Abstract
The present study investigated the effect of various concentrations and combinations of plant growth regulators on callus induction, shoot proliferation and root regeneration of three potato (Solanum tuberosum L.) cultivars by using potato nodal and leaf explants under long-day conditions. The minimum days for callus induction (8.33 d), the maximum callus formation percentage (87.50%), the largest callus diameter (1.90 cm) and the maximum callus weight (2.04 g) were recorded on 1.0 × Murashige and Skoog (MS) medium containing 3.0 mg L−1 benzyl amino purine (BAP) + 2.0 mg L−1 naphthalene acedic acid (NAA). Nodal explants demonstrated a better performance for callus induction compared to leaf segments. Nodal and leaf derived calli differentiated into shoot promordia when subcultured on MS media supplemented with different concentration of BAP, kinetin and thidiazuran as well as gibberellic acid (GA3) and NAA. The maximum shoot regeneration percentage (90.00%) and the maximum number of shoots (6.42) were obtained on 1.0 × MS medium containing 2.0 mg L−1 BAP + 0.25 mg L−1 GA3.
Introduction
Plant tissue culture is the one of the most powerful tools for induction of fast crop improvements in modern plant breeding age. Recent developments in plant science have clearly shown that biotechnological approaches have contributed the most for the improvements in potato (Solanum tuberosum L.) compared to other crops.[Citation1] Potato callus induction and regeneration through nodal and leaf cuttings is an efficient way for mass propagation of potato plantlets under in vitro conditions. Reliable callus induction and subsequent plant regeneration by plant growth regulators (PGRs) accelerate the multiplication of young and strong plantlets throughout the year without depending on the season. This allows a fast commercial propagation of new potato cultivars. Callus culture can be used to study the stress physiology and genetic improvement of potatoes at the cellular level and also to select mutants in vitro. This would enable the selection of new improved potato lines with better stress tolerance to be used in potato breeding programmes.[Citation2,Citation3] Studies have been carried out to enhance callus induction and subsequent plantlet regeneration from callus. They have investigated the factors affecting the plant regeneration by using different potato explants, like tuber discs, petioles with intact leaflets, leaves, meristems, anthers and nodal or internodal segments of the stem.[Citation4,Citation5]
The process of shoot induction, either through direct or indirect organogenesis is genotype dependent, therefore, the establishment of efficient protocols for callus induction for each potato cultivar is a prerequisite.[Citation6] Previous studies indicate that callus induction and subsequent plantlet regeneration in potato require the presence of appropriate amount and various concentrations of auxins and cytokinins alone or in combination with each other in Murashige and Skoog (MS) medium.[Citation7,Citation8] Theoretically, it has been postulated that equal amounts of auxin and cytokinin promote callus induction; however, in practice, this differs to a great extent, due to variations in the endogenous levels of phytohormones in individual plants.[Citation9]
6-Benzylaminopurine (BAP) is beneficial for its effect on callogenesis, followed by organogenesis of potato; it brings significant improvement in multiple shoot inductions, when used in moderate concentrations.[Citation10] It has been suggested that naphthalene acetic acid (NAA) is essential for callus initiation and callus induction. MS medium containing the combination of NAA and BAP produced the highest callus development in short time and the highest percentage of shoot regeneration from leaf [Citation7] and internodal explants [Citation11] of potato. It has been reported that the combination of 6-furfurylamino-purine (kinetin; KIN) + NAA is more effective for shoot regeneration from internodal derived calli.[Citation8]
Since it was revealed in many studies that the combination of cytokinins (BAP and kinetin) and auxins [NAA, indole-3-acetic acid (IAA) and indole-3-butyric acid (IBA)] produced better results compared to the use of auxins or cytokinins alone,[Citation7,Citation8] the present study aimed to establish a suitable combination of BAP and NAA with kinetin, IAA and IBA to promote callus induction under long-day conditions. Therefore, the purpose of the present study was to determine an effective protocol for callus induction and rapid plant regeneration from leaf and nodal cutting explants of potato cultivars Caspar, Pasinler and Granola under 16 h long day-conditions.
