ABSTRACT
The first step in plant breeding is to identify suitable genotypes containing the desired genes among existing varieties, or to create one if it is not found in nature. In nature, variation occurs mainly as a result of mutations and without it, plant breeding would be impossible. In this context, the major aim in mutation-based breeding is to develop and improve well-adapted plant varieties by modifying one or two major traits to increase their productivity or quality. Both physical and chemical mutagenesis is used in inducing mutations in seeds and other planting materials. Then, selection for agronomic traits is done in the first generation, whereby most mutant lines may be discarded. The agronomic traits are confirmed in the second and third generations through evident phenotypic stability, while other evaluations are carried out in the subsequent generations. Finally, only the mutant lines with desirable traits are selected as a new variety or as a parent line for cross breeding. New varieties derived by induced mutatgenesis are used worldwide: rice in Vietnam, Thailand, China and the United States; durum wheat in Italy and Bulgaria; barley in Peru and European nations; soybean in Vietnam and China; wheat in China; as well as leguminous food crops in Pakistan and India. This paper integrates available data about the impact of mutation breeding-derived crop varieties around the world and highlights the potential of mutation breeding as a flexible and practicable approach applicable to any crop provided that appropriate objectives and selection methods are used.
Introduction
It is well known that evolution and practical breeding depend on genetic variation. The variations that are found in nature do not represent the original spectra of spontaneous mutations. Rather, they are the result of genotypes recombining within populations and their continuous interaction with environmental factors.[Citation1] Green plants are essential for human existence as a source of food, clothing and energy resources. As highlighted in [Citation1], prehistoric hunter-gatherers depended on their hunting skills and on abundant natural vegetation to get non-poisonous and nutritious fruits, tubers, seeds and other food stuffs. With the growth of human population, larger and safer food supplies had to be found and gradually large-scale production systems based on plant domestication were developed. The means by which new plant varieties were developed for cultivation and used by humans is called plant breeding. Primarily, simple selection of desirable offspring was the first method of breeding and this utilized the occurrence of spontaneous mutations.[Citation1,Citation2]
Genetics became a fundamental science of plant breeding after Gregor Johann Mendel discovered the laws of heredity in the nineteenth century. However, further advancements in plant breeding took place when the hybridization methodology was developed. Its aim was to combine desirable genes found in two or more different varieties in order to produce pure-line progeny superior to the parental types in many respects.[Citation1] Cross breeding (or recombinant breeding), based on crossing of different genotypes followed by trait selection, has become a common practice in plant breeding. Later, the work on the induction of genetic alterations through X-rays by Lewis John Stadler in the late 1920s and early 1930s laid the foundation of another type of plant breeding known as mutation breeding.[Citation2] The variation so created is further amplified by recombination of alleles on homologous chromosomes and their independent assortment at meiosis. Mutations are the primary source of all genetic variations existing in any organism, including plants.[Citation3] The resulting variation provides the raw material for natural selection and is also a driving force in evolution. Spontaneous mutations are very rare and random in terms of time of occurrence, which makes them more difficult to use in plant breeding programmes.[Citation4] In this way, mutant forms showing both large and small effects on the phenotype arise for all kinds of traits.[Citation3] Mutation breeding involves the development of new varieties by generating and utilizing genetic variability through chemical and physical mutagenesis. It is now a pillar of modern plant breeding, along with recombinant breeding and transgenic breeding.[Citation2] As underlined by Novak and Brunner,[Citation1] this method, often supplemented by germplasm derived from induced mutation, has become the most common one for breeding plants through sexual reproduction.
In crops that do not produce seeds, e.g. edible banana or seedless grapes, mutation induction may be the only productive way of increasing variability for developing new cultivars.[Citation5–7] This also applies to many root and tuber crops,[Citation5,Citation6,Citation8] and the development of novel colours and variations in ornamental plant species propagated vegetatively.[Citation6,Citation9] Crossbreeding in vegetatively propagated perennial crop species, such as many fruit crops, is also subject to constraints of time, growing space and clonal identity. In this case, mutation induction can be a valuable breeding strategy.[Citation6,Citation8,Citation10,] (Reviewed in [Citation11]) For plants that are fully sterile without seeds, alternative approaches have to be developed. This is the technique of somatic tissue manipulation by mutation breeding and biotechnology.[Citation1]
Mutation breeding
Mutagenesis is the process whereby sudden heritable changes occur in the genetic information of an organism not caused by genetic segregation or genetic recombination, but induced by chemical, physical or biological agents.[Citation12] Mutation breeding employs three types of mutagenesis. These are induced mutagenesis, in which mutations occur as a result of irradiation (gamma rays, X-rays, ion beam, etc.) or treatment with chemical mutagens; site-directed mutagenesis, which is the process of creating a mutation at a defined site in a DNA molecule; and insertion mutagenesis, which is due to DNA insertions, either through genetic transformation and insertion of T-DNA or activation of transposable elements.[Citation13,Citation14] Plant breeding requires genetic variation of useful traits for crop improvements.[Citation1] However, multiple mutant alleles are the sources of genetic diversity for crop breeding as well as functional analysis of the targeted gene in many cases. The key point in mutation breeding is the process of identifying individuals with a target mutation, which involves two major steps: mutant screening and mutant confirmation.[Citation14] Mutant screening is a process involving selection of individuals from a large mutated population that meet specific selection criteria, e.g. early flowering, disease resistance as compared to the parent. However, these selections are often regarded as putative mutants or false mutants. Mutant confirmation, on the other hand, is the process of re-evaluating the putative mutants under a controlled and replicated environment using large samples. Through this process, many putative mutants are revealed to be false mutants. In general, the mutations that are important in crop improvement usually involve single bases and may or may not affect protein synthesis.[Citation15]
History of plant mutagenesis
It has been suggested that the history of plant mutation could be traced back to 300 BC with reports of mutant crops in China.[Citation3,Citation16] For a detailed review, see [Citation3]. Mutations as a mechanism of creating variability were first identified by Hugo de Vries in the late nineteenth century, while experimenting on the ‘rediscovery’ of Mendel's laws of inheritance.[Citation3] He considered this variability as heritable changes by mechanisms very distinctive from segregation and recombination. He described this occurrence as swift changes in organisms, which were hereditary and thus produced relatively large effects on the phenotypic appearance of organism. He then coined the term ‘mutation’ and presented an integrated concept concerning the occurrence of sudden, shock-like changes (leaps) of existing traits which leads to development of a new species and variation. Radiation-induced mutations as a tool for generating novel genetic variability in plants advanced as a field after the discovery of the mutagenic action of X-rays demonstrated in maize, barley and wheat by Stadler.[Citation17] The first commercial mutant variety was produced in tobacco in 1934. Prior to 1995, Acquaah [Citation18] reported 77 cultivars that were developed via mutagenesis. In 1995, the number of commercially released varieties increased to 484. This number has sharply increased since with new mutant varieties being continuously reported in different continents (). Some of the plants include fruit trees (e.g., apple, citrus, peach), ornamentals (e.g., chrysanthemum, dahlia, poinsettia), food crops (e.g., rice, barley, wheat, corn, pea), etc. Agronomic traits modified due to mutation breeding include lodging resistance, early maturity, winter hardiness, product quality (e.g., protein and lysine content) and numerous ornamental mutants. As a breeding tool, mutagenesis became very popular from the 1950s onwards when a large range of crop and ornamental plant species were predominantly treated by irradiation to increase trait variation.[Citation19–21]
Mutagenic agents
Agents of artificial mutations are called mutagens. They are generally grouped into two broad categories, namely chemical mutagens and physical mutagens.[Citation18,Citation22] Traditionally, to induce mutations in crops, planting materials are exposed to physical and chemical mutagenic agents. Mutagenesis can be performed with all types of planting materials, e.g. whole plants, usually seedlings, and in vitro cultured cells. Nevertheless, the most commonly used plant material is seed. Multiple forms of plant propagules, such as bulbs, tubers, corms and rhizomes [Citation23] and more recently, the induction of mutations in vegetatively propagated plants is becoming more efficient as scientists take advantage of totipotency (ability of a single cell to divide and produce all of the differentiated cells in an organism to regenerate into whole plants) using single cells and other forms of in vitro cultured plant tissues.[Citation15] The starting materials for the induction of mutations are vegetative cuttings, scions, or in vitro cultured tissues like leaf and stem explants, anthers, calli, cell cultures, microspores, ovules, protoplasts, etc. Gametes, usually inside the inflorescences, are also targeted for mutagenic treatments through immersion of spikes, tassels, etc. [Citation23] Whereas chemical mutagens are preferably used to induce point mutations, physical mutagens induce gross lesions, such as chromosomal abbreviation or rearrangements.[Citation13] As pointed out by Mba,[Citation15] it is noteworthy that the frequency and types of mutations are direct results of the dosage and rate of exposure or administration of the mutagen rather than its type. In the end, the choice of a mutagen will be based more often than not on the particular researcher's circumstances, such as safety of usage, ease of use, availability of the mutagens, effectiveness in inducing certain genetic alterations, suitable tissue, cost and available infrastructure among other factors.
