 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Saccharomyces boulardii preparations are promising probiotics and clinical agents for animals and humans. This work focused on optimizing the nutritional conditions for the production of S. boulardii in solid-state fermentation by using classical and statistical methods. In single-factor experiments, the S. boulardii production was significantly increased by the addition of glucoamylase and the optimal carbon and nitrogen sources were found to be soluble starch and NH4Cl, respectively. The effects of the glucoamylase, soluble starch and NH4Cl on S. boulardii production were evaluated by a three-level three-factor Box–Behnken design and response surface methodology (RSM). The maximal yeast count (4.50 ×109CFU/g) was obtained under the optimized conditions (198 U/g glucoamylase, 2.37% soluble starch and 0.9% NH4Cl), which was in a good agreement with the predicted value of the model. This study has provided useful information on how to improve the accumulation of yeast cells by RSM.
Introduction
Saccharomyces boulardii is a common type of yeast, which has been extensively studied for its probiotic effects,[Citation1] such as the prevention and treatment of infectious enteritis, Clostridium difficile-associated enterocolopathies, Crohn's disease and ulcerative colitis. Thus, S. boulardii is widely used in the clinic and animal feed and has achieved significant results.[Citation2]
Solid-state fermentation (SSF) is defined as a fermentation process, which involves solid matrix in the absence or near absence of free water.[Citation3] SSF has many advantages, such as high product yield with little risk of bacterial contamination, extended stability of products, low wastewater generation and low production costs.[Citation4–7] Because of these advantages, in recent years, SSF has received more interest from researchers and has been applied in various areas, such as biotransformation of crops and crop residues for microbial preparation, nutritional enrichment [Citation3] and production of a range of high value-added products.[Citation8–12]
Generally, two main methods are used for the process of optimization, which are usually referred to as the classical and statistical methods. The classical method is based on the ‘one-factor-at-a-time’ method, in which one independent variable is observed, whereas the other factors are kept at a fixed level. However, this method cannot guarantee the determination of optimal conditions and is unable to study the interactions between the factors, thus probably leads to unreliable results and inaccurate conclusions.[Citation13] Response surface methodology (RSM), the statistical optimization method, uses the data from a few sets of experiments to determine equations. This method can overcome the limitations of the classical method and has been proved to be a powerful tool for designing experiments, building models, evaluating the effects of factors and analysing optimal conditions of factors for desirable responses.[Citation11,Citation14,Citation15] Therefore, RSM has been successfully applied for the optimization of fermentation processes.
S. boulardii is usually produced by liquid-state fermentation and only a few reports are available about the production of S. boulardii in SSF. This study aimed to optimize the S. boulardii production in SSF with wheat bran as the substrate medium by using both classical and statistical methods. Single-factor experiments, steepest ascent experiments, three-level three-factor Box–Behnken design (BBD) and RSM were performed to identify the factors that influence the fermentation process and the optimal medium for yeast growth.
Materials and methods
Strain and inoculum preparation
The yeast S. boulardii SH94 (CCTCC M2014211) was obtained from the State Key Laboratory of Agricultural Microbiology of Huazhong Agricultural University, Wuhan, China. It was maintained at 4 °C on yeast extract peptone dextrose (YPD) agar (glucose 2.0%, peptone 2.0%, yeast extract 1.0% and agar 1.8% (w/v); it was autoclaved at 115 °C for 20 min). Inocula were prepared in 250-mL conical flasks containing 100 mL of YPD liquid medium at 30 °C for 34 h.
Yeast count
After fermentation, the samples (fermentation products) were removed from the jar, cut into similar size particles and dried at 45 °C. Then, 10 g of dry samples was dissolved in a 250 mL flask with 90 mL of sterile saline water (0.9%) and some sterile glass beads by shaking for 30 min (30 °C, 200 r/min). Finally, the gradient dilution (10−5 to 10−7) was performed for total yeasts by using the plate counting method (YPD agar, which was supplemented with ampicillin and the final concentration was 100 μg/mL before use; 30 °C, 48 h).
Single-factor experiments
Initial fermentation conditions were set as follows: the solid medium containing 30 g of dry wheat bran (dispensing in 360 mL jar), with a water-to-material ratio of 1.0 and sealing with four-layer gauze, was sterilized at 115 °C for 20 min. After cooling, the material was inoculated with yeast 1 × 108 colony-forming units (CFU) per gram of dry wheat bran, with a natural pH value, followed by incubating all the jars at 30 °C. Once the process of fermentation was completed, 10 g of the sample was used for the assay of yeast count and 5 g of the sample was used to determine the moisture content by drying at 105 °C.