Materials and methods
Preperation and concentrations of plant growth regulators
1.0 × MS medium (MS medium without any other chemicals) supplemented with 3% (w/v) sucrose and 0.8% (w/v) agar was used in the study. The pH was adjusted to 5.7 ± 0.1 by using 1 mol L−1 HCl or 1 mol L−1 NaOH after adding all medium components, except agar. The media were prepared as follows:
For the callus induction experiment – treatment (T) T0: no hormone, T1: 3.0 mg L−1 BAP + 2.0 mg L−1 NAA, T2: 3.0 mg L−1 BAP + 2.0 mg L−1 IBA, T3: 3.0 mg L−1 BAP + 2.0 mg L−1 KIN, T4: 3.0 mg L−1 BAP + 2.0 mg L−1 IAA, T5: 3.0 mg L−1 NAA + 2.0 mg L−1 BAP, T6: 3.0 mg L−1 NAA + 2.0 mg L−1 IBA, T7: 3.0 mg L−1 NAA + 2.0 mg L−1 KIN, T8: 3.0 mg L−1 NAA + 2.0 mg L−1 IAA.
For the shoot regeneration experiment – T0: no hormone,T1: 2.0 mg L−1 BAP + 0.25 mg L−1 gibberellic acid (GA3), T2: 2.0 mg L−1 KIN + 0.25 mg L−1 GA3, T3: 2.0 mg L−1 thidiazuron (TDZ) + 0.25 mg L−1 GA3, T4: 2.0 mg L−1 BAP + 0.25 mg L−1 NAA, T5: 2.0 mg L−1 KIN + 0.25 mg L−1 NAA, T6: 2.0 mg L−1 TDZ + 0.25 mg L−1 NAA.
For the root regeneration experiment: T0: 0.1 mg L−1 GA3, T1: 0.5 mg L−1 NAA + 0.1 mg L−1 GA3, T2: 0.5 mg L−1 IBA + 0.1 mg L−1 GA3, T3: 1.0 mg L−1 NAA + 0.1 mg L−1 GA3 T4: 1.0 mg L−1 IBA + 0.1 mg L−1 GA3.
Since PGRs are thermolabile, these were not autoclaved; they were filter sterilized by passing through 0.2 μm Millipore filters (Schleicher & Schuell, FP 30/0.2 CA-S; 0.2 μm; 7 bar max) inside a laminar flow cabin before they were added to the medium. The other constituents were sterilized by autoclaving at 121 °C for 15 min and 104 kPa pressure.
Plant materials and micropropagation of explants
Most of the potato callus studies in vitro have a three-stage regeneration system. The first stage stimulates the callus formation, the second stage is designed to induce de novo shoot outgrowth and the third stage includes root regeneration and acclimatization to ex vitro conditions. Therefore, three experiments were conducted: one on in vitro callus induction, one on regeneration of plantlets from callus culture and another one on rooting of three potato cultivars. Stem node and leaf segments of in vitro grown potato cultivars Pasinler (locally improved and registered mid-early maturing cultivar), Granola (mid-late maturing) and Caspar (late-maturing) were used for callus induction and plant regeneration. Binodal (hereinafter called stem node) cuttings about 5–7 mm long and leaf explants dissected from developed plants were aseptically cultured in the 1.0 × MS medium containing 60 explants per treatment, with 10 explants per replicate. Only the first five internodes from the top of the plantlets were excised. Each replicate consisted of 10 Pyrex glass tubes and all experiments were replicated six times. Explants were kept under 1000 lux light intensity with 16 h of day light (at a temperature of 24 ± 2 °C) photoperiod for six weeks. After a sufficient callus induction, well-developed calli were selected and subcultured on freshly prepared and sterilized MS medium supplemented with the combinations of BAP, KIN, TDZ and GA3 or NAA for shoot regeneration. For shoot initiation, the cultures were incubated under 22 ± 2 °C temperature and 2500 lux white light. Regenerated shoots were rooted on MS medium supplemented with IBA and NAA with a lower concentration of GA3. After the first callus and shoot initiation, observations were recorded and the other callus and shoot formation parameters were recorded at the end of the six weeks. When the shoots grew to 3–4 cm in length, they were rooted aseptically, separating single shoots on freshly prepared root induction medium containing 0.5 mg L−1 and 1.0 mg L−1 NAA or IBA + 0.25 mg L−1 GA3. The rooted plantlets successfully acclimatizated to ex vitro conditions and then they were eventually transferred to the greenhouse. For the acclimatization, the rooted plantlets were removed from the culture bottles, washed throughly to remove the adhering agar residues from roots and transplanted to small pots containing sterilized soil, sand and manure mixture (2:1:1). Plants were covered with transparent bottles to ensure high humidity. They were irrigated regularly and were kept under controlled conditions for three weeks. Later on, the plantlets were transferred to shaded greenhouse, where the plants showed morphologically uniform and normal leaf form, shape and growth pattern.