Physical mutagenesis
In the past 80 years, physical mutagens, mostly ionizing radiations, have been used widely for inducing hereditary aberrations and more than 70% of mutant varieties were developed using physical mutagenesis (reviewed in [Citation15,Citation24]). Radiation is defined as energy travelling through a distance in the form of waves or particles. These are relatively high-energy levels of electromagnetic (EM) spectrum that are capable of dislodging electrons from the nuclear orbits of the atoms that they impact upon. The impacted atoms, therefore, become ions. Hence, the term ionizing radiation. These ionizing components of the EM include cosmic, gamma (γ) and X-rays.[Citation15] The most commonly used physical mutagens are shown in . X-rays were the first to be used to induce mutations. Since then, various subatomic particles (neutrons, protons, beta particles and alpha particles) have been generated using nuclear reactors.[Citation18] Gamma radiation from radioactive cobalt (60Co) is widely used. It has high penetrating potential and is hazardous. However, it can be used for irradiating whole plants and delicate materials, such as pollen grains. Various mutants have been developed through gamma radiation.[Citation25] The mutagenic effect results mostly from DNA double-strand breaks. The mutants show higher potential for improving plant architecture leading to better crop improvement and are used as a complementary tool in plant breeding [Citation26]. Gamma rays have a shorter wave length and therefore, possess more energy than protons and X-rays, which gives them ability to penetrate deeper into the tissue.[Citation27] Neutrons are hazardous and hence have less penetrating abilities, but they are known to cause serious damage to the chromosomes. They are best used for materials, such as dry seeds.[Citation18] Various forms of neutrons were also studied extensively for their use in mutagenesis in the 1960s and 1970s. Though it has been proved to be an effective mutagen, particularly for producing large DNA fragment deletions, the application of neutrons in induced mutagenesis is limited.[Citation3] The mutagenic effect of ultraviolet light was discovered by Altenbung [Citation28] through irradiation of the polar cap cells of fruit fly eggs. The mutagenic potential of these rays have since been confirmed in many organisms. In those organisms, germ tissue could be easily exposed to the low-penetrating ultraviolet light which resulted in covalent dimerization of adjacent pyrimidine.[Citation3,Citation29] Emission of UV light (250–290 nm) has a modest capacity to infiltrate tissues as compared with ionizing radiation. Ionizing emission goes deeper into the tissue and can cause a great number of variations in the chemical composition. The major advantage of using physical mutagenesis compared to chemical mutagenesis is the degree of accuracy and sufficient reproducibility, particularly for gamma rays, which have a uniform penetrating power in the tissue.[Citation30] During the past two decades, ion beams either through implantation or irradiation have become a new type of physical mutagen instead of the widely used gamma rays, X-rays and neutrons.[Citation31–33] They consist of particles travelling along a path that vary in mass from a simple proton to a uranium atom which are generated through particle accelerators.[Citation34] The positively charged ions are accelerated at a high speed (about 20%–80% of the speed of light) and form high linear energy transfer (LET) radiation. LET radiation causes significant biological effects, such as chromosomal aberration, lethality, etc., as compared to other types of radiation used in physical mutagenesis. The damage caused by ion beams to DNA double strands is less repairable when compared to that induced by gamma rays due to deletion of DNA fragments of various sizes.[Citation35] More recently, to study the intricacies of mutation induction in space, plant materials have been sent out into aerospace.[Citation3] It has been speculated that the special environment of space flight, such as cosmic radiation, microgravity, weak geomagnetic field, etc. contains the potential agents of mutation induction. However, knowledge of the underlying genetics of aerospace mutagenesis is so far scarce.[Citation3]
Table 1. Examples of commonly used physical mutagens.
From the past to the present, doses that lead to 50% lethality (LD50) have often been chosen.[Citation19] As noted by Oldach,[Citation19] it can be argued that LD50 is quite arbitrary and might lead to a high number of (mostly deleterious) mutations in every plant. This could go to the extent that desirable mutations are either lost or overlooked due to either plant mortality or poor agronomic performance in generations following the mutagenesis. Therefore, a mutation rate targeting a lower LD (e.g. LD20) with a survival rate of 80% appears to be more suitable for mutation breeding in selfing plant species. Maluszynski et al.[Citation36] also suggested that the final doses for mutagenic treatment should be rather low if the aim is to add new traits to an already high-quality genetic background, such as varieties or elite breeding lines. They conclude that the doses with an LD50 generally applied in the mutation breeding programmes of the 1960s and 1970s were too high and thus did not lead to the success expected from this technology (reviewed in [Citation19]).
Ionizing radiations cause mutations by breaking chemical bonds in the DNA molecule, deleting a nucleotide, or substituting it with a new one.[Citation18] Acquaah [Citation18] also pointed out the importance of radiation being applied at the proper dose, a factor that depends on radiation intensity and duration of exposure. The dosage of radiation is commonly measured in roentgen (r or R) units. The exposure may be chronic (continuous low dose administered for a long period) or acute (high dose over a short period). The quality of mutation (proportion of useful mutations) is not necessarily positively correlated with dose rate. It is common knowledge that a high dose does not necessarily yield the best results [Citation18]. The mutagen dose used should be a compromise between mutation load and the chance to find desirable mutations, and this greatly depends on the cost effectiveness of selection. Screening of larger mutant populations that originate from a lower mutagen dose may be feasible for traits with simple phenotypic selection criteria, such as early maturity. On the other hand, screening the same population for complex phenotypic traits, such as seed protein quality would not be feasible.
Chemical mutagenesis
The effect of chemical mutagens on plant materials is generally considered milder.[Citation18] An advantage of chemical mutagenic agents is that they can be applied without complicated equipment or facilities. The ratio of mutational to undesirable modifications is generally higher for chemical mutagens than for physical mutagens (reviewed in [Citation18]). Usually, the material is soaked in a solution of the mutagen to induce mutations. However, chemical mutagens are generally carcinogenic and therefore, extra care must be taken for health protection during the procedure. Material and safety data sheets for the specific chemical mutagen chosen should be carefully read and the agent should be appropriately inactivated before disposal. Despite the large number of mutagenic compounds, only a small number has been tested in plants.[Citation23] Among them, only a very restricted group of alkylating agents has found large application in plant experimental mutagenesis and plant mutation breeding. Over 80% of the registered new mutant plant varieties reported in the International Atomic Energy Association (IAEA) database [Citation37] obtained via chemical mutagenesis were induced by alkylating agents. Of these, three compounds are significant: ethyl methane sulphonate (EMS), 1-methyl-1-nitrosourea and 1-ethyl-1-nitrosourea, which account for 64% of these varieties. For review, see [Citation23].
