 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The aim of our study was to evaluate in vitro antioxidant potential of methanolic extracts (ME) of 14 medicinal plants, 8 of which are endemic species of Anatolia. Scavenging activity was tested by 1,1-diphenyl-2-picrylhydrazyl (DPPH) method and the inhibitory effect on lipid peroxidation was examined by the ferric thiocyanate (FTC) and thiobarbituric acid (TBA) methods. The obtained results showed significant differences in the antioxidant potential amongst the tested methanolic plant extracts. Among the plant samples, Crataegus microphylla C. Koch, Salvia hypargeia Fisch. & Mey., Cotinus coggygria Scop., Origanum sipyleum L. and Rosa damascena Miller exhibited the highest DPPH scavenging activity. Five extracts (Centaurea nerimaniae Ş. Kültür, C. coggygria, Scorzonera tomentosa L., R. damascena and Colchicum sanguicolle K.M. Perss) showed strong antioxidant activity in the FTC and TBA tests, with per cent inhibition ranges of 72%–84% and 84%–92%, respectively. The ME of C. coggygria and R. damascena exhibited potent antioxidant activity by the DPPH, FTC and TBA methods.
Introduction
Herbal medicine has gained popularity in health care; according to the World Health Organization, about 65%–80% of the world's population which lives in developing countries depends essentially on plants for primary health care.[Citation1] Since the middle of the nineteenth century, different classes of bioactive compounds have been isolated and characterized. Many of these are used as the active ingredients of modern medicines, or as the lead compounds for new drugs discovery. Several plant-derived medicines are rich in phenolic compounds, flavonoids, alkaloids, tannins etc., used in the treatment of various degenerative ailments.[Citation2,Citation3]
Overproduction of free radicals or reactive oxygen species (ROS) contributes to oxidative stress, which leads to damage of proteins, DNA and lipids that is associated with chronic degenerative diseases, including cancer, coronary artery diseases, hypertension and diabetes etc.[Citation4,Citation5] The human body has an elaborate antioxidant defence system. Antioxidants are manufactured within the body and can also be extracted from the food humans eat such as fruits, vegetables, seeds, nuts, meats and oil. For review, see [Citation6]. There are two lines of antioxidant defence within the cell. The first line, found in the fat soluble cellular membrane consists of vitamin E, β-carotene and co-enzyme Q; of these, vitamin E is considered the most potent chain-breaking antioxidant within the membrane of the cell. Inside the cell, water-soluble antioxidant scavengers are present.[Citation6,Citation7] Antioxidants occurring naturally in leafy vegetables and seeds, such as ascorbic acid, vitamin E and phenolic compounds possess the ability to reduce the oxidative damage associated with many diseases. That is why many researchers have focused on natural antioxidants and, in the plant kingdom, numerous crude extracts and pure natural compounds have been reported to have antioxidant properties.[Citation4] A large number of medicinal plants has been investigated for their antioxidant properties. Natural antioxidants either in the form of raw extracts or their chemical constituents are very effective to prevent the destructive processes caused by oxidative stress.[Citation8,Citation9]
Our aim was to evaluate in vitro antioxidant activity of the crude methanolic extracts (ME) of 14 medicinal plants, 8 of which are endemic species to Anatolia, by commonly used methods [1,1-diphenyl-2-picrylhydrazyl (DPPH), ferric thiocyanate (FTC) and thiobarbituric acid (TBA) methods].
Materials and methods
Plant selection and collection
Fourteen medicinal plants were chosen for in vitro antioxidant activity testing based on literature reviews and ethnobotanical information. The selected plants, some of which are endemic, belong to different family groups and were collected from different districts of Turkey (). Information about the plant chemical constituents with antioxidant activity is shown in
Table 1. List of plants used in the study.
Table 2. Plants and their chemical constituents with known antioxidant activity.
