ABSTRACT
Synthetic dyes are known to be highly toxic to mammalian cells and mutagenic and carcinogenic to humans and, therefore, should be detoxified and removed from industrial effluents. Different approaches for removal and detoxication are extensively sought. Biochemical methods are considered the most economical and effective method of dye decolourization. In this research, the laccase gene from Laccaria bicolor was modified and expressed in Pichia pastoris. The properties of the recombinant laccase and its ability to degrade synthetic dyes were studied. The laccase activity was optimal at pH 2.2 and 50 °C. Its Km value was 0.187 mmol/L for ABTS [2,2'-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid)]. The laccase obtained was shown to decolorize the synthetic dyes, malachite green, crystal violet and orange G, with ABTS as a mediator. These results indicated that the laccase obtained may be used to treat industrial effluents containing artificial dyes.
Introduction
Laccases (benzenediol:oxygen oxidoreductases, EC 1.10.3.2), members of the blue multi-copper oxidase family, catalyse the oxidation of a wide range of organic substrates, including phenols, polyphenols, anilines and even certain inorganic compounds. They were first discovered in plants and have been subsequently identified in fungi, insects and some bacteria. Laccases are involved in lignification, delignification, sporulation and plant pathogenesis. They can catalyse the four-electron reduction of O2 to H2O with concomitant oxidation typically of a phenolic substrate. The free radical of the substrate is unstable and may undergo a second enzyme-catalysed reaction or a non-enzymatic reaction.
The production of natural laccases is low and cannot meet the requirements of industrial applications. It is feasible to recombine cloned and modified laccase genes into Pichia pastoris, which is a well-established host organism, and express them. A lot of heterologous laccases have been reported, for example, in Fome lignosus,[Citation1] Pycnoporus cinnabarinus,[Citation2] Pleurotus sajor-caju [Citation3] and Pycnoporus sanguineus.[Citation4] The expression level of heterologous laccases can be enhanced by using the methanol-inducible alcohol oxidase 1 (AOX1) promoter and applicable vectors in P. pastoris, and protein production can also be raised by increasing the copy number of the laccase gene. Besides, P. pastoris can grow to high cell density during fermentation, which is extremely useful in industrial fermentation.
Some industries widely use synthetic dyes, which are released into the aquatic environment as a component of waste waters. However, they are cytotoxic agents, mutagens and carcinogens to fish and mammalians. In the USA and Europe, although malachite green (MG) and crystal violet (CV), as typical triphenylmethane dyes, are banned in food fish because of significant health risks, these dyes are persistently used in different countries as an effective fungicide, bactericide, ectoparasiticide and antiprotozoan in fish.[Citation5] That is why it is necessary to investigate various methods for the treatment of triphenylmethane dyes. Decolourization and detoxification of triphenylmethane dyes by laccase have been reported.[Citation6] To our knowledge, fungal laccases are normally difficult to use to decolorize azo dyes without a mediator. Taking account of the low cost, high efficiency, simple procedure and reduced generation of secondary pollution, biochemical methods are considered the most economical and effective method of dye decolourization and degradation.[Citation7]
In this study, we synthesized a gene according to the protein sequence of LBLCC3I, and expressed it in P. pastoris. The enzymatic characteristics and the ability of the enzyme to degrade synthetic dyes were investigated.
Materials and methods
Microorganisms, reagents and culture conditions
The Escherichia coli strain DH5α (Invitrogen, USA) was applied to DNA and plasmid manipulations. P. pastoris strain GS115 (Invitrogen, USA) was used for heterologous expression of laccase. pMD18-T (Takara) and modified pPIC9K vectors (our lab) were used as a cloning and expression vectors, respectively. All enzymes used in DNA manipulation procedures were purchased from TaKaRa (Dalian, China). Bradford protein quantitative test kit was purchased from Shanghai Generay Biotech Co., Ltd. The synthetic dyes, MG, CV and orange G, were purchased from Shanghai Sangon Co., Ltd. The media used for the expression of recombinant protein were prepared following the Multi-Copy Pichia Expression Kit (Invitrogen, USA).