Observed parameters
The observed parameters for the callus induction experiment were degree of callus formation, texture and colour of callus, days for callus induction, callus induction percentage (%), diameter (cm) and weight (g) of callus. The observation of the number of days till the callus initiation was started following the explants' inoculation, and the other data were recorded six weeks later. The observed parameters for the shoot regeneration experiment were shoot regeneration percentage (%), days for shoot induction, length (cm) of shoots and number of shoots, nodes and leaves. The observed parameters of the root induction experiment were root induction percentage (%), days for root induction, number and length (cm) of roots.
Statistical analysis
A completely randomized design was used for the evaluation of the three cultivars, two explant sources, nine (for callus induction), seven (for shoot regeneration) and five (for root induction) PGR combinations with six replications. Data were subjected to analysis of variance and the means were separated by Duncan's multiple range test using SPSS software. P < 0.05 was considered statistically significant. The interaction effect of all applications was also determined for each study.
Results and discussion
The effects of different cultivars, explant sources, combinations of PGRs and their interactions on callogenesis are presented in and . There was a wide range of variations in days for callus initiation, callus induction percentage (%), callus diameter (cm) and callus weight (g), degree of callus formation, texture of callus and callus colour, depending on various concentrations of auxins and cytokinins, explant source (node or leaf) and cultivars (P < 0.01). The results showed that there was a remarkable and pronounced variation among calli, depending on hormone applications. The effects of PGRs, cultivars and explant sources on callus induction and shoot proliferation are described below.
Table 1. Effects of different explant sources and plant growth regulators on callus formation.
Table 2. The effect of plant growth regulators on callus induction parameters.
Callus formation, texture and colour
The final data of the experiment were taken after six weeks of culture. The experimental results clearly showed that the callus induction percentage, texture, colour and degree of callus formation, days for callus initiation, diameter and weight of callus, differed, based on the extent of interaction between the combinations of PGRs, explant sources and potato cultivars. The callus induction percentage and friability of the produced callus varied widely between explants. The callus formation from node explants was more pronounced compared to the callus formation from leaf explants ( and ).
The application of cytokinins affected callogenesis by resulting in decrease of the cell wall lignification, facilitating callus initiation and growth in vitro. It has been observed that callus proliferation usually started from the cut surface of the explant and finally covered the whole explant.[Citation12] At the beginning, the colours of calli ranged from colourless to yellowish and later turned into light green. After two weeks of incubation, the colours of calli changed from light green to dark green. The colour of the nodal explant derived callus was also darker compared to leaf explants derived callus; dense and dark green calli were observed on 3.0 mg L−1 BAP + 2.0 mg L−1 NAA and 3.0 mg L−1 NAA + 2.0 mg L−1 BAP, including calli induced on regeneration medium for stem node explants. However, yellowish light green and friable calli were noted on leaf derived explants () with hyperhydric look. Calli with hyperhydric exudates induced necrosis soon after. The growth of some calli showed high lignification, including of their hard texture, whereas others were embryogenic and separated easily into small fragments (). Differently coloured and structured calli from various explant sources from number of cultivars have been reported by Ehsandar et al. [Citation13] and Abd-Elaleem et al.[Citation14] Iqbal et al. [Citation15] obtained the most necrotic and brownish calli on cv. Desiree, whereas, the most colourless calli were obtained on cv. Cardinal. They suggested that dark green and brownish calli have a compact structure and a good regeneration ability, and could be used easily for shoot proliferation.[Citation15]