In the single-factor experiments, first, the optimal values of incubation time (12, 24, 36, 48, 60 and 72 h), incubation temperature (25 °C, 28 °C, 30 °C, 34 °C and 37 °C), water-to-material ratio [0.5, 1.0, 1.5, 2.0 and 2.5 (v/m)] and glucoamylase concentration [0 (control), 50, 100, 150, 200 and 250 U/g dry wheat bran; enzyme activity, 18,100 U/g; Yuanye Biotechnology Company, Shanghai, China] were determined one after another. Then, five different carbon sources (soluble starch, lactose, maltose, sucrose and glucose) were selected for further study. The carbon sources (1%, w/w) were first mixed with water and then introduced into the wheat bran. The medium without carbon source supplementation served as control. Finally, nitrogen sources experiments were carried out. Five different nitrogen sources [NH4Cl, yeast powder, peptone, urea and (NH4)2SO4; 1%, w/w] were first mixed with water and then added into the wheat bran. The medium without nitrogen source supplementation served as control.
The steepest ascent experiment
According to the results of single-factor experiments, the factors including glucoamylase, soluble starch and ammonium chloride were selected for further optimization by the steepest ascent experiment. The path of the steepest ascent experiment was adopted to find the proper levels of key factors and ensure the validity and correctness of direction. The path started at the centre of the current design space and stretched well outside the design space, followed by the selection of a sequence of equally spaced locations along the path to form a set of experiments.[Citation16] If a maximal value was found, that point would be near the optimal range and would be set as the centre point of BBD. lists the experimental design and results of the steepest ascent path.
Table 1. Steepest ascent experiment design and results.
Box–Behnken design and response surface methodology
Three key factors affecting the yeast growth were further studied by RSM. A BBD with 3 factors at 3 levels and a total of 17 runs were performed for the study, with glucoamylase (X1), soluble starch (X2) and NH4Cl (X3) as individual parameters ().
Table 2. Box–Behnken design matrix and responses.
The role of each variable, their interactions and statistical analysis in obtaining predicted yields was explained by the following quadratic polynomial model:(1)
(1) where Y is the predicted response (yeast count); β0 is the offset term; βi is the linear effect; βii is the squared effect; βij is the interaction effect and Xi is the dimensionless coded value of the variable.
Software used for experimental design and statistical analysis
All experiments and measurements were performed in triplicate. Conventional statistical methods were used to calculate means and standard deviations. Design Expert software (version 8.0; Stat-Ease Inc., Minneapolis, USA) was used for the experimental design, data analysis and quadratic model building. The quadratic regression model was evaluated by analysis of variance.The quality of fit for the regression model equation was expressed by the coefficient of determination R2 and its statistical significance was determined by the F-test.
Results and discussion
Single-factor experiments
One of the most important factors affecting the value of yeast products is the biomass after fermentation, which can be measured by yeast count.[Citation12] In single-factor experiments, the yeast count was evaluated separately by observing the effects of different values of incubation time, incubation temperature, ratio of water-to-material and glucoamylase concentration, as well as the effects of five different carbon and nitrogen sources.
In the experiments with different incubation times, as indicated in A), the yeast count kept increasing until reaching the maximal value of 1.06 × 109 CFU/g at 60 h, followed by a steady decrease. Thus, 60 h was selected as the optimal incubation time.
Figure 1. Results of the single-factor experiment. (A) Incubation time; (B) incubation temperature; (C) ratio of water-to-material; (D) glucoamylase concentration; (E) addition of five different carbon sources; (F) addition of five different nitrogen sources.
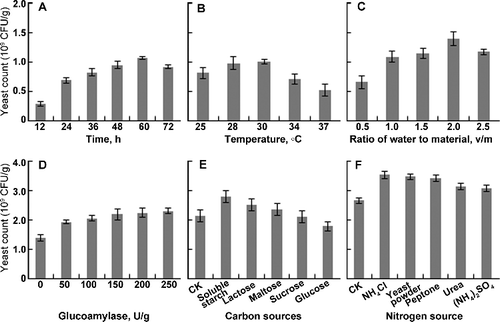
It is known that temperature is one of the most critical factors influencing the industrial fermentation, including specific growth rate and biomass yield.[Citation17,Citation18] B) shows that the maximal yeast count (1.01×109 CFU/g) was obtained at 30 °C. After that, with increasing the temperature, the yeast count decreased rapidly. Thus, 30 °C was defined as the optimal temperature.