Alkylating agents can be found among a large array of classes of compounds, including sulphur mustards, nitrogen mustards, epoxides, ethyleneimines, ethyleneimides, alkyl methanesulphonates, alkylnitrosoureas, alkylnitrosoamines, alkylnitrosoamides, alkyl halides, alkyl sulphates, alkyl phosphates, chloroethyl sulphides, chloroethylamines, diazoalkanes, etc.[Citation23] One of the most effective chemical mutagenic groups is the group of alkylating agents (these react with the DNA by alkylating the phosphate groups as well as the purines and pyrimidines).[Citation18] Another group is that of the base analogues (they are closely related to the DNA bases and can be wrongly incorporated during replication). Examples are 5-bromouracil and maleic hydrazide (). A clear advantage of the point mutations created by chemical mutagens is their potential to generate not only loss-of-function but also gain-of-function phenotypes if the mutation leads to a modified protein activity or affinity, like tolerance to the herbicide glyphosate [Citation38] or sulphonylurea shown in the legume Medicago truncatula.[Citation39] The concentration of the mutagen, the length of treatment and the temperature at which the experiment is carried out affect the efficiency of mutagenesis. As chemical mutagens are very reactive, it is important to use fresh batches of the chemical(s) that have been appropriately stored.
Table 2. Examples of commonly used chemical mutagens.
Types of mutations
Mutations can be broadly divided into intragenic or point mutations (occurring within a gene in the DNA sequence); intergenic or structural mutations within chromosomes (inversions, translocations, duplications and deletions) and mutations leading to changes in the chromosome number (polyploidy, aneuploidy and haploidy).[Citation11] In addition, it is important to distinguish between nuclear and extranuclear or plasmone (mainly chloroplast and mitochondrial) mutations, which are of considerable interest to agriculture.[Citation11]
A neat and concise outline of the types of mutations is given in [Citation3]. Among the various kinds of mutational changes at the molecular level are base substitutions, a term meaning nucleotide changes that involve substitution of one base for another. This can happen through mis-pairing of the base analogue in the treated DNA during replication, leading to mutation through transitions when exchanges occur either between purines (A–G) or between pyrimidines (T–C) and transversions when purines are exchanged for pyrimidines or vice versa (A, G–T, C).[Citation3]
Basically, transitions and transversions are the simplest kinds of base pair changes, but they may result in phenotypically visible mutations. There are no restrictions on the different kinds of sequence changes in the DNA of a gene following different types of misprints during replication. Another common error would be addition or deletion of a nucleotide base pair when one of the bases manages to pair with two bases or fails to pair at all. These kinds of sequence changes resulting in an alteration in the reading frame of the gene's DNA are known as frame-shift mutations. They are more drastic in their effect as they may completely change the message of the gene starting with the point of deletion/addition. Some of the mutations occur from rearrangement of bases in the DNA. A small or large sequence of bases may be inverted as a result of chromosome breakage, and reunion of the broken ends may involve different DNA molecules in a reciprocal rearrangement or in loss of a fragment. Duplication of a DNA sequence is another common mechanism for change in the structure of a gene leading to gene mutation.[Citation3]
Practical considerations in induced crop mutagenesis
The dose of a mutagen that achieves the optimum mutation frequency with the least possible unintended damage is regarded as the optimal dose for induced mutagenesis.[Citation22] For physical mutagens, this is estimated by carrying out tests of radiosensitivity (from radiation sensitivity), a term described as a relative measure that gives an indication of the quantity of recognizable effects of radiation exposure on the irradiated subject.[Citation16] Its predictive value, therefore, guides the researcher in the choice of optimal exposure dosage depending on the plant materials and the desired outcome. Mba et al.[Citation22] further describe the steps and procedures used for induction of mutations in vegetatively propagated plants and seeds, based on established protocols validated for cassava and rice, respectively. These protocols are for chemical mutagenesis (using EMS) and physical mutagenesis (using gamma rays) and include a list of the required equipment and reagents. The protocols cover the procedures ranging from pre-treatment to mutagenic treatments to post-treatment handling of the propagules materials, as well as the methodologies for data collection and analysis.[Citation15,Citation22]
Important factors influencing the outcome of mutagenesis using chemical mutagens include the condition of the mutagenic solution; inherent characteristics of the targeted tissue; the environment; concentration of mutagen; treatment volume; treatment duration; temperature; presoaking of seeds; pH (7.0); catalytic agents (Cu2+ and Zn2+) and post-treatment handling. Factors influencing the outcome of mutagenesis using physical mutagens includes oxygen; moisture content; temperature; physical ionizing agents (EM and ionizing radiation); dust and fibres (e.g. from asbestos); biological and infectious agents (both viral and bacterial).[Citation22] In general, the steps required for inducing and detecting mutations vary among sexually and asexually propagated plants/crops but there are some basic principles that they share in common. The common practical considerations that need to be taken into account in induction and detection of mutations as summarized by Mba [Citation15] include the following.
A perfect understanding of the genetic makeup of the traits to be improved is very important. For example, a trait controlled by many genes (i.e., polygenic) has less chances of inducing modification compared to a trait that is governed by a single gene (i.e., monogenic).
Understanding the mode of reproduction of the target crop is also a prerequisite, whether asexually or sexually propagated. If it is asexually propagated, then the method to employ is the next question: whether it will be in vitro or in vivo. If the crop is seed propagated, the question will be on the type of fertilization (self or cross-fertilization) to be used.
The determination of the material that is to be used for the propagation prior to treatment, i.e. gametes or seeds for sexually propagated crops; and stem cuttings, buds, nodal segments or twigs for asexually propagated ones.
Knowledge of the number of sets of chromosomes in the nucleus of a cell (ploidy) of the target crop, especially when it relates to how hybridization barriers could impact on the predicted effectiveness of the induced mutants.
Determinations of the genetic pedigree of the target crop for inducing mutations, i.e. selecting homozygous plants and the best genotype that is deficient in a single trait.
Selection of an appropriate mutagen (physical or chemical mutagens) and dose (duration and concentration of mutagens). That is why a pilot assay is advisable to be carried out prior to the large-scale treatment of propagules.
Identification of infrastructure (irradiation house, laboratories, screen/glass house, fields, etc.) for successful selection of desired mutants.
Screening techniques for dissociation of chimeras from stable mutants.
Mutation breeding strategy for obtaining mutants
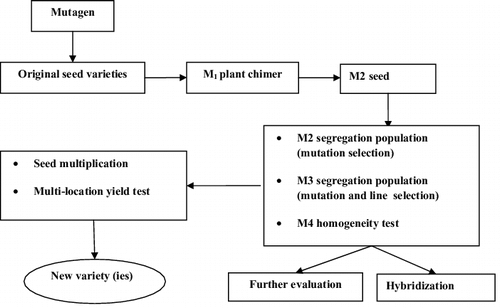