Preparation of extracts
The dried plant material () was percolated with methanol (95%) at room temperature. The ME were evaporated to dryness under pressure and controlled temperature (40 °C–50 °C) in a rotary evaporator. The dried cormus of Colchicum sanguicolle were extracted with methanol (95%) in a Soxhlet apparatus. All the extracts were kept at –20 °C and then were lyophilized. The crude ME, thus, obtained were dissolved in distilled water.
In vitro antioxidant activity tests
DPPH radical method
In evaluating the free-radical-scavenging activity of crude extracts and single compounds, the agent of choice is often the DPPH radical.[Citation16,Citation17] Here, the radical scavenging activities of the crude ME of 14 different plants were assayed by measuring the decrease in absorbance of DPPH in the presence or absence of the extract in the assay mixture. The method used was adapted from Ko et al. [Citation16], Hossain et al. [Citation18] and Lakshmi et al. [Citation3]. Equal volumes of 10−4 mol/L DPPH (in methanol) and crude ME (E1–14) or reference solution [10 µg/mL and 100 µg/mL vitamin E and ascorbic acid as natural antioxidants and 3-tert-butyl-4-hydroxyanisol (BHA) as a synthetic antioxidant] were mixed and incubated at room temperature for 15 min in darkness. The control was prepared as above without any extract and methanol was used for the baseline correction. The absorbance of samples was measured at 517 nm. Decrease in absorbance indicated the antioxidant activity. Radical scavenging activity was expressed as percentage inhibition of DPPH and was calculated as follows:[Citation7,Citation19]where Acontrol is the absorbance of the control reaction and Asample is the absorbance of the tested extract samples.
Ferric thiocyanate (FTC) method
In this assay, hydroperoxide produced by linoleic acid added to the reaction mixture, which has been oxidized by air during the experimental period, is indirectly measured.[Citation19–22] An assay mixture of 2 mL sample [or methanol (as blank) or BHA/vitamin E (as reference)], 2.05 mL of 2.51% linoleic acid in 99.8% ethanol, 4 mL of 0.05 mol/L phosphate buffer (pH 7.0) and 1.95 mL of distilled water was placed in an Erlenmeyer flask in a rotary incubator (150 r/min, 40 °C) in a dark place. To measure the antioxidant activity, 0.1 mL of the reaction mixture was transferred into a test tube. Then, 9.7 mL of 75% ethanol was added to it, followed by 0.1 mL of 30% ammonium thiocyanate, and 0.1 mL of 2 × 10−2 mol/L ferrous chloride in 3.5% hydrochloric acid. Three minutes after the addition of ferrous chloride to the reaction mixture, the absorbance was measured at 500 nm (µ Quant Universal Microplate Spectrophotometer, Bio-Tek). Measurements were taken every 24 h until the absorbance of the control reached its maximum value. This mixture was also prepared without linoleic acid as a negative control. Vitamin E and BHA were used as positive controls. Antioxidant activity was calculated using the following equation:[Citation23,Citation24]where Acontrol is the absorbance of the control reaction and Asample is the absorbance of the tested extract samples.
Thiobarbituric acid (TBA) method
The TBA method was used to represent the inhibition of the production of carbonyl compounds degraded from the peroxides at a later stage. TBA test is used to measure the second product of peroxide oxidation such as aldehyde and ketone.[Citation22] Briefly, 2 mL of 20% trichloroacetic acid and 1 mL of 0.67% TBA were added to 2 mL of the assay mixture, which was prepared and incubated as described for the FTC method.[Citation19–22] This mixture was placed in a boiling water bath for 10 min and after cooling was centrifuged at 3000 r/min for 10 min (Thermo Scientific Heraeus Megafuge® 40/40R). The absorbance of the supernatant was recorded at 532 nm. Antioxidant activity was calculated using the following equation:[Citation23,Citation24]where Acontrol is the absorbance of the control reaction and Asample is the absorbance of the tested extract samples.