Effects of plant growth regulators on callus formation
Days for callus initiation, percentage of callus formation, callus diameter and callus weight were 8.33–24.04 d, 35.83%–87.50%, 0.25–1.90 cm and 0.30–2.04 g on different combinations of PGRs, respectively. MS medium containing 3.0 mg L−1 BAP + 2.0 mg L−1 NAA induced callusing in minimum days (8.33 d), followed by 3.0 mg L−1 NAA + 2.0 mg L−1 BAP (9.83 d), 3.0 mg L−1 BAP + 2.0 mg L−1 KIN (11.92 d) and 3.0 mg L−1 NAA + 2.0 mg L−1 KIN (12.88 d). MS medium supplemented with 3.0 mg L−1 BAP + 2.0 mg L−1 NAA and 3.0 mg L−1 NAA + 2.0 mg L−1 BAP concentrations was also found to be effective on the callus induction percentage (87.50% and 83.33%), increasing the callus diameter (1.90 and 1.59 cm) and callus weight (2.04 and 1.81 g), respectively. The treatment of 2.0 mg L−1 IAA with 3.0 mg L−1 BAP or 3.0 mg L−1 NAA took maximum days for callusing (21.96 and 24.04 d) with the minimum callus induction percentage (40.00% and 35.83%) and callus diameter (0.33 and 0.25 cm), and with a mean callus weight of 0.39 and 0.30 g, respectively. No callus induction was noted on culture medium without any PGRs (). The combination of 2.0 mg L−1 KIN with 3.0 mg L−1 BAP or 3.0 mg L−1 NAA had a significant effect on callus induction, suggesting that kinetin, in combination with BAP or NAA, improved the callogenesis. However, the interaction of IAA with BAP or NAA was found ineffective on callogenesis ( and ).
Khatun et al. [Citation16] obtained 83.33% callus formation on MS medium containing 5.0 mg L−1 BAP. Yasmin et al. [Citation7] reported 95% callus induction percentage and 8.13 d minimum time for callus induction on MS medium containing 2.5 mg L−1 NAA + 2.0 mg L−1 BAP. Nasrin et al. [Citation11] noted that the highest callus development was determined on 1 mg L−1 BAP + 1 mg L−1 NAA including MS medium from nodal explants and on 0.5 mg L−1 BAP + 1 mg L−1 NAA from internodal explants of potato cvs. Multa and Diamant. The results of Iqbal et al. [Citation15] revealed that the combination of 5 mg L−1 benzyl adenine (BA) + 4 mg L−1 NAA produced maximum callus from nodal explants. Yasmin et al. [Citation17] obtained minimum days for callus induction (4.9 d) on 5.0 mg L−1 BAP containing medium. In contrast, Laboney et al. [Citation2] reported that 2.0 mg L−1 2,4-dichlorophenoxyacetic acid (2,4-D) supplemented MS media gave the best callus induction percentage (95%) in 10 d, the highest number of callus (4.75) and weight of callus (0.074 g) in potato cv. Granola. Kumar et al. [Citation9] noted the highest callus induction parameters from leaf explants of Kufri Chipsona-3 and MP-97/644 potato cultivars on MS medium containing 3.0 mg L−1 2,4-D + 1.0 mg L−1 KIN.
Effects of cultivars on callus formation
The minimum days for callus initiation of in vitro cultured explants was noted on cv. Caspar (12.51 d), followed by cv. Pasinler (12.98 d) and cv. Granola (13.81 d). It was clear from the results that there was no relationship between callus formation and the earliness of potato cultivars. The maximum callus induction percentage of 66.11%, the largest callus diameter of 1.10 cm and the highest callus weight of 1.27 g were noted on cv. Caspar (). It has been shown that the response of the explant types and the concentration of auxins and cytokinins used were genotype dependent.[Citation10] Banerjee et al. [Citation18] obtained the highest callus induction (86%) from leaf explants after seven days of incubation on MS basal medium with 5.0 mg L−1 NAA and 0.1 mg L−1 BAP. Onamu et al. [Citation6] determined the highest callus induction (83.33%) from both leaf and nodal explants of cv. Alfa on MS medium supplemented with 4.0 mg L−1 BA and 1.0 mg L−1 NAA.
Table 3. The effect of cultivars and explant sources on callus induction parameters.