Any mutation breeding strategy requires several sequential steps. The effectiveness of mutation breeding over other breeding methods depends upon the efficacy of selection of useful variant mutants in the second (M2) or third (M3) generation as summarized in . The first step in mutation breeding is to reduce the number of potential variants among the mutagenized seeds or other propagules of the first (M1) plant generation to a significant level to allow close evaluation and analysis.[Citation12] Determination of the target population size in the first generation of mutants is a prerequisite for potential success in any mutation breeding programme. The targeted population should be fixed so as to allow a high number of mutation measurements. Thus, the population size should be managed effectively by the breeder. It should be noted that the population size depends on the inheritance pattern of the target gene. Therefore, it is advisable to select mutagens that give a high mutation frequency so as to reduce the population size of the M1 generation.[Citation12] Genetically, Ml mutant plants are heterozygous. This is because only one allele is affected by one mutation during treatment. However, the probability of having a mutation on both the alleles concurrently is a product of individual probability of mutation. Therefore, its occurrence is extremely low. Moreover, in Ml, only dominant mutations can be identified, while it is impossible to identify a recessive mutation expression at this stage. In this case, a plant breeder should attempt screening mutations in subsequent generations where segregation will occur.[Citation12] Consequently, the plant breeder generates homozygotes for dominant or recessive alleles. Caution should be taken to prevent cross pollination among the M1 population as this would lead to generation of new variation which will be difficult to differentiate from the effect of mutation.[Citation12,Citation40] Screening and selection start in the M2 generation. Roychowdhury et al.[Citation41] discuss three main types of screening/selection techniques. These are physical/mechanical, visual/phenotypic and other methods. Physical or mechanical selection can be used efficiently to determine the shape, size, weight, density of seeds, etc., using appropriate sieving machinery. Visual screening is the most effective and efficient method for identifying mutant phenotypes. Visual/phenotypic selection is often used in selection for plant height, adaptation to soil, growing period, disease resistance, colour changes, earliness in maturity, ion-shattering, climate adaptation, etc. In the category of ‘others’, physiological, biochemical, chemical, physio-chemical procedures for screening may be used for selection of certain types of mutants. When a mutant line appears to possess a promising character, the next stage is seed multiplication for extensive field trials. In this case, the mutant line, the mother cultivar and other varieties will be tested. The methods for comparative trials of mutants are the same as those for any other newly developed varieties. The purpose of field trials is to find whether the mutant promises to become a commercial variety that is superior to the mother cultivar. Prior to release as a commercial variety, the promising mutant should be studied for combinations of different characters like growth habit, structure and yield components in a wide range of environments under varying water availability, plant density, sowing dates, etc.[Citation12]
Figure 2. Distribution of mutant crop varieties by continents (Accessed on 15th July, 2015). (Source: Joint Division of the Food and Agriculture Organization of the United Nations and the International Atomic Energy Agency [Joint FAO/IAEA, 2015], [IAEA mutant database, http://mvgs.iaea.org/] Reproduced with permission)
![Figure 2. Distribution of mutant crop varieties by continents (Accessed on 15th July, 2015). (Source: Joint Division of the Food and Agriculture Organization of the United Nations and the International Atomic Energy Agency [Joint FAO/IAEA, 2015], [IAEA mutant database, http://mvgs.iaea.org/] Reproduced with permission)](/cms/asset/1fc6d671-6e67-4351-833f-2446211cd4e7/tbeq_a_1087333_f0002_c.jpg)
Impact of mutant cultivars
Genetic variability as a result of induced mutation by various mutagens has contributed to modern plant breeding. Over the past five decades, it has played a major role all over the world in the development of superior plant varieties [Citation13] with characteristics of high-yield, early maturity, lodging resistance among others. Global impact of developed and released varieties in major crops all over the world has been reviewed by Ahloowalia et al.[Citation42] Several achievements in crop improvement through mutation breeding have resulted in two major outcomes: improved varieties that are directly used for commercial cultivation and new genetic stocks with improved characters or with better combining ability of traits.[Citation12] These traits could be increased yield, enhanced nutritional quality, resistance to pest and disease, early maturity, drought and salt tolerance, etc. Although the development of new cultivars has been the primary objective of mutation breeding, the genetic stocks developed can have numerous applications in plant breeding, from being used as a donor parent in conventional breeding programmes or as a parent in hybrid breeding programmes. Apart from these, mutation research itself has also a very different objective, i.e., mapping of genes.[Citation12] The technique of identification of a gene by knockdown of the phenotypic expression through induced mutagenesis is a major component of research on molecular genetics and genomics today. However, the discussion of this technique is beyond the scope of this paper. This paper primarily concentrates on the application of mutation breeding towards crop improvement. IAEA has categorized its mutant variety database [Citation37] of 3222 (July 2015) varieties according to four breeding methods, namely
direct use of a mutant line that is developed through physical and chemical mutagenesis, or somaclonal variation;
indirect use of a mutant line/lines, which is/are used as a parental variety/varieties in cross breeding (cross between mutant lines or with a commercial variety/varieties);
use of mutant gene allele (trait), e.g. the Calrose 76 sd1 allele (semi-dwarf 1 trait) in rice and
use of wild species' genes translocated into plant genomes through irradiation-facilitated translocations, e.g. genes of wheat wild relative species.
Among the other classes, more success has been achieved by using mutant lines as a parent in breeding programmes as well as mutagenesis in breeding nurseries. As reviewed by Kharkwal and Shu,[Citation13] induced mutations have contributed to significant increase in crop production at locations people could directly access. The worldwide uses of new varieties derived directly or indirectly from induced mutants are rice in China, Thailand, Vietnam and the United States; barley in European countries and Peru; durum wheat in Bulgaria and Italy, wheat in China; soybean in China and Vietnam; as well as other food legumes in India and Pakistan.
Impact of mutation breeding in different countries
Over 232 different crops and plant species have been subjected to mutation breeding, including various essential crops, such as wheat, rice, grapefruit, rapeseed, sunflower, cotton and banana.[Citation37] The recent database of Food and Agriculture Organization of the United Nations (FAO)/IAEA [Citation37] indicates that 3222 mutant varieties with improved characters have been released officially as summarized in . More than 67% of the varieties were obtained through direct mutation.[Citation42,Citation43] The induced mutant varieties possess both agronomic and nutritional quality traits that make them the most preferred varieties on the market. Some examples of different applications of induced mutagenesis for biotic stress resistance in plant breeding are shown in . For instance, Peiris et al.[Citation44] described a tomato cultivar resistant to bacterial wilt (Ralstonia solanacearum). It was obtained by irradiation with 320 Gy gamma-rays in Sri Lanka. Similarly, there have been released numerous cultivars of rice, maize, wheat, cotton, chickpea, rapeseed, mungbean, sesame, apple and durum wheat that are resistant to different bacteria, viruses and pathogens.[Citation44–52] Tolerant and resistant varieties to various abiotic stresses [Citation53–73] have been released through induced mutagenesis as well (). These include lodging resistance, acid sulphate soil tolerance in rice,[Citation13,Citation42,Citation53] salinity tolerance in barley and sugarcane,[Citation72,Citation73] etc. Other targets of mutation breeding programmes in different plants include improvement of crop quality and various nutritional traits (), such as oil and protein quality,[Citation74–82] amylose, phytate, protein content, etc.[Citation83–87]
Table 3. Officially released mutant varieties in the FAO/IAEA Mutant Varieties Database, July 2015.[Citation37]
Table 4. Applications of induced mutagenesis for biotic stress resistance in plant breeding.
Table 5. Applications of induced mutagenesis for abiotic stress resistance in plant breeding.
Table 6. Applications of induced mutagenesis in improvement of crop quality and nutritional traits in plant breeding.
All these examples come to show that mutation breeding has had an ever-growing impact in crop production, especially in rice, as it is considered the most important food crop in the world. The advancements in plant mutation breeding have had such a significant socio-economic impact that an international symposium was especially dedicated to the topic of Induced Plant Mutations in the Genomics Era. It was held in 2009 in Vienna (Austria) and was organized by the IAEA and the FAO of the United Nations through the Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture. Details about some of the leading rice varieties obtained by mutation breeding in different countries are summarized in [Citation12,Citation13,Citation35,Citation42,Citation88–Citation92]
Table 7. Leading rice varieties obtained by mutation breeding.