Data analysis
All experiments were performed in triplicate. Values presented in tables and figures are means with standard deviation. Statistical analysis was performed using GraphPad Prism version 5.0 (San Diego, California, USA) and KCJunior software.
Results and discussion
Substantial evidence has accumulated and indicated key roles for ROS and other oxidants in causing numerous disorders and diseases.[Citation25] As previously highlighted,[Citation9] the evidence has brought the attention of scientists to an appreciation of antioxidants for prevention and treatment of diseases and maintenance of human health.[Citation25]
There are several techniques that are commonly used to determine the antioxidant activity in vitro for the purpose of rapid screening of substances, since substances that have low antioxidants activity in vitro will probably show little activity in vivo.[Citation9,Citation17] In this work, three different methods based on various aspects of ROS action and scavenging were used to evaluate and compare the antioxidant potential of the studied plant extracts.
It is known that the presence of three aromatic rings in DPPH makes its molecule very stable.[Citation26] This radical has an absorbance maximum at 517 nm. Every substance that can scavenge DPPH, decreases the absorbance in this wavelength, therefore, the DPPH assay could be considered an appropriate method for evaluation of the potential of samples to scavenge free radicals.[Citation26] The radical scavenging activity of the standards (BHA, vitamin E and ascorbic acid) and ME tested is summarized in . As shown in , five ME (Crataegus microphylla (E1), Salvia hypargeia (E5), Cotinus coggygria (E6), Origanum sipyleum (E11) and Rosa damascena (E12)) demonstrated potent free-radical-scavenging activity with 86.64%–95.69% DPPH radicals scavenged. Interestingly, the radical scavenging capacity of these five extracts was found to be higher even than that of the reference natural antioxidant vitamin E.
Table 3. DPPH radical-scavenging activity of the methanolic extracts.
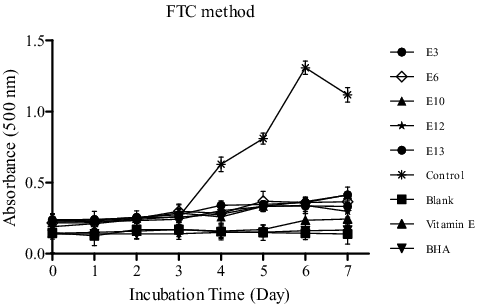
The FTC method was used to evaluate the level of lipid peroxidation by measuring the absorbance of hydroperoxide of linoleic acid. In this method, the concentration of peroxide decreases as the antioxidant activity increases.[Citation22,Citation26,Citation28] illustrates the absorbance versus incubation time, showing the activities of five active ME compared to standards at different time intervals. It was found that hydroperoxide formation started at day 3 and reached the maximum value at day 6. The highest per cent inhibition was shown by the R. damascena (E12) extract (84.27%) followed by the extract of Centaurea nerimaniae (E3), an endemic species, (80.68%).
Figure 1. Antioxidant activity of five methanolic plant extracts as measured by the FTC method at 500 nm and compared to standards (vitamin E and BHA).
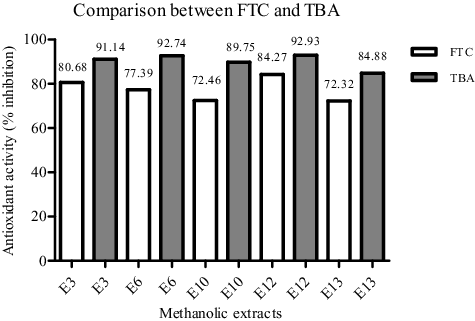
The third (TBA) method was used to evaluate the inhibition of the production of carbonyl compounds degraded from the peroxides at a later stage. The results showed that the same five active extracts caused a decrease in the production of carbonyl compounds (). Three out of these five active extracts were most effective and showed the antioxidant potential comparable to that of the synthetic antioxidant BHA. Comparative analysis of the results obtained by the FTC and TBA methods () showed that the antioxidant activity of the five extracts was found to be slightly higher by the TBA method than by the FTC one. The ME that exhibited potent antioxidant activity in all the three assays used, DPPH, FTC and TBA assay, were those of C. coggygria (E6) and R. damascena (E12).