Moisture content is a crucial factor in SSF, which affects the physical properties of the solid substrate, the growth of microbes and the success of the bioprocess.[Citation12,Citation19] As shown by the effect of water-to-material ratio (v/m) on yeast count in C), the yeast count increased linearly within the ratio range of 0.5–2.0, and the maximal count was 1.40×109 CFU/g. As the ratio reached 2.5, the yeast count decreased. Thus, 2.0 was determined as the optimal ratio. High moisture causes decreased porosity, lowers the oxygen transfer and alters the substrate structure, whereas low moisture decreases the solubility of the solid substrate, lowers the degree of swelling and reduces the solubility of the nutrients of the solid substrate.[Citation19,Citation20]
Glucoamylase can hydrolyse starch almost completely into glucose. Its supplementation promoted the degradation of starch in wheat bran, especially at the beginning of the fermentation.[Citation21] Moreover, yeast could produce large amounts of amylase to sustain the degradation and growth rate.[Citation12] As shown in D), the yeast count was significantly increased by the addition of glucoamylase, and it reached 1.92 × 109 CFU/g, 1.4-fold of the control, at a dose of 50 U/g. However, within the dosage range of 50–250 U/g, the yeast count increased slightly. When the content of glucoamylase reached 150 U/g, the yeast count was 2.17×109CFU/g. Considering the cost and benefit, 150 U/g was selected as the optimal dose of glucoamylase.
In the experiment with five different carbon sources (soluble starch, lactose, maltose, sucrose and glucose), the result showed that the yeast count increased with the addition of soluble starch, lactose and maltose, however, a negative effect occurred with the addition of sucrose or glucose (E)). Furthermore, the soluble starch was found to be more favourable for the yeast growth, which was in accordance with a previous study.[Citation12] Therefore, the soluble starch was selected as the supplemental carbon source for further experiments.
In the experiment with different nitrogen sources [NH4Cl, yeast powder, peptone, urea and (NH4)2SO4], the highest yeast count was obtained with the addition of NH4Cl, followed by yeast powder and peptone (F)). Thus, NH4Cl was selected as the supplemental nitrogen source for further experiments.
In single-factor experiments, the effect of optimization was remarkable and the obtained optimal conditions were as follows: incubation time 60 h, temperature 30 °C, water-to-material ratio 2.0, glucoamylase 150 U/g, with the addition of soluble starch, and NH4Cl. The yeast count was significantly increased by the addition of glucoamylase. Furthermore, the soluble starch and NH4Cl are cheap and easy to obtain. Therefore, three factors (glucoamylase, soluble starch and NH4Cl) were selected for the steepest ascent and BBD experiments.
Steepest ascent experiments
To ensure the optimal region inside the current design space, a path of steepest ascent experiment was performed. shows the five sets of experiment design of the steepest ascent and the corresponding results. The highest yeast count was obtained in Run 4 (175 U/g glucoamylase, 2.33% soluble starch and 1.10% NH4Cl), suggesting that it was near the region of maximal response, and thus, this point was chosen as the centre point of BBD ().
Box–Behnken design results and response surface analysis
lists the three levels of the significant variables and the results of BBD design consisting of 17 experimental runs including 5 runs under the same conditions. By applying the multiple regression analysis of the experimental data, a second-order polynomial equation was obtained:(2)
(2) where Y is the predicted yeast count, X1, X2, and X3 are the coded values of glucoamylase, soluble starch and NH4Cl, respectively.
The regression coefficients and corresponding P-values for the model are presented in , indicating that the model was highly significant because of its very low P-value (P < 0.0001). The confidence level of X2 (P = 0.0006), X3 (P < 0.0001), X1X3 (P = 0.0118), X2X3 (P = 0.0024), X12 (P = 0.0311), X22 (P = 0.0003) and X32 (P< 0.0001) were above 95% (P < 0.05), suggesting that they had a significant effect on the response Y (yeast count) (). Specifically, linear terms of X2, X3, interactive terms X1X3, X2X3, and quadratic terms of X12, X22 and X32 had a significant effect on the yeast growth, whereas the effects of other terms (X1 and X1X2) were not significant.
Table 3. Analysis of variance for the response surface quadratic model.