Synthesis, cloning and vector construction
According to the amino acid sequence of LBLCC3 of L. bicolor (GenBank: FJ432086.1), we designed and synthesized the gene, and renamed it LBLCC3I. Bam HI and a Sac I site were added at the 5′-end and 3′-end, respectively. The synthetic gene was cloned into the pMD18-T vector and sequenced. This plasmid was digested by Bam HI and Sac I, and the fragment of the LBLCC3I gene was inserted directly into a modified pPIC9k vector to form the LBLCC3I gene expression vector. The Sac I site in the AOX 1 promoter and Xho I site in the G418 cassette of the modified pPIC9K vector were removed by site-directed mutagenesis, and a 6×His-tag was added after Kex2 protease cleavage at the site of the MF α-signal sequence.
Transformation, screening and expression of LBLCC3I in P. pastoris
The expression vector was transformed into E. coli DH5α and extracted according to the standard method. Ten microliter plasmid was digested into two fragments with restriction enzyme Bgl II, and then transformed into P. pastoris GS115 competent cells by electroporation (Bio-Rad Genepulser, Hercules, CA, USA). Then, P. pastoris cells were spread on selective regeneration dextrose medium plates and incubated at 28 °C until colonies appeared. The colony(ies) was(were) picked and streaked on BMMY (SD, 0.000004% biotin) plates containing ABTS [2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid)]. If the LBLCC3I gene was recombined into yeast genomic DNA and expressed successfully, the medium around the yeast colony would turn blue. The blue colonies were selected and further confirmed by polymerase chain reaction (PCR).
A single colony was picked and inoculated in 50 mL of BMGY (SD, 1% yeast extract, 2% peptone, 1.34% YNB, 0.000004% biotin, 1% glycerol) medium at 28 °C in a shaking incubator until the culture reached to OD600 of 2–6. The cells were harvested by centrifugation (5000 r/min for 5 min, 4 °C) and resuspended in 100 mL of BMGY, BMM (buffered minimal methanol medium) or MM (minimal methanol medium) medium supplemented with 0.5 mmol/L copper sulphate and returned to the incubator to grow for three days. Methanol was added to a final concentration of 1% every 24 h.
Purification and characterization of recombinant laccase
The concentration of purified protein was quantified by the Bradford method protein quantification kit with bovine serum albumin as a standard. The gel was stained with Coomassie brilliant blue. The crude enzyme in the supernatant of the inducted culture was precipitated by 40% and 70% ammonium sulphate. Phosphate-buffered saline (PBS; pH 6.0) was used to dissolve and dialyse the precipitate. Then, the desalted solution was purified by nickel-charged iminodiacetic acid column (Sigma-Aldrich), according to the operation manual. The molecular mass of the laccase was estimated by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) with a 12% polyacrylamide gel.
The activity measurement was performed with 0.5 mmol/L ABTS in 0.2 mol/L citrate-phosphate buffer at 37 °C in a total of 200 μL. The assay mixture was incubated at 37 °C for 10 min before the enzyme was added. The reaction was terminated by addition of 50 μL 1.0 mol/L NaF. The absorbance of the reactant was measured at 420 nm (Tecan) (ϵ = 12,000 L/(mol cm)). One unit (U) of laccase activity was defined as the amount of enzyme catalysing the formation of 1.0 μmol of product per minute under the assay conditions. The optimum pH was determined spectrophotometrically with ABTS in 0.2 mol/L citrate--phosphate buffer (pH 2.2–7.8) at 37 °C. The pH stability of LBLCC3I was assessed by enzyme incubation at 4 °C in various buffers for 24 h. The optimum temperature was investigated between 30 and 80 °C at 5 °C increments. Thermostability assays were determined by incubating the enzyme in McIIvaine buffer (pH 2.2) at 20, 30, 40, 50, 60