The country that ranks first in development of new varieties through induced mutagenesis is China. It is well ahead of other countries in number of released varieties.[Citation12] In China, there have been developed major commercial mutant varieties of rice, wheat, maize, barley, millet, mulberry, rapeseed, soybean, pepper, cotton, tomato and groundnut. During the last few decades, many mutant varieties (810) belonging to 46 different species have been developed, released (or approved) for commercial production and used as donor parents in cross breeding as well. Combined use of mutation induction and in vitro bio-techniques has been employed to enhance wide-cross and to introduce alien genes into receptor species.[Citation93]
In view of practical breeding, many achievements have been made in mutation breeding in Japan over the past years. About 242 direct-use mutant varieties generated by using irradiation, chemical mutagenesis and somaclonal variations have been registered. About 61% of these were induced by gamma-ray irradiation, largely due to successful collaboration with the Institute of Radiation Breeding.[Citation13] This high percentage of gamma-ray irradiated mutants indicates that mutation breeding via gamma-ray irradiation is an effective and highly successful approach for the generation of commercial cultivars.[Citation53] Some mutant cultivars of Japanese pear exhibit resistance to diseases induced by gamma-ray irradiation and the development of a unique bioassay by using toxins of fungi has been discussed by Nakagawa.[Citation53] In addition, 228 indirect-use (hybrid) mutant varieties primarily generated in rice and soybean have found value as parental breeding germplasm resources in Japan. In 2005, the total cultivated area of mutant cultivars there was 210,692 ha and it was 12.4% of the total cultivated area of rice fields in Japan.[Citation53] The total crude income from mutant cultivars increased as the cultivated area increased. The total crude income from mutant cultivars was estimated to be approximately 250 billion Yen (2.34 billion US dollars) in 2005 though the price of grain differed on a yearly basis.[Citation53] Nakagawa [Citation49] reported a set of 17 mutant varieties, which were cultivated on more than 956,383 ha from 2001 to 2005. Also another set of 5 mutant cultivars were planted on a large total area of 2,886,378 ha as reported by Nakagawa.[Citation94] Comprehensive details regarding these varieties are provided in [Citation94]. For soybean, similar gamma-ray-induced mutants are cultivated in nearly 9.4% of the total cultivation area of soybean in Japan. In general, in 1961–2008, Japan released 481 registered mutant varieties.[Citation37]
In India, the first attempts to induce mutations date back to the 1930s, and a few spontaneous mutants were released as new cultivars in the 1940s. Sustained efforts to use induced mutations for genetic improvement of crop plants were initiated in the late 1950s.[Citation92] From 1950 to 2009, India has developed about 329 mutant varieties of different crops through direct mutagenesis of which major varieties have been developed for rice, wheat, barley, pearl millet, jute, groundnut, soybean, chickpea, mung bean, cowpea, black gram, sugarcane, chrysanthemum, portulaca, tobacco and Dahlia. Out of these 329 mutant varieties, about 50 varieties have been developed through using mutant lines in breeding programmes. The Indian mutation breeding programme became successful in the 1950s.[Citation35] The primary research centres and institutes in India that participated in the development and release of various mutants include the Indian Agricultural Research Institute (IARI), the Bhabha Atomic Research Centre, Tamil Nadu Agricultural University, and the National Botanical Research Institute.[Citation23] The efforts of these centres have brought a great breakthrough for India. The IARI has been a primary institution in India for research concerning induced mutations since 1957 and has released many mutant varieties of crops and ornamentals.[Citation42] Several gamma radiation-induced rice mutants were released in India as high-yielding varieties under the series ‘PNR’. Some of these varieties are short and mature early.[Citation95] Among these varieties, two early ripening and aromatic mutation-derived rice varieties, ‘PNR–381’ and ‘PNR–102’, are currently popular with farmers in Haryana and Utter Pradesh states. No data are available regarding the actual area planted with these varieties. However, based on the rate of fresh seed replacement by farmers, the distribution of breeder seeds, foundation seeds, certified seeds and the data obtained from the IARI, the value of rice (paddy) production would be 1748 million US dollars per year.[Citation42]
The wide use of high-yielding varieties has allowed Vietnam to become the second largest exporter of rice, exporting 4.3 million tons per year.[Citation96]. At present, mutant varieties yield about 15% of the annual rice production in Vietnam. With the great achievements attributed to the use of nuclear techniques, more than 55 mutant varieties have been developed, most of which are cereal crops, especially rice.[Citation97] These varieties substantially contribute to food self-sufficiency. Mutant rice varieties have been planted on over 1.0 million ha in areas, including Hatay, Bacgiang, Nghean Vinhphuc, Hanam, Thaibinh and Hanoi of northern Vietnam, and they have already produced significant social and economic effects, contributing to poverty relief in some areas.[Citation42] Besides yield varieties, numerous other varieties have been developed, e.g. ones with good performance in quality (aroma, protein, amylase content), as well as tolerance to harmful environmental conditions, such as salinity, cold or high temperatures, drought, lodging variety, etc. It has been estimated that mutant varieties cover over 2,540,000 ha cultivated area since the time of release with an added return of 374.4 million USD.[Citation69]
In Thailand, the work on induced mutations in rice was initiated in 1965 and was stimulated in cooperation with IAEA.[Citation42] Two aromatic indica-type varieties of rice, ‘RD6’ and ‘RD15’, which were developed by gamma irradiation of a popular rice variety, ‘Khao Dawk Mali 105’ (‘KDML 105’), were released in 1977 and 1978, respectively. Today, over 30 years after their release, these varieties remain extensively grown in Thailand. RD6 has glutinous endosperm and retains all of the grain characters, including the aroma of its parent variety. In contrast, RD15 is non-glutinous and aromatic, similar to the parent, but ripens 10 days earlier than the parent. This is a prized advantage for harvesting before the outset of the rainy season in wet areas. According to the Bureau of Economic and Agricultural Statistics of Bangkok, during 1995–1996, RD6 was grown on 2,429,361 ha, covering 26.4% of the area under rice in Thailand and produced 4,343,549 tons paddy.[Citation13,Citation42]
In Bangladesh, more than 44 mutant varieties belonging to 12 different crop species have been released through mutation breeding.[Citation12] The Bangladesh Institute of Nuclear Agriculture in Mymensingh, Bangladesh, is the primary centre of mutation breeding and has released up to eight mutant rice varieties [Citation37]. Rice mutants, including Binasail, Iratom-24 and Binadhan-6, were all planted in a cumulative area of 795,000 ha and contributed substantially towards food security in Bangladesh.[Citation13]
The United States of America have produced a semi-dwarf gene allele, sd1, through gamma-ray mutagenesis.[Citation13] This triggered the American version of the ‘Green Revolution’ in rice. Details regarding the sd1 allele and its contribution to rice production in the United States are discussed by Rutger.[Citation98]
In Pakistan, a mutation programme was initiated at the Nuclear Institute for Agriculture and Biology to improve important food and fibre crops. The crops selected for improvement include rice, chickpea, mungbean and cotton. Improvement has been sought in plant architecture, maturity period, disease resistance, etc.[Citation99] The results achieved so far have helped to evolve better varieties/germplasm in these crops. The primary triumph of the Nuclear Institute of Agriculture is the release of four improved varieties of rice that were obtained using induced mutagenesis. These varieties have contributed to a meaningful improvement in the socio-economic conditions of farmers and to an increase in the yield per hectare in Pakistan.[Citation99]
In Malaysia, the mutation breeding approach has had limited application. However, this technique has brought about the release of several rice mutant varieties, such as Q 34,[Citation100] SPM 130 and SPM 142,[Citation101] and MRQ 50 [Citation102]. Targets for improvement include plant architecture, maturity period and resistance to biotic and abiotic factors. These attempts can be regarded as successful, as they have made significant contributions to the varietal development in Malaysia.
Mutation breeding programmes have also been conducted in different European countries. For example, in Bulgaria, more than 76 new cultivars have been developed using induced mutagenesis, namely maize (26), durum wheat (9), tomato (6), barley (5), wheat (5), soybean (5), pepper (4), lentil (4), sunflower (3), cotton (2), tobacco (2), bean (2) and pea (1). Maize has the largest number of varieties developed through mutation breeding, amounting to 26 varieties released so far. These varieties show high grain yield and productivity; tolerance to dense sowing; early ripening; drought tolerance; high protein content; high biomass dry matter; shifts in the flowering time; white-colour grain; strong stem; altered ear length; increased number of rows.[Citation66] A maize hybrid, Kneja 509, has become a leading cultivar occupying up to 50% of the growing area of this crop.[Citation66]
In other European countries, development of short-height and high-yielding mutant cultivars of barley ‘Golden Promise’ and ‘Diamant’ have made a major impact on the brewing industry. These two mutants have also been used as parents for many leading barley cultivars across Europe, North America and Asia. For example, it was reported by Ahloowalia et al.[Citation42] that more than 150 leading barley cultivars were derived from crosses involving ‘Diamant’. This mutant cultivar was officially released in Czechoslovakia in 1965 through gamma-ray irradiation of ‘Valticky’. ‘Diamant’ has 12% increased grain yield and is 15 cm shorter than the parent cultivar and was cultivated on more than 43% of the barley growing area in Czechoslovakia in 1972. Bouma and Ohnoutka [Citation103] report spring barley cultivars that had mutated Diamant's gene grown on 2.86 million ha across Europe in 1972. In contrast, the Golden Promise cultivar was developed through gamma-ray irradiation of malting cultivar ‘Maythorpe’. Even after 30 years of release, Golden Promise is still popular and is widely used by the brewing industry in Ireland, Scotland and the United Kingdom for the production of beers and whisky. This cultivar is characterized by improved malting quality, average yield of 4.5 t/ha and stiff straw. Recent studies also show that this cultivar is salt-tolerant as compared with the parent cultivar Maythorpe, which is salt-sensitive.[Citation104,Citation105]
There is no doubt that there are many more cultivars/varieties derived by means of mutation breeding all over the world. Albeit not completely comprehensive, the examples reviewed here could serve to illustrate the remarkable effect that this technique has had on world agriculture both from a historical and contemporary perspective. Against the backdrop of the still ongoing debates concerning the safety of genetically modified organisms, crop varieties and cultivars that have been derived by mutation breeding in the last 50 years, continue to play a key role in present-day agriculture.