Figure 2. Antioxidant activity of five methanolic plant extracts as measured by the TBA method at 532 nm and compared to standards (vitamin E and BHA).
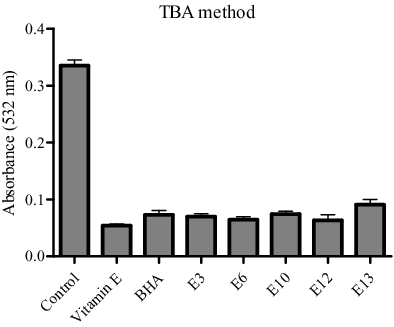
Figure 3. Comparison between the antioxidant activity of the five extracts as evaluated by the FTC and TBA methods.
A review of the known chemical constituents of the eight plants, whose extracts demonstrated most pronounced antioxidant activity by DPPH and/or FTC and/or TBA methods, revealed that most of them contain flavonoids (). Flavonoids are known to be related to antioxidant activity.[Citation19,Citation21,Citation29] Additionally, ME have been reported to contain a significantly higher concentration of flavonoids than extracts prepared in other solvents.[Citation19,Citation21] For two endemic species, C. nerimaniae and C. sanguicolle, no data related to their chemical constituents and biological activities were found in the available literature. The antioxidant activity of these two endemic species was, to the best of our knowledge, determined for the first time in this study. Further studies should be directed at identifying the active antioxidant constituents in these extracts, as it is well known that there may be different interactions (additive, synergistic or antagonistic) among components of a given extract.[Citation30,Citation31]
In recent years, considerable effort has been directed towards identifying naturally occurring substances that can protect against oxidative stress.[Citation3] Antioxidants stabilize or deactivate free radicals, often before they attack targets in living cells.[Citation17,Citation32] Free radical reactions are well known to play a role in disease pathology, e.g. in many acute and chronic disorders in humans, such as diabetes, atherosclerosis, aging, immunosuppression and neurodegeneration.[Citation33] Antioxidants, such as phenolics, flavonoids, tannins and proanthocyanidins, have been reported to be present in many medicinal plants. It is generally considered that the antioxidant contents of medicinal plants may contribute to the protection against diseases. For instance, the ingestion of natural antioxidants has been inversely associated with morbidity and mortality from degenerative disorders.[Citation9,Citation34]Today, there is a growing interest in antioxidant-based drugs/formulations for the prevention and treatment of various complex diseases. Hence, the increased use of natural sources of antioxidants. Natural resources have been reported to be the main sources of medicinal plants which are used as antioxidants.[Citation18] Antioxidants, however, when applied at high dosages, may begin to function as mutagens and tumour promoters. This effect appears to be expressed to a higher extent for synthetic antioxidants than for natural ones. Thus, natural antioxidants could be considered to give a greater degree of safety, even at higher dosages. Natural antioxidants can protect the human body against free radicals, and have also been shown to delay the progress of a variety of chronic diseases such as cancer, heart disease and diabetes.[Citation16,Citation35]
The results from our study indicated that the following plants can be considered potent antioxidant sources: C. microphylla, S. hypargeia (endemic species), C. coggygria, O. sipyleum (endemic species) and R. damascena, according to the DPPH method; and C. nerimaniae (endemic species), C. coggygria, Scorzonera tomentosa (endemic species), R. damascena and C. sanguicolle (endemic species), according to the FTC and TBA methods. These plant ME may be used for discovery of new drugs in future.
