflammatory cells. Based on the results of the present study at all evaluated tissue levels, the presence of small (<10 µm) and large (>30 µm) metallic particles was confirmed, consistent with the results of the studies by Flatebo et al. [Citation10] and Olmedo et al.,[Citation8] indicating signs of release of metals from implants into the gingival tissues overlying the implants in a large number of patients.
One of the concerns in relation to dental implants is the local corrosion of the cover screw components into its overlying soft tissues and the possible complications, such as induction of allergic and inflammatory reactions, or the premature exposure of the implant into the oral cavity. Those complications are considered to be a result of the biological interactions of the body with the cover screw components, as a foreign material.
Despite the hard evidences in relation to the biocompatibility of implant components and their relative neutrality, there are suspicions that the implant manufacturers might be encouraged to use low-cost alloys in the manufacture of accessory components, such as the cover screws, in order to decrease the cost of the implants manufacturing process. The reason for this may be based on the justification that these components come into contact with body tissues for a short period of time, compared to the fixture itself; therefore, they are less important than the implant fixture.
In the studies carried out by Flatebo et al. [Citation10] and Olmedo et al.,[Citation8] the connective tissue adjacent to the cover screw exhibited a maximum number of metallic particles. In this study, the presence of metallic particles with different sizes in the most superficial layer of the soft tissue overlying the cover screw indicated that the corrosion of the components of the cover screw was not confined to the contact area of the overlying connective tissue. This means that the particles could be transferred to the more superficial layers of the epithelium. Lupi et al. [Citation11] reported that such a transfer of metallic particles is carried out by keratinocytes. Since the transfer of corrosion products is not confined to the contact area of the connective tissue with the cover screw and it is possible for these products to be transferred to other areas, including the superficial layers of the epithelium, it is also possible that these components have allergic properties, if they are not biocompatible. Therefore, hypersensitivity or inflammatory reactions might occur with varying severity in the adjacent tissues.
Mercan et al. [Citation12] evaluated the presence of titanium in gingival tissues after contact with the cover screws of dental implants for three months using laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) technique and reported a higher amount of titanium in the experimental group (50.4 µg/g) compared to the control group (37.1 µg/g). The control group consisted of gingival tissues from subjects, who had no dental implants and had undergone gingivoplasty. The results indicated that titanium had undergone corrosion in a short period of time. The results of the present study are consistent with those of the study above, in relation to the presence of metallic particles resulting from corrosion of the titanium alloy in the gingival tissue in a short period of time.
It should be pointed out that due to the dense distribution of particles in the histological samples around the cover screws in the present study and the large size of the observed particles, there is a very low possibility that these particles have a systemic origin. More probably, they have originated from the corrosion of metallic components of cover screws.
Based on the results of the present study, in relation to the presence of metallic particles with different sizes in the gingival tissue, the future implant science should be directed towards minimizing wear and corrosion of the metallic implant components, so that less corrosion products would be transferred to tissues overlying the implants. Metallic corrosion might decrease the resistance of metals to fatigue, which might even result in the fracture of the implant body.[Citation13,Citation14]
It has been reported that penetration of saliva between the superstructure (nickel–chromium–molybdenum alloy) and the implant (pure titanium) can result in galvanic corrosion due to the differences in the electrochemical potentials of these metals. Such process leads to the release of ions, such as nickel and chromium, from the alloys of the crown or bridges and implants into the tissues surrounding the implant, finally resulting in bone loss.[Citation15]
In the present study, some samples exhibited tissue mineralization areas. They are supposed to consist of mineralized particles resulting from the bone chips remained after the drilling stage or produced during bone morphogenic induction procedure during local formation of bone. In this context, such a phenomenon was observed in 9 samples (9.4%) at epithelium‒connective tissue junction, in 16 samples (16.7%) within the connective tissue and in 28 samples (29.2%) at connective tissue‒cover screw surface junction.