Conclusions
The database on released mutant cultivars since 1950 shows specific trends of the activity on radiation-induced mutations in over 70 countries. Of 3222 registered mutant varieties in more than 232 different crops and plant species released through induced mutation, the largest number is in China (810), followed by Japan (481) and India (330). On a crop basis, the maximum released mutant cultivars are rice varieties (815), being the most important food crop in the world. Induced mutagenesis and its breeding strategies are potential tools for improving both quantitative and qualitative traits in crops within a much shorter period of time than conventional breeding. Because of its relative simplicity and low cost, mutagenic treatment of seeds and other parts of the plant remains a useful tool for isolating the desired variants and developing resistance to biotic and abiotic stresses in various crops. Thus, the released mutant cultivars are already a part of the overall strategy and commitment of the Joint FAO/IAEA Division to contribute towards the global food security. Therefore, the impact of mutation breeding-derived crop varieties around the world demonstrates the potential of mutation breeding as a flexible and practicable approach applicable to any crop provided that appropriate objectives and selection methods are followed accordingly.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Novak FJ, Brunner H. Plant breeding: induced mutation technology for crop improvement. IAEA Bull. 1992;4:25–33.
- Shu QY, Forster BP, Nakagawa H. Principles and applications of plant mutation breeding. In: Shu QY, Forster BP, Nakagawa H, editors. Plant mutation breeding and biotechnology. Wallingford: CABI; 2012. p. 301–325.
- Kharkwal MC. A brief history of plant mutagenesis. In: Shu QY, Forster BP, Nakagawa H, editors. Plant mutation breeding and biotechnology. Wallingford: CABI; 2012. p. 21–30.
- Lönnig WE. Mutation breeding, evolution, and the law of recurrent variation. Recent Res Dev Genetics Breeding. 2005;2:45–70.
- Ahloowalia BS, Maluszynski M. Induced mutations – a new paradigm in plant breeding. Euphytica. 2001;118(2):167–73.
- Broertjes C, Van Harten AM. Applied mutation breeding for vegetatively propagated crops. Vol.12, Developments in crop science. Amsterdam: Elsevier Publ.; 1988. p. 197–204.
- Chai M, Ho YW, Liew KW, et al. Biotechnology and in vitro mutagenesis for banana improvement. In: Jain SM, Swennen R, editors. Banana improvement: cellular, molecular biology, and induced mutations. Enfield (NH): Science Publishers, Inc.; 2004. p. 59–77.
- Predieri S. Mutation induction and tissue culture in improving fruits. Plant Cell Tissue Organ Culture. 2000;64(2–3):185–210.
- Kondo E, Nakayama M, Kameari N, et al. Red–purple flower due to delphinidin 3,5–diglucoside, a novel pigment for Cyclamen spp., generated by ion-beam irradiation. Plant Biotechnol. 2009;26(5):565–569.
- Hensz RA. ‘Ray Ruby’ grapefruit, a mutant of ‘Ruby Red’, with redder flesh and peel color. J Rio Grande Valley Horticultural Society. 1978;32:39–41.
- Pathirana R. Plant mutation breeding in agriculture. In: Hemming D, editor. Plant sciences reviews 2011. Cambridge: CABI; 2012. p. 107–125.
- Roychowdhury R, Tah J. Mutagenesis–a potential approach for crop improvement. In: Hakeem KR, Ahmad P, Ozturk M, editors. Crop improvement: new approaches and modern techniques. New York (NY): Springer; 2013. p. 149–187.
- Kharkwal MC, Shu QY. The role of induced mutations in world food security. In: Shu QY, editor. Induced plant mutations in the genomics era. Rome: Food and Agriculture Organization of the United Nations; 2009. p. 33–38.
- Forster BP, Shu QY. Plant mutagenesis in crop improvement: basic terms and applications. In: Shu QY, Forster BP, Nakagawa H, editors. Plant mutation breeding and biotechnology. Wallingford: CABI; 2012. p. 9–20.
- Mba C. Induced mutations unleash the potentials of plant genetic resources for food and agriculture. Agronomy. 2013;3(1);200–231.
- Van Harten AM. Mutation breeding: theory and practical applications. Cambridge: Cambridge University Press; 1998.
- Stadler LJ. Some genetic effects of X–rays in plants. J Heredity. 1930;2:3–20.
- Acquaah G. Principles of plant genetics and breeding. Chichester: Wiley-Blackwell; 2006.
- Oldach KH. Mutagenesis. In: Pratap A, Kumar J, editors. Biology and breeding of food legumes. Wallingford: CABI; 2011. p. 208–219.
- Nencheva D. In vitro propagation of Chrysanthemum. In: Jain SM, Sergio O, editors. Protocols for in vitro propagation of ornamental plants. Humana Press; 2010. p. 177–185.
- Leitāo JM. Chemical mutagenesis. In: Shu QY, Forster BP, Nakagawa H, editors. Plant mutation breeding and biotechnology. Wallingford: CABI; 2012. p. 135–158.
- Mba C, Afza R, Bado S, et al. Induced mutagenesis in plants using physical and chemical agents. In: Davey MR, Anthony P, editors. Plant cell culture: essential methods. Chichester: John Wiley & Sons, Ltd.; 2010. p. 111–130.
- Wani MR, Kozgar MI, Tomlekova N, et al. Mutation breeding: a novel technique for genetic improvement of pulse crops particularly Chickpea (Cicer arietinum L.). In: Parvaiz A, Wani MR, Azooz MM, Lam-son PT, editors. Improvement of crops in the era of climatic changes. New York (NY): Springer; 2014. p. 217–248.
- Mba C, Afza R, Shu QY. Mutagenic radiations: X–rays, ionizing particles and ultraviolet. In: Shu QY, Forster BP, Nakagawa H, editors. Plant mutation breeding and biotechnology. Wallingford: CABI; 2012. p. 83–90.
- Mahadevappa M, Ikehashi H, Coffman WR, et al. Improvement of native rice for earliness though induced mutagenesis. Oryza. 1983;20(1):40–46.
- Khin TN. Rice mutation breeding for varietal improvement in Myanmar. Plant Mutation Rep. 2006;1:34–36.
- Amano E. Use of induced mutants in rice breeding in Japan. Plant Mutation Rep. 2006;1(1):21–24.
- Altenbung E. The artificial production of mutations by ultraviolent light. Am Naturalist. 1934;68:491–507.
- Naito K, Kusaba M, Shikazono N, et al. Transmissible and nontransmissible mutations induced by irradiating Arabidopsis thaliana pollen with γ–rays and carbon ions. Genetics. 2005;169(2):881–889.
- Yamaguchi H, Nagatomi S, Morishita T, et al. Mutation induced with ion-beam irradiation in rose. Nucl Instr Meth Phys Res. 2003;206:561–564.
- Watanabe H. Significance and expectations of ion beam breeding. Gamma Field Symposia. 2001;40:15–19.
- Shikazono N, Yokota Y, Kitamura S, et al. Mutation rate and novel tt mutants of Arabidopsis thaliana induced by carbon ions. Genetics. 2003;163:1449–1455.
- Matsumura A, Nomitzu T, Furukani N, et al. Ray florets colour and shape mutants induced by 12C5+ ion beam irradiation in chrysanthemum. Scientia Horticulturae. 2010;123:558–561.
- Abe T, Matsuyama T, Sekido S, et al. Chlorophyll-deficient mutants of rice demonstrated the deletion of a DNA fragment by heavy–ion irradiation. J Radiat Res. 2002;43(Suppl):S157–S161.
- Bughio HR, Asad MA, Odhano IA, et al. Sustainable rice production through the use of mutation breeding. Pakistan J Bot. 2007;39:2457–2461.