Conclusions
Five ME (C. microphylla C. Koch, S. hypargeia Fisch. & Mey. (endemic species), C. coggygria Scop., O. sipyleum L. (endemic species) and R. damascena Miller) have potent free-radical-scavenging activity. The plant ME of C. nerimaniae Ş. Kültür (endemic species), C. coggygria, S. tomentosa L. (endemic species), R. damascena and C. sanguicolle K.M. Perss (endemic species) showed strong antioxidant activity in the FTC and TBA methods, whereas the ME of C. coggygria and R. damascena exhibited potent antioxidant activity in the DPPH, FTC and TBA methods. The results indicated that the eight plants in this study could be considered promising antioxidant sources. These plants extracts could be used for isolating the active compound(s), which in turn could be used for discovery of new drugs in future.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Johnson OO, Ayoola GA. Antioxidant activity among selected medicinal plants combinations (multi-component herbal preparation). Int J Res Health Sci. 2015;3(1):526–532.
- Uddin G, Rauf A. Phytochemical screening and biological activity of the aerial parts of Elaeagnus umbellate. Sci Res Essays. 2012;7:3690–3694.
- Lakshmi GM, Bhuvaneshwari V, Amsaveni R, et al. Antioxidant and antibacterial activity from whole plant of Eclipta alba (L.) - an in vitro model. IJBSANS. 2015;2(1):1–8.
- Santharam E, Ganesh P, Soranam R, et al. Evaluation of in vitro free radical scavenging potential of various extracts of whole plant of Calycopteris floribunda (Lam). J Chem Pharm Res. 2015;7(1):860–864.
- Gafrikova M, Galova E, Sevcovicova A, et al. Extract from Armoracia rusticana and its flavonoid components protect human lymphocytes against oxidative damage ınduced by hydrogen peroxide. Molecules. 2014;19(3):3160–3172.
- Raju SM, Nageshwara Nao JS. Jaypee's review of medical biochemistry. New Delhi: Jaypee Brothers Publishers; 2005. Chapter 7, Vitamins; p. 65–87.
- Marinal S, Viji Stella Boi G. In vitro antioxidant activity and total phenolic content of leaf extracts of Limonia crenulata (Roxb). J Nat Prod Plant Resour. 2012;2(1):209–214.
- Zengin G, Cakmak YS, Guler GO, et al. Antioxidant properties of methanolic extract and fatty acid composition of Centaurea urvillei DC. subsp. hayekiana Wagenitz. Rec Nat Prod. 2011;5:123–132.
- Sayed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic total and flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med. 2012;12:1–12.
- Melikoğlu G, Bitiş L, Meriçli AH. Flavonoids of Crataegus microphylla. Nat Prod Res. 2004;18(3):211–213.
- Topcu G, Turkmen Z, Schilling JK, et al. Cytotoxic activity of some anatolian Salvia extracts and isolated abietane diterpenoids. J Pharm Biol. 2008;46(3):180–184.
- Marčetić M, Božić D, Milenković M, et al. Antimicrobial, antioxidant and anti-inflammatory activity of young shoots of the smoke tree, Cotinus coggygria Scop. PTR. 2013;27(11):1658–1663.
- Sarı A, Zidorn C, Ellmerer EP, et al. Phenolic compounds from Scorzonera tomentosa L. HCA. 2007;90(2):311–317.
- Ozkan G, Sagdic O, Ekici L, et al. Phenolic compounds of Origanum sipyleum L. extract, and its antioxidant and antibacterial activities. J Food Lipids. 2007;14(2):157–169.
- Basim E, Basim H. Antibacterial activity of Rosa damascena essential oil. Fitoterapia. 2003;74(4):394–396.
- Ko EY, Kim D, Roh SW, et al. Evaluation on antioxidant properties of sixteen plant species from Jeju Island in Korea. EXCLI J. 2015;14:133–145.