Effects of explant sources on callus formation
Stem node explants showed better performance (12.08 d) for callus induction compared to leaf segments (14.13 d) in terms of days for callus induction. Nodal explants also induced higher callus regeneration percentage (64.07%), bigger callus (1.08 cm) and higher callus weight (1.27 g) compared to leaf explants – 56.48%, 0.92 cm and 1.03 g, respectively (). The results revealed that the binodal explants illustrated the best results for callus production in combination with cytokinins and auxins. It is very easy to work with binodal explants, which are less sensitive to injuries during the different manipulation steps.
No previous reports have suggested callus induction from binodal explant. Internodal segment, as an explant source proved to be the best for callus induction in potato.[Citation8] Huda et al. [Citation19] have also noted that internode explants for earlier callusing produced significantly higher callus size (quite massive) and established the longer shoots because of its thicker structure. They also emphasized that, since the leaf explant with lower thickness could dry rapidly depending on light and temperature, the size of leaf derived callus may be smaller compared to nodal explants.[Citation19] On the contrary, Haque et al. [Citation3] showed that leaf explant derived callus had the best diameter and weight. Dhital et al. [Citation20] also reported that the frequency of callus induction from leaf explants was higher compared to the frequency of induction from internode and petiole explants.
Effects of plant growth regulators, potato cultivars and explant sources on shoot proliferation from callus
Nodal and leaf derived calli from potato were subcultured on MS medium supplemented with different concentrations of BAP, KIN and TDZ as well as GA3 and NAA for shoot proliferation (). Nutrient medium supplemented with a combination of cytokinin and auxin lowered the days for shoot induction, enhanced the shoot growth with multiple shooting (the number of shoots), increased the number of nodes and leaves per plantlet. Dark green and compact calli were used for the shoot regeneration process, since these calli were capable of regenerating into shoots. In the current experiment, the regeneration of shoots started within 14–32 d of subculture and the other shoot parameters were recorded at regular time intervals. The results showed that the combination of BAP and KIN with GA3 and NAA was essential and very effective for the regeneration of callus. The best shoot regeneration percentage was observed on MS medium containing 2.0 mg L−1 BAP + 0.25 mg L−1 GA3 (90%) and followed by 2.0 mg L−1 KIN + 0.25 mg L−1 GA3 (82.50%), 2.0 mg L−1 BAP + 0.25 mg L−1 NAA (72.50%) and 2.0 mg L−1 KIN + 0.25 mg L−1 NAA (67.50%) ().
Figure 2. Effects of different growth regulators on shoot regeneraton from callus (a), in vitro rooting (b) and acclimatization of potato plantlets to ex vitro conditions (c, d).

Table 4. The effect of plant growth regulators on shoot regeneration parameters from callus.
It was clear from the results that nutrient medium supplemented with low concentrations of GA3 and NAA along with the high concentrations of BAP and KIN enhanced shoot growth with multiple shooting and increased the number of leaves and nodes per plantlet. The promotion of growth in terms of shoot length and number of shoots, nodes and leaves, resulted in increased plasticity of the cell walls, followed by the hydrolysis of starch to sugars, which lowered the water potential of the cells, resulting in the entry of water into the cell, causing cell elongation. Dhital et al. [Citation20] pointed out that internode explants sourced calli showed higher frequency of shoot regeneration and higher number of shoots per explants, when compared to leaf and petiole explants sourced calli. The reason for this is referred to the mature and more vascular internodes' tissue. Caspar was the most responsive genotype in terms of shooting parameters from callus among the three cultivars (). Significant differences were determined among cultivars and between explant sources in terms of shoot induction percentage, days to shoot induction, shoot length and the number of shoots, nodes and leaves (P < 0.01).
Table 5. The effect of cultivars and explant sources on shoot regeneration parameters from callus.