- Maluszynski M, Szarejko I, Bhatia CR, et al. Methodologies for generating variability. In: Ceccarelli S, Guimar EP, Weltzien E, editors. Plant breeding and farmer participation. Rome: Food and Agriculture Organization (FAO); 2009. Available from: http://www.fao.org/docrep/012/i1070e/i1070e00.htm
- IAEA. IAEA mutant database. Vienna: International Atomic Energy Agency; c2015 [accessed July 2015]. Available from: http://mvd.iaea.org/
- Bradshaw LD, Padgette SR, Kimball SL, et al. Perspectives on glyphosate resistance. Weed Technol. 1997;11:189–198.
- Oldach KH, Peck DM, Cheong J, et al. Identification of a chemically induced point mutation mediating herbicide tolerance in annual medics (Medicago spp.). Annals Bot. 2008;101:997–1005.
- Roychowdhury R, Bandyopadhyay A, Dalal T, et al. Biometrical analysis for some agro-economic characters in M1 generation of Dianthus caryophyllus. Plant Arch. 2011;11(2):989–994.
- Roychowdhury R, Datta S, Gupta P, et al. Analysis of genetic parameters on mutant populations of mungbean (Vigna radiata L.) after ethyl methane sulphonate treatment. Not Sci Biol. 2012;4(1):137–143.
- Ahloowalia BS, Maluszynski M, Nichterlein K. Global impact of mutation-derived varieties. Euphytica. 2004;135:187–204.
- Maluszynski M, Gustafson P, Maluszynska J, et al. Advanced breeding for germplasm enhancement and yield improvement. In: Minas KP, Frank JD, Edward MH, editors. Yield gap and productivity decline in rice production. Rome: FAO; 2001. p. 191–224.
- Peiris R, Wickramasinghe TK, Indrasena SP. M 127–A promising tomato variety developed through induced mutation technique. In: Shu QY, editor. Induced plant mutations in the genomics era. Proceedings of an International Joint FAO/IAEA Symposium. Rome: Food and Agriculture Organization of the United Nations; 2008. p. 379–380.
- Liu L, Shi S, Wu J, et al. Mutation induction and in vitro screening for stem rot resistant/tolerant materials with oxalic acid in rape (Brassica napus L.). Chinese J Oil Crop Sci. 2003;25(1):5–8.
- Haq MA, Sadiq M, Hassan M. ‘CM 98’ (CM31–1/85) – a very high yielding disease resistant mutant variety of chickpea. Int Chickpea Pigeonpea Newsletter. 1999;6: 9–10.
- Haq MA, Sadiq M, Hassan M. CM88 – a multiple disease resistant chickpea mutant variety. Mutat Breed Newsletter. 2001;45:5–6.
- Luzi-Kihupi A, Shao-Mwalyego F, Zakayo JA, et al. Mwangaza – a new early maturing, RYMV resistant rice mutant released in the United Republic of Tanzania. Plant Mutat Rep. 2008;2(1):13–5.
- Iqbal RMS, Chaudhry MB, Aslam M, et al. Economic and agricultural impact of mutation breeding in cotton in Pakistan–a review. Plant mutation breeding for crop improvement. Vol. 1. Vienna: International Atomic Energy Agency (IAEA); 1991. p. 187–201.
- Pathirana R, Weerasena LA, Bandara P. Development and release of gamma ray induced sesame mutant ANK–S2 in Sri Lanka. Trop Agric Res Extension. 2000;3(1):19–24.
- Pathirana R. Gamma-ray-induced field tolerance to phytophthora blight in sesame. Plant Breeding. 1992;108:314–319.
- Kiruki S, Onek LA, Limo M. Azide-based mutagenesis suppresses Striga hermonthica seed germination and parasitism on maize varieties. Afr J Biotechnol. 2006;5(10):866–870.
- Nakagawa, H. Induced mutations in plant breeding and biological researches in Japan. In: Shu QY, editor. Induced plant mutations in the genomics era. Proceedings of an International Joint FAO/IAEA Symposium. Rome: Food and Agriculture Organization of the United Nations; 2009. p. 48–54.
- Patnaik D, Chaudhary D, Rao GJN. Genetic improvement of long grain aromatic rices through mutation approach. Plant Mutation Rep. 2006;1(1):11–16.
- Rutger JN. Thirty years of induction, evaluation, and integration of useful mutants in rice genetics and breeding. Plant Mutation Rep. 2006;1(2):4–13.
- Oladosu Y, Rafii MY, Abdullah N, et al. Genetic variability and selection criteria in rice mutant lines as revealed by quantitative traits [Internet]. Scientific World J. 2014;2014:190531. Available from: http://dx.doi.org/10.1155/2014/190531.
- Rutger JN, Peterson MI, Hu CH. Registration of Calrose 76 rice. Crop Sci. 1976;17:978.
- Foster KW, Rutger JN. Inheritance of semidwarfism in rice Oryza sativa L. Genetics. 1978;88:559–574.
- Soldatov KI, Kalaidzhan AA. Production of dwarf and semidwarf mutant forms of sunflower. Khimicheskii Mutagenez i Problemy Selektsii. Moscow: Nauka; 1991. p. 208–212. Russian.
- Ismachin M, Sobrizal A. Significant contribution of mutation techniques to rice breeding in Indonesia. Plant Mutation Rep. 2006;1:18–21.
- Mohamad O, Mohd. Nazir B, Alias I, et al. Development of improved rice varieties through the use of induced mutations in Malaysia. Plant Mutation Rep. 2006;1:27–34.
- Haq MA. Development of mutant varieties of crop plants at NIAB and the impact on agricultural production in Pakistan. In: Shu QY, editor. Induced plant mutations in the genomics era. Proceedings of an International Joint FAO/IAEA Symposium. Rome: Food and Agriculture Organization of the United Nations; 2009. p. 61–64.
- Oladosu Y, Rafii MY, Abdullah N, et al. Genetic variability and diversity of mutant rice revealed by quantitative traits and molecular markers. Agrociencia. 2015;49(3):249–266.
- Rutger JN, Bryant RJ. Registration of aromatic se rice germplasm. Crop Sci. 2004;44(1):363–364.
- Lal JP, Tomer AK. Genetic enhancement of lentil (Lens culinaris Medikus) for drought tolerance through induced mutations. In: Shu QY, editor. Induced plant mutations in the genomics era. Proceedings of an International Joint FAO/IAEA Symposium. Rome: Food and Agriculture Organization of the United Nations; 2009. p. 151–154.
- Tomlekova NB. Induced mutagenesis for crop improvement in Bulgaria. Plant Mutation Rep. 2010;2(2): 4–27.
- Cheema AA. Mutation breeding for rice improvement in Pakistan: achievements and impact. Plant Mutation Rep. 2006;1:36–39.
- Balooch AW, Soomro AM, Naqvi MH, et al. Sustainable enhancement of rice (Oryza sativa L.) production through the use of mutation breeding. Plant Mutat Rep. 2006;1:40–42.
- Tran DQ, Dao TTB, Nguyen HD, et al. Rice mutation breeding in Institute of Agricultural Genetics, Viet Nam. Plant Mutation Rep. 2006;1:47–49.
- Do KT, Dao MS, Hung PQ, et al. Rice mutation improvement for short duration, high yield and tolerance to adverse conditions in Mekong Delta of Viet Nam. Plant Mutation Rep. 2006;1:49–51.
- González MC, Pérez N, Cristo E, et al. Development of salinity-tolerant rice varieties using biotechnological and nuclear techniques. In: Shu QY, editor. Induced plant mutations in the genomics era. Proceedings of an International Joint FAO/IAEA Symposium. Rome: Food and Agriculture Organization of the United Nations; 2009. p. 138–140.
- Moustafa RAK. Development of salt–tolerant high–yielding barley lines via crossing between a mutant induced by EMS and a local cultivar. In: Shu QY, editor. Induced plant mutations in the genomics era. Proceedings of an International Joint FAO/IAEA Symposium. Rome: Food and Agriculture Organization of the United Nations; 2009. p. 148–150.