- Nunes PX, Silva SF, da S Almeida JRG, et al. Biological oxidations and antioxidant activity of natural products. In: Rao V, editor. Phytochemicals as nutraceuticals - global approaches to their role in nutrition and health. Rijeka: InTech; 2012. p. 1–20. Available from: http://www.intechopen.com/books/phytochemicals-as-nutraceuticals-global-approaches-to-their-role-in-nutrition-and-health/biological-oxidations-and-antioxidant-activity-of-natural-products
- Hossain AA, Hossain S, Fatema K, et al. An evaluation on antioxidant activity, total phenolic and total flavanoid contents of extracts from Adina cordifolia (Roxb.). Am J Plant Sci. 2015;6(5):633–639.
- Stanković MS. Total phenolic content, flavonoid concentration and antioxidant activity of Marrubium peregrinum L. Extracts. Kragujevac J Sci. 2011;33:63–72.
- Inatani R, Nakatani N, Fuwa H. Antioxidative effect of the constituents of rosemary (Rosmarinus officinalis L.) and their derivatives. Agric Biol Chem. 1983;47(3):521–528.
- Rezaeizadeh A, Zuki ABZ, Abdollahi M, et al. Determination of antioxidant activity in methanolic and cloroformic extract of Momordica charantia. Afr J Biotechnol. 2011;10(24):4932–4940.
- Khalili RMA, Shafekh SE, Norhayati AH, et al. Total phenolic content and in vitro antioxidant activity of winged bean (Psophocarpus tetragonolobus). Pak J Nutr. 2013;12(5):416–422.
- Saripah RSA, Sunalti M, Norizan A, et al. Phenolic content and antioxidant activity of fruits of Ficus deltoidea var angustifolia sp. MJAS. 2009;13(2):146–150.
- Sharma S. In vitro evaluation of antioxidant activity of methanolic and petroleum ether extracts from seeds of Benincasa hispida. J Nat Plant Resour. 2014;4(4):31–34.
- Halliwell B, Gutteridge JMC. Formation of thiobarbituric acid reactive substances from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS Lett. 1981;128(2):347–352.
- Motamed SM, Motlagh SS, Bagherzadeh H, et al. Evaluation of antioxidant activity of Ruta graveolus L. extract on inhibition of lipid peroxidation and DPPH radicals and the effects of some external factors on plant extracts potency. Res J Phcog. 2014;1:45–50.
- Artun FT, Karagoz A, Ozcan G, et al. In vitro anticancer and cytotoxic activities of some plant extracts on cancer and normal cell lines. Mitt-Klosterneuburg. 2015;65(4):55–64.
- Emynur SS, Catherine ZA, Siti Syakiroh A, et al. Total antioxidant activity, total phenolic content and free radical scavenging activity of methanol, chloroform and thyl acetate extracts of Vigna sinesis. Int Food Res J. 2012;19:1393–1400.
- Kaliq A, Sabir AM, Ahmad SD, et al. Antioxidant activities and phenolic composition of Olive (Olea europaea) leaves. J Appl Bot Food Qual. 2015;88:16–21
- Nedamani ER, Mahoonak AS, Ghorbani M, et al. Antioxidant properties of individual vs. combined extracts of Rosemary leaves and Oak fruit. J Agr Sci Tech. 2014;16:1575–1586.
- Kirakosyan A, Seymour EM, Noon KR, et al. Interactions of antioxidants isolated from tart cherry (Prunus cerasus) fruits. Food Chem. 2010;122(1):78–83.
- Das N, Islam ME, Jahan N, et al. Antioxidant activities of ethanol extracts and fractions of Crescentia cujete leaves and stem bark and the involvement of phenolic compounds. BMC Complement Altern Med. 2014;45:1–9.
- Harman D. Free radical theory of aging. Mutat Res. 1992;275(3–6):257–266.
- Gulcin I. Antioxidant activity of food constituents: an overview. Arch Toxicol. 2012;86(3):345–391.
- Siriwardhana N, Lee KW, Jeon YJ. Radical scavenging potential of hydrophilic phlorotannins of Hizikia fusiformis. Algae. 2005;20(1):69–75.