The fit of the model was checked by the coefficient of determination (R2) and the adjusted coefficient of determination (Adj-R2). The R2 value is a measure of how much variability can be explained by the experimental parameters and their interactions.[Citation16,Citation22] The ability of models to predict response values increases as the R2 value approaches 1.0.[Citation23] Thus, high R2 values indicate that the model equations can adequately predict the responses.[Citation16] In this study, the value of the determination coefficient R2 was 97.22%, indicating that 97.22% of the sample variation is attributed to the factors and only 2.78% can occur due to chance. The Adj-R2 value is a modification of R2 based on the number of variables used in the model, which was 98.39%, indicating that the regression equation fitted the data very well. The P-value of ‘lack-of-fit’ was 0.8485 (>0.05), implying that the ‘lack-of-fit’ was not significantly relative to the pure error and the model was fairly stable. All these findings indicated that the models were useful in describing the yeast production.
Three-dimensional (3D) response surfaces and two-dimensional (2D) contour plots were developed based on the equations to describe the interactions between the variables and to determine the optimal concentration of each component. A)–2(C) present the effect of two variables on yeast growth when the third variable was kept at the zero level. The shapes of response surfaces and contour plots indicated the nature and extent of the interactions of different components.[Citation24] The peaks in the 3D response surface and 2D contour plots suggested that the optimal points were within the design limits. The 3D plot showed that the interactions among the three independent variables significantly influenced the yeast production, indicating the superiority of the responses. As shown in , the yeast count rapidly increased with increasing the concentration of soluble starch and NH4Cl until reaching the optimal conditions, followed by an unbalanced increase between the yeast count and the concentrations of soluble starch and NH4Cl. Meanwhile, the yeast count slightly increased with increasing the glucoamylase concentration.
Figure 2. Three-dimensional response surface plots of the interaction of (A) soluble starch and glucoamylase, (B) NH4Cl and glucoamylase, (C) NH4Cl and soluble starch on yeast count.
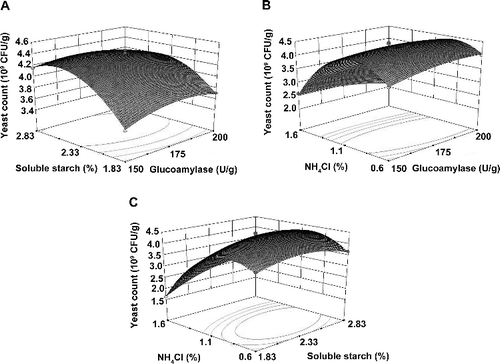
The optimal levels of the variables were calculated from the data obtained from the RSM, which were 198 U/g glucoamylase, 2.37% soluble starch and 0.9% NH4Cl. Under the optimized conditions, the maximal predicted value of the yeast count was 4.41×109 CFU/g.
Verification of the model
To verify the predicted results of the model, further experiments were carried out under the determined optimal conditions and the maximal yeast count was 4.50 × 109 CFU/g. This result was obviously in close agreement with the predicted value of 4.41 × 109 CFU/g, indicating the validity and precision of the model. The yeast count in mixed agro-industrial wastes fermented by Saccharomyces cerevisiae was reported to be 3.6 × 109 CFU/mL,[Citation25] and the yeast counts in yeast products were 1.3 × 109 CFU/g [Citation26] and 1.58 × 109 CFU/g.[Citation12] Compared with these three reported results, the value obtained in this study was significantly higher.
Conclusions
In this study, single-factor experiments, steepest ascent experiments, BBD and RSM were performed to optimize the production of S. boulardii in SSF. The individual and interactive roles of the three factors (glucoamylase, soluble starch and NH4Cl) were investigated by BBD and RSM. The maximal predicted value of the yeast count was 4.41×109 CFU/g and a mean value of 4.50×109 CFU/g was achieved in the experiment under optimal conditions, which was in a close agreement with the model prediction. This study provided useful information on how to improve the accumulation of yeast cells by fermentation optimization by using classical and statistical methods.
Acknowledgments
Yuanliang Hu and Huanhuan Qin contributed equally to the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Czerucka D, Piche T, Rampal P. Yeast as probiotics - Saccharomyces boulardii. Aliment Pharm Therap. 2007;26:767–778.
- Robinson P, Erasmus LJ. Effects of analyzable diet components on responses of lactating dairy cows to Saccharomyces cerevisiae based yeast products: a systematic review of the literature. Anim Feed Sci Tech. 2009;149:185–198.
- Singhania RR, Patel AK, Soccol CR, et al.Recent advances in solid-state fermentation. Biochem Eng J. 2009;44:13–18.
- Barrios-Gonzalez J. Solid-state fermentation: physiology of solid medium, its molecular basis and applications. Process Biochem.2012;47:175–185.
- Toscano L, Montero G, Stoytcheva M, et al. Lipase production through solid-state fermentation using agro-industrial residues as substrates and newly isolated fungal strains. Biotechnol Biotechnol Equip. 2013;27:4074–4077.