GA3 plays a relatively broad spectrum role for cell enlargement and cell division, when it is added to MS medium. GA3 stimulates shoot elongation and node enlargement at high concentrations, and it produces narrow and elongated shoots, depending on cultivars. Absence of GA3 promotes nodule-like structures that usually do not develop further.[Citation20] It is well known that cytokinins stimulate plant cell division and participate in the release of lateral bud dormancy, in the induction of adventitious bud formation and in the growth of lateral buds.[Citation9,Citation21–24] The vital role of BAP was well documented and it demonstrated a better response in terms of shoots per explant, shoot length and number of leaves and nodes in different potato varieties.[Citation10,Citation12] Laboney et al. [Citation2] obtained results that the combination of 1.0 mg L−1 BAP and 0.5 mg L−1 GA3 showed minimum days for shoot initiation (15 d), highest shoot regeneration (93.33) and highest number of shoots (4.67) and leaves (3.67), and longest shoots (5.33 cm) from callus derived explants. Haque et al. [Citation3] obtained an increased shoot length and number of leaves on node and internode explants on a medium containing 1.0 mg L−1 BAP + 0.1 mg L−1 GA3. Yasmin et al. [Citation7] obtained minimum days for shoot regeneration (25 d) on MS medium containing 5.0 mg L−1 BAP. Ehsandar et al. [Citation12] demonstrated that calli were differentiated into shoot primordia when subcultured on MS medium supplemented with different concentrations of BA and the maximum number of shoots per callus (3.75) was recorded on 2 mg L−1 BA. The effective TDZ applications in other studies [Citation14,Citation25] turned out to be ineffective in the present study.
Effects of plant growth regulators and cultivars on rooting and acclimatization to ex vitro conditions
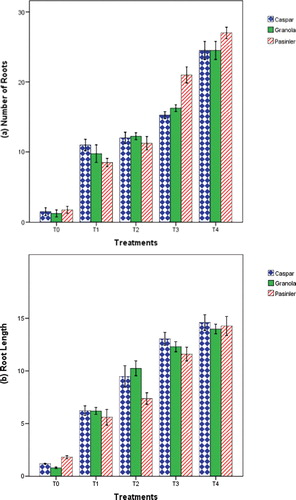
The results of the present study illustrated that nutrient medium supplemented with a combination of auxin and GA3 lowered the days for shoot induction, induced variability of root growth and increased the length and number of roots per plantlet. In vitro root induction of callus-regenerated shoots was studied with the addition of various concentrations of NAA and IBA with GA3 in MS medium. Statistically significant effects were recorded for all PGR applications on the number of days for root induction (P < 0.01) in the present experiment. Cultivars Caspar and Granola appeared with the highest root induction percentage (75%) followed by cv. Pasinler (73.75%). Addition of 0.1 mg L−1 GA3 + 1 mg L−1 IBA or 1 mg L−1 NAA was capable of inducing 100% rooting in the regenerated plantlets, followed by root induction on 0.1 mg L−1 GA3 + 0.5 mg L−1 IBA (89.60%) and 0.1 mg L−1 GA3 + 0.5 mg L−1 NAA (83.33%) (). The minimum number of days required for root induction was noted on 1.0 × MS medium containing 0.1 mg L−1 GA3 + 1.0 mg L−1 IBA (10.50 d) for cv Pasinler, 11.50 d for cv. Granola and 11.75 d for cv. Caspar (). The beneficial effect of IBA supplemented MS medium has already been reported by Khatun et al.[Citation16] The maximum number of roots was observed on cv. Pasinler (27.00), followed by cvs. Granola and Caspar (both 24.00) on 1.0 × MS medium supplemented with 0.1 mg L−1 GA3 + 1.0 mg L−1 IBA (). The longest roots were observed on cv. Caspar (14.60 cm), followed by cv. Pasinler (14.28 cm) and cv. Granola (13.98 cm) using 1.0 × MS medium supplemented with 0.1 mg L−1 GA3 + 1.0 mg L−1 IBA ().
Figure 3. Effects of 1.0 × MS medium containing NAA and IBA with GA3 on the root induction percentage (%) (a) and days for root induction (b) of cvs. Caspar, Granola and Pasinler.
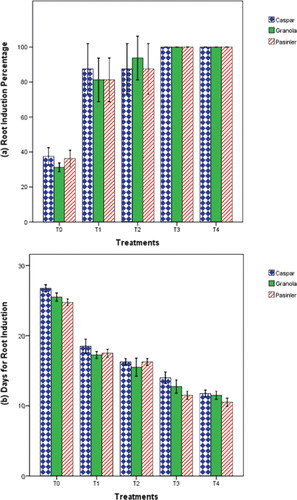
Figure 4. Effects of 1.0 × MS medium containing NAA and IBA with GA3 on the number of roots (a) and root length (b) of cvs. Caspar, Granola and Pasinler.