- Suprasanna P, Patade VY, Vaidya ER, et al. Radiation induced in vitro mutagenesis, selection for salt tolerance and characterization in sugarcane. In: Shu QY, editor. Induced plant mutations in the genomics era. Proceedings of an International Joint FAO/IAEA Symposium. Rome: Food and Agriculture Organization of the United Nations; 2009. p. 145–147.
- Fehr WR, Hammond EG. U.S. Patent No. 6,133,509. Washington (DC): U.S. Patent and Trademark Office; 2000.
- Hammond EG, Fehr WR. Registration of A5 germplasm line of soybean. Crop Sci. 1983;23:192–193.
- Patil A, Taware SP, Rao VS. Development of a high oleic soybean mutant and its stability across the environments. In: Shu QY, editor. Induced plant mutations in the genomics era. Proceedings of an International Joint FAO/IAEA Symposium. Rome: Food and Agriculture Organization of the United Nations; 2009. p. 200–202.
- Landge SP, Barve YY, Gupta RK, et al. Development of B. napus canola quality varieties suitable for Indian agro-climatic conditions by induced mutations. In: Shu QY, editor. Induced plant mutations in the genomics era. Proceedings of an International Joint FAO/IAEA Symposium. Rome: Food and Agriculture Organization of the United Nations; 2009. p. 322–326.
- D'Souza SF, Reddy KS, Badigannavar AM, et al. Mutation breeding in oilseeds and grain legumes in India: accomplishments and socio-economic impact. In: Shu QY, editor. Induced plant mutations in the genomics era. Proceedings of an International Joint FAO/IAEA Symposium. Rome: Food and Agriculture Organization of the United Nations; 2009. p. 55–57.
- Mondal S, Badigannavar AM, D'Souza SF. Induced variability for fatty acid profile and molecular characterization of high oleate mutant in cultivated groundnut (Arachis hypogaea L.). Plant Breeding. 2011;130(2):242–247.
- Kaveri SB, Nadaf HL. Induced mutagenesis for oil quality enhancement in peanut (Arachis hypogaea L.). In: Shu QY, editor. Induced plant mutations in the genomics era. Proceedings of an International Joint FAO/IAEA Symposium. Rome: Food and Agriculture Organization of the United Nations; 2009. p. 191–194.
- Fernández–Martínez JM, Pérez–Vich B, Velasco L. Mutation breeding for oil quality improvement in sunflower. In: Shu QY, editor. Induced plant mutations in the genomics era. Proceedings of an International Joint FAO/IAEA Symposium. Rome: Food and Agriculture Organization of the United Nations; 2009. p. 177–181.
- Yathaputanon C, Kumsueb B, Malipan A, et al. Protein content in high-protein soybean mutants in Thailand. In: Shu QY, editor. Induced plant mutations in the genomics era. Proceedings of an International Joint FAO/IAEA Symposium. Rome: Food and Agriculture Organization of the United Nations; 2009. p. 195–196.
- Ceballos H, Sanchez T, Denyer K, et al. Induction and identification of a small-granule, high-amylose mutant in cassava (Manihot esculenta Crantz). J Agr Food Chem. 2008;56(16):7215–7222.
- Landau-Ellis D, Pantalone VR. Marker-assisted backcrossing to incorporate two low phytate alleles into the Tennessee soybean cultivar 5601T. In: Shu QY, editor. Induced plant mutations in the genomics era. Proceedings of an International Joint FAO/IAEA Symposium. Rome: Food and Agriculture Organization of the United Nations; 2009. p. 316–318.
- Bregitzer P, Raboy V, Obert DE, et al. Registration of ‘Clearwater’ low-phytate hulless spring barley. J Plant Regist. 2008;2(1):1–4.
- Chen X, Liu X, Wu D, et al. Recent progress of rice mutation breeding and germplasm enhancement in China. Plant Mutation Rep. 2006;1(1):4–6.
- Hamid MA, Azad MAK, Howlidar MAR. Development of three varieties of groundnut with improved quantitative and qualitative traits through induced mutation. Plant Mutation Rep. 2006;1(2):14–17.
- Shu Q, Wu D, Xia Y. The most widely cultivated rice variety ‘Zhefu 802’ in China and its geneology. MBNL. 1997;43:3–5.
- Wang H, Qiu S, Zheng J, et al. Generation of new rice cultivars from mature pollen treated with Gamma–radiation. In: Shu QY, editor. Induced plant mutations in the genomics era. Proceedings of an International Joint FAO/IAEA Symposium. Rome: Food and Agriculture Organization of the United Nations; 2009. p. 231–234.
- Ro PV, At DH. Improvement of traditional local varieties through induced mutations using nuclear techniques. In: Quy TD, Dong NH, Thanh LD, Quyen NHM, Truc PD, Ro PV, editors. Seminar on Methodology for Plant Mutation Breeding for Quality: Effective use of physical/chemical Mutagens, Oct 9–13; Hanoi: Japan Atomic Energy Research Institute, JERI; 2000. p. 90–94.
- Badawi AT. Yield gap and productivity decline in rice production in Egypt. Yield gap and productivity decline in rice production. Expert consultation. Proceedings; 2000 Sep 5–7; Rome. Rome: Food and Agriculture Organization of the United Nations; 2001.
- Bhatia CR. Economic impact of mutant varieties in India. Plant Mutation Breed Crop Improvement. 1991;1:33–45.
- Linqing W. Induced mutation for crop improvement in China; a review. Plant mutation breeding for crop improvement. Vol. 1. Vienna: International Atomic Energy Agency (IAEA); 1991. p. 9–32.
- Nakagawa H. Mutation breeding, status quo and future. Techno Innovation. 2008;68:6–12.
- Chakrabarti SN. Mutation breeding in India with particular reference to PNR rice varieties. J Nucl Agric Biol. 1995;24:73–82.
- Vinh MQ, Thinh DK, Bang DT, et al. Current status and research directions of induced mutation application to seed crops improvement in Vietnam. In: Shu QY, editor. Induced plant mutations in the genomics era. Proceedings of an International Joint FAO/IAEA Symposium. Rome: Food and Agriculture Organization of the United Nation; 2009. p. 341–345.
- Do KT. Socio-economic impacts of mutant rice varieties in Southern Vietnam. In: Shu QY, editor. Induced plant mutations in the genomics era. Proceedings of an International Joint FAO/IAEA Symposium. Rome: Food and Agriculture Organization of the United Nation; 2009 p. 65–70.
- Rutger JN. The induced SD1 mutant and other useful mutant genes in modern rice varieties. In: Shu QY, editor. Induced plant mutations in the genomics era. Proceedings of an International Joint FAO/IAEA Symposium. Rome: Food and Agriculture Organization of the United Nation; 2009. p. 44–47.
- Awan MA. Use of induced mutations for crop improvement in Pakistan. Plant mutation breeding for crop improvement. Vol. 1. Vienna: International Atomic Energy Agency (IAEA); 1991. p. 67–72.
- Hadzim K, Othman O, Ajimilah NH. Height reduction of mutant Mahsuri and identification of Q34. Teknol Padi. 1994;10:17–23.
- Azlan S, Alias I, Saad A, et al. Performance of potential mutant lines of MR 180. In: Sivaprasagam, editor. Modern rice farming. Proceedings of International Rice Conference Serdang, Malaysia: MARDI; 2004. p. 293–296.
- Phang FK, Darman WA, Othman O. Commercial production of high quality rice - The MRQ 50 rice estate in Rompin, Pahang. In: Sivaprasagam, et al. editor. Modern rice farming. Proceedings of International Rice Conference Serdang; Malaysia. MARDI; 2004. p. 251–259.
- Bouma J, Ohnoutka Z. Importance and application of the mutant ‘Diamant’ in spring barley breeding. Plant mutation breeding for crop improvement. Vol. 1. Vienna: IAEA; 1991. p. 127–133.
- Wei W, Bilsborrow P, Hooley P, et al. Variation between two near isogenic barley (Hordeum vulgare) cultivars in expression of the B subunit of the vacuolar ARPase in response to salinity. Hereditas. 2001;135:227–231.
- Forster BP. Mutation genetics of salt tolerance in barley: an assessment of Golden Promise and other semi-dwarf mutants. Euphytica. 2001;120:317–328.