- Toscano L, Montero G, Cervantes L, et al.Production and partial characterization of extracellular lipase from Trichodermaharzianum by solid-state fermentation. Biotechnol Biotechnol Equip. 2013;27:3776–3781.
- Zhao S, Hu N, Huang J, et al. High-yield spore production from Bacillus licheniformis by solid state fermentation. Biotechnol Lett. 2008;30:295–297.
- Quevedo-Hidalgo B, Monsalve-Marín F, Narváez-Rincón PC, et al. Ethanol production by Saccharomyces cerevisiae using lignocellulosic hydrolysate from Chrysanthemum waste degradation.World J Microbiol Biotechnol. 2013;29:459–466.
- Aggelopoulos T, Katsieris K, Bekatorou A, et al. Solid state fermentation of food waste mixtures for single cell protein, aroma volatiles and fat production. Food Chem. 2014;145:710–716.
- Fleuri LF, de Oliveira MC, Campos Arcuri MdL, et al. Production of fungal lipases using wheat bran and soybean bran and incorporation of sugarcane bagasse as a co-substrate in solid-state fermentation. Food Sci Biotechnol. 2014;23:1199–2205.
- Wu W, Zhao S, Chen C, et al. Optimization of production conditions for antioxidant peptides from walnut protein meal using solid-state fermentation. Food Sci Biotechnol. 2014;23:1941–1949.
- Hu Y, Pan L, Dun Y, et al. Conversion of yellow wine lees into high-protein yeast culture by solid-state fermentation. Biotechnol Biotechnol Equip. 2014;28:1–7.
- Long C, Ou Y, Guo P, et al. Cellulase production by solid state fermentation using bagasse with Penicillium decumbens L-06. Ann Microbiol. 2009;59:517–523.
- Li Y, Cui F, Liu Z, et al. Improvement of xylanase production by Penicillium oxalicum ZH-30 using response surface methodology. Enzyme Microbiol Technol. 2007;40:1381–1388.
- Lin SS, Dou WF, Xu HY, et al. Optimization of medium composition for the production of alkaline beta-mannanase by alkaliphilic Bacillus sp. N16-5 using response surface methodology. Appl Microbiol Biotechnol. 2007;75:1015–1022.
- Qiu J, Song F, Qiu Y, et al. Optimization of the medium composition of a biphasic production system for mycelial growth and spore production of Aschersonia placenta using response surface methodology. J Invertebr Pathol. 2013;112:108–115.
- Ginovart M, Prats C, Portell X, et al. Exploring the lag phase and growth initiation of a yeast culture by means of an individual-based model. Food Microbiol. 2011;28:810–817.
- Chi Z, Zhao S. Optimization of medium and cultivation conditions for pullulan production by a new pullulan-producing yeast strain. Enzyme Microb Technol. 2003;33:206–211.
- Mandviwala T, Khire J. Production of high activity thermostablephytase from thermotolerant Aspergillus niger in solid state fermentation. J Ind Microbiol Biotechnol. 2000;24:237–243.
- Mahadik ND, Puntambekar US, Bastawde KB, et al. Production of acidic lipase by Aspergillus niger in solid state fermentation. Process Biochem. 2002;38:715–721.
- Norouzian D, Akbarzadeh A, Scharer JM, et al. Fungal glucoamylases. Biotechnol Adv. 2006;24:80–85.
- Sridevi V, Mahanti CLV, Adimadhyam SVN, et al. Statistical optimization of process variables by response surface methodology to enhance phenol degradation by Pseudomonas putida (NCIM 2102). Adv Biosci Biotechnol. 2011;2:175–181.
- Acikel U, Ersan M, Acikel YS. Optimization of critical medium components using response surface methodology for lipase production by Rhizopus delemar. Food Bioprod Process. 2010;88:31–39.
- Prakash GVSB, Padmaja V, Kiran RRS. Statistical, optimization of process variables for the large-scale production of Metarhizium anisopliae conidiospores in solid-state fermentation. Bioresource Technol. 2008;99:1530–1537.
- Aggelopoulos T, Bekatorou A, Pandey A, et al. Discarded oranges and brewer's spent grains as promoting ingredients for microbial growth by submerged and solid state fermentation of agro-industrial waste mixtures. Appl Biochem Biotech. 2013;170:1885–1895.
- Zhang L, Wu W, Li C, et al. Research on the solid state fermentation medium of yeast culture additive. J Anhui Agric Sci. 2008;36:2787–2788.