It is a known fact that the leaves of microplants have a very poor water retention capacity because of poorly functioning stomata. Thus, it is vital that the new root system of the microplants begins to function as soon as possible after the transfer to ex vitro conditions, so that the extensive water loss from the leaves can be compensated by water taken up by the roots. Often, microplants do not require the application of auxin to achieve rooting, but treatment with auxin increased the rooting percentage, the number of roots and the speed and synchronicity of rooting.[Citation26] The roots have an essential role and function in plant growth and development, supplying water and nutrients to the potato plants from the environment.[Citation13] Sanavy and Moeini [Citation27] emphasized that the increase in root length and number is very important for the acclimization to ex vitro conditions, as well as for the water and nutrient uptake in potato plantlets.
The rooted plantlets in the present study showed morphologically uniform and normal leaf form, shape and growth pattern (). All plants transferred to the ex vitro conditions showed a high homogeneity without an obvious morphological avoidance of somaclonal variation.[Citation28]
Conclusions
A well-developed callus from different explants and PGRs may be used for the development of new potato lines and overall improvement of potato genotypes through biotechnological approach. The regeneration of shoots is also considered as a limiting step for the successful use of modern techniques in genetic improvement of potato crop. For the effective utilization of genetic and cellular techniques, successful application of callus culture and plantlet regeneration is the first step that should be considered. The presented results suggested that the most favourable medium for shoot induction from callus was MS medium supplemented with the higher concentrations of BAP and kinetin (2.0 mg L−1) along with the lower concentrations of GA3 and NAA (0.25 mg L−1). Those combinations improved the process of shoot organogenesis. Caspar cultivar and binodal explants showed a better regeneration performance than the other cultivars and leaf explants on callus induction and shoot proliferation. The rooting experiments revealed that 1.0 × MS medium containing 1 mg L−1 IBA + 0.1 mg L−1 GA3 and 1 mg L−1 NAA + 0.1 mg L−1 GA3 decreased the number of days for root induction. These combinations also induced the highest rooting percentage and the maximum number of roots, which were also the longest. Further studies should be established to determine the best concentrations and combinations of PGRs for breeding potato lines through a biotechnological approach. The results could be used for the large-scale commercial production of disease-free and healthy potato seed tubers.
Disclosure statement
The authors reported no potential conflict of interest.
Additional information
Funding
References
- Srivastava AK, Diengdoh LC, Rai R, et al. Tissue culture-technology harnessed for potato seed production. Keanen J Sci. 2012;1:80–86.
- Laboney UZ, Biswas GC, Abdullah-Al-Shoeb M, et al. Callus induction and regeneration of potato shoot tip culture. Int J Agric Sci. 2013;3:40–45.
- Haque AU, Samad MA, Shapla TL. In vitro callus initiation and regeneration of potato. Bangladesh J Agric Res. 2009;34:449–456.
- Bordallo PN, Silva DH, Maria J, et al. Somaclonal variation in in vitro callus cultures of potato cultivars. Hortic Bras. 2004;22:34–44.
- Badoni A, Chauhan JS. Single node callus culture: improvement for micropropagation of Solanum tuberosum (cv. Kufri Himalini). Nat Sci. 2009;7:99–103.
- Onamu R, Legaria JP, Sahagun JC, et al. In vitro regeneration and Agrobacterium-mediated transformation of potato (Solanum tuberosum L.) cultivars grown in Mexico. Plant Tissue Cult Biotechnol. 2012;22:93–105.
- Yasmin S, Nasiruddin KM, Begum R, et al. Regeneration and establishment of potato plantlet through callus formation with BAP and NAA. Asian J Plant Sci. 2003;2(12):936–940.
- Shirin F, Hossain M, Kabir MF, et al. Callus induction and plant regeneration from internodal and leaf explants of potato (Solanum tuberosum L.) cultivars. World J Agric Sci. 2007;3:1–6.