The authors believe that one of the advantages of the present study, over the similar previous studies, was the evaluation and determination of the inflammation severity in the samples. Based on the results of the present study, mild and moderate inflammations were detected in 63 (65.62%) and 32 (33.3%) samples from the epithelium‒connective tissue junction, respectively (). Mild, moderate and severe inflammation were observed in 57 (59.4%), 32 (33.3%) and 5 (5.2%) samples from the connective tissue‒cover screw surface junction, respectively (). On the other hand, the inflammation was significantly more severe in samples with metallic particles at epithelium‒connective tissue junction, within the connective tissue and connective tissue‒cover screw surface junction.
Another advantage of the present study was the use of the EDS technique to determine the exact nature of the metallic particles in the samples. However, a disadvantage of this technique was its inability to determine the nature of particles measuring under 1 µm. The authors believe that one of the factors affecting the induction of immunologic reactions is the extent of trauma inflicted on the gingival tissue during the different surgical steps of implant placement, including incision, retraction and suturing steps. Such confounding factors could not be controlled in the present study, because the implants had been placed by different surgeons and dental practitioners.
Future studies should take into account the type of the periodontium of the subjects as a confounding factor because thick and thin periodontium can affect the incidence and severity of the biologic reactions after implant placement. In this way, inflammatory reactions can be minimized after implant placement.
Cover screws in different implant systems are marketed with colour for the following reasons:
to decrease corrosion of the metallic components of the cover screw into the overlying gingiva;
to prevent osseointegration around the metallic structure and to facilitate its unscrewing during the second-stage surgery;
to classify them based on size with different colour-coding designation.
Therefore, it should be pointed out that this coloured layer itself might lead to chemical reactions during its placement in the biological environment of the body by releasing some of its constituents into the overlying gingival tissue.
In the research conducted by Wilson et al.,[Citation16] the microscopic analysis of soft tissue biopsies taken from around dental implants with cemented restorations suffering from peri-implantitis revealed a mixture of subacute and chronic inflammation immunologic factors, dominated by plasma cells. Foreign bodies primarily consisting of titanium and dental cement were found to be associated with an inflammatory infiltrate. In 34 of the 36 biopsies analysed in this study, the results revealed this initial hypothesis. Corroded dental titanium particles around peri-implant tissue can lead to activation of peri-implantitis process. Anyway, further studies need to be done until the accuracy of this hypothesis is proved.[Citation16]
Bruno et al. [Citation17] hypothesized that due to corrosion, the dental titanium implant's surface can be a potential source of the release of microsized particles (MPs) and nanosized particles (NPs) into the body's biological environment. This work sought to evaluate the biokinetics of different sized titanium dioxide particles (TiO2) and their potential to cause organ's cell damage. Wistar rats were intraperitoneally injected with 150, 10 or 5 nm TiO2 elements. The presence of TiO2 particles was evaluated in histological specimens of the liver, lung and kidney and in blood cells at 3 and 12 months. This study indicated if corroded titanium's particles were present around the peri-implant tissue. Achieving entrance into the biological environment and other distant organs could have probable adverse effects on cells and the physiology of the organs. Nowadays, the use of dental implants in fully and partially edentulous patients is spreading very fast. This is why, the implementation of more clinical and laboratory studies for the detection of any adverse effects of titanium's particles on the body's cells is highly suggested.[Citation17]
Royhman et al. [Citation18] evaluated the corrosion effect of nicotine on Ti-6Al-4V under physiological conditions. It was hypothesized that nicotine in artificial saliva would have an adverse side effect on the corrosion of Ti-6Al-4V. As it has been clearly shown, cigarette smoking is one of the most important risk factors for dental implant's failure. The accurate mechanism of the adverse effects of smoking in decreasing the implant success and survival rate is still not obvious. The results of this study can probably describe the relation of titanium particles' corrosion with dental implant failure. This research may assist in formulating strategies to improve dental implant success and survival rates in patients, who consume nicotine products routinely.[Citation18]
Flatebo et al [Citation19] demonstrated the presence of corrosion titanium particles in the tissue adjacent to Ti cover screws. However, Ti particles may be released during insertion of the fixture and may be retained in the covering soft tissue. Accumulation of large metal particles in the peri-implant soft tissue surrounding the titanium fixture with screws has been previously demonstrated by microscopy and by scanning electron microscopy.[Citation19]
Conclusions
Histological evaluation of the gingival tissue overlying the cover screw in the two-stage implant placement revealed the presence of small and large metallic particles in the gingival tissue after placement of the cover screw in a number of samples. The use of EDS method to determine the nature of metallic particles has some advantages, such as positive cost–benefit ratio, less time consumption, bigger accuracy and safety. Therefore, further evaluation of the biological implications, caused by the presence of metals in the gingival tissues overlying dental implants, is suggested.