- Kumar V, Rashmi D, Banerjee M. Callus induction and plant regeneration in Solanum tuberosum L. cultivars (Kufri Chipsona 3 and MP-97/644) via leaf explants. Int Res J Biol Sci. 2014;3:66–72.
- Sarker RH, Mustafa B. Regeneration and Agrobacterium-mediated genetic transformation of two indigenous potato varieties of Bangladesh. Plant Tissue Cult. 2002;12:69–77.
- Nasrin S, Hossain MM, Anjumarana K, et al. Induction and evaluation of somaclonal variation in potato (Solanum tuberosum L.). J Biol Sci. 2003;3(2):183–190.
- Hoque A, Nahar A, Razvy MA, et al. Micropropagation of water chestnut (Trapa sp.) through local varieties of Rajshahi Division. Asian J Plant Sci. 2006;5(3):409–413.
- Ehsandar S, Majd A, Choukan R. Callus formation and regeneration of the first modified Iranian potato cultivar (Savalan). Adv Crop Sci. 2013;3:201–208.
- Abd-Elaleem KG, Modawi RS, Khalafalla MM. Effect of plant growth regulators on callus induction and plant regeneration in tuber segment culture of potato (Solanum tuberosum L.) cultivar Diamant. Afr J Biotechnol. 2009;8:2529–2534.
- Iqbal M, Jaskani MJ, Rafique R, et al. Effect of plant growth regulators on callus formation in potato. J Agric Food Appl Sci. 2014;2:77–81.
- Khatun N, Bari MA, Islam R, et al. Callus induction and regeneration from nodal segment of potato cultivar Diamant. J Biol Sci. 2003;3:1101–1106.12
- Yasmin A, Jalbani AA, Kumar R. Regeneration potential of 6-benzyl amino purine (BAP) induced calli of Solanum tuberosum. Pak J Agric Agric Eng Vet Sci. 2011;27:13–17.
- Banerjee AK, Prat S, Hannapel DJ. Efficient production of transgenic potato (S. tuberosum L. ssp. andigena) plants via Agrobacterium tumefaciens mediated transformation. Plant Sci. 2006;170:732–738.
- Huda MS, Hossain MM, Haq MZ, et al. Effect of explants on callus formation of potato. Ecofriendly Agric J. 2013;6:146–149.
- Dhital SP, Lim HT, Manandhar HK. Direct and efficient plant regeneration from different explant sources of potato cultivars as influenced by plant growth regulators. Nepal J Sci Technol. 2010;12:1–6.
- Kumlay A. Combination of the auxins NAA, IBA, and IAA with GA3 improves the commercial seed-tuber production of potato (Solanum tuberosum L.) under in vitro conditions. BioMed Res Int. 2014;2014:439259.
- Gaspar T, Kevers C, Penel C, et al. Plant hormones and plant growth regulators in plant tissue culture. In Vitro Cell Dev Biol Plant. 1996;32(4):272–289.
- Gaspar TH, Kevers C, Faivre-Rampant O, et al. Changing concepts in plant hormone action. In Vitro Cell Dev Biol Plant. 2003;39(2):85–106.
- Arias AMG, Valverde JM, Fonseca PR, et al. In vitro plant regeneration system for common bean (Phaseolus vulgaris): effect of N6-benzylaminopurine and adenine sulphate. Electron J Biotechnol. 2010;13(1):1–7.
- Khalafalla M, Khadiga GA, Rasheid SM. Callus formation and organogenesis of potato (Solanum tuberosum L.) cultivar Almera. J Phytol. 2010;2:40–46.
- de Klerk GJ. Rooting of micropropagules. In: Waisel Y, Eshel A, Kafkafi U, editors. Plant roots – the hidden half. 3rd ed. New York (NY): Marcel Dekker; 2002; p. 349–357.
- Sanavy SAMM, Moeini MJ. Effects of different hormone combinations and planting beds on growth and plantlets from potato meristem culture. Plant Tissue Cult. 2003;13:145–150.
- Aslam J, Mujib A, Sharma MP. In vitro micropropagation of Dracaena sanderiana Sander ex Mast: an important indoor ornamental plant. Saudi J Biol Sci. 2013;20(1):63–68.