This article was originally published with errors. This version has been corrected. Please see Erratum (http://dx.doi.org/10.1080/13102818.2015.1093284).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Olmedo D, Fernández MM, Guglielmotti MB, et al. Macrophages related to dental implant failure. Implant Dent. 2003;12(1):75–80.
- Olmedo DG, Duffo G, Cabrini RL, et al. Local effect of titanium implant corrosion: an experimental study in rats. Int J Oral Maxillofac Surg. 2008;37(11):1032–1038.
- Olmedo D, Tasat D, Evelson P, et al. Biological response of tissues with macrophagic activity to titanium dioxide. J Biomed Mater Res A. 2008;84(4):1087–1093.
- Olmedo D, Paparella M, Brandizzi D, et al. Reactive lesions of peri-implant mucosa associated with titanium dental implants: a report of 2 cases. Int J Oral Maxillofac Surg. 2010;39(5):503–507.
- Black J, Sherk H, Bonini J, et al. Metallosis associated with a stable titanium-alloy femoral component in total hip replacement: a case report. J Bone Joint Surg. 1990;72(1):126–130.
- Bullough PJ. Metallosis. J Bone Joint Surg. 1994;76(5):687–688.
- Olmedo D, Paparella M, Brandizzi D, et al. Response of oral mucosa associated to titanium implants. J Dent Res. 2007;86, Spec Iss Letter B: 83.
- Olmedo DG, Paparella ML, Spielberg M, et al. Oral mucosa tissue response to titanium cover screws. J Periodontol. 2012;83(8):973–980.
- Kärrholm J, Frech W, Nivbrant B, et al. Fixation and metal release from the Tifit femoral stem prosthesis: 5-year follow-up of 64 cases. Acta Orthop. 1998;69(4):369–378.
- Flatebø RS, Johannessen AC, Grønningsaeter AG, et al. Host response to titanium dental implant placement evaluated in a human oral model. J Periodontol. 2006;77(7):1201–1210.
- Lupi SM, Zaffe D, Rodriguez y Baena R, et al. Cytopathological and chemico-physical analyses of smears of mucosa surrounding oral piercing. Oral Dis. 2010;16(2):160–166.
- Mercan S, Bolukbasi N, Bolukbasi M, et al. Titanium element level in peri-implant mucosa. Biotechnol Biotechnol Equip. 2013;27(4):4002–4005.
- Adya N, Alam M, Ravindranath T, et al. Corrosion in titanium dental implants: literature review. J Indian Prosthodont Soc. 2005;5:126–131.
- Nikolopoulou F. Saliva and dental implants. Implant Dent. 2006;15(4):372–376.
- Tagger Green N, Machtei E, Horwitz J, et al. Fracture of dental implants: literature review and report of a case. Implant Dent. 2002;11(2):137–143.
- Wilson TG Jr, Valderrama P, Burbano M, et al. Foreign bodies associated with peri-implantitis human biopsies. J Periodontol. 2015;86(1):9–15.
- Bruno ME, Tasat DR, Ramos E, et al. Impact through time of different sized titanium dioxide particles on biochemical and histopathological parameters. J Biomed Mater Res A. 2014;102(5):1439–1448
- Royhman D, Dominguez-Benetton X, Yuan JC, et al. The role of nicotine in the corrosive behavior of a Ti-6Al-4V dental implant. Clin Implant Dent Relat Res. Forthcoming 2015.
- Flatebø RS, Høl PJ, Leknes KN, et al. Mapping of titanium particles in peri-implant oral mucosa by laser ablation inductively coupled plasma mass spectrometry and high-resolution optical darkfield microscopy. J Oral Pathol Med. 2011;40(5):412–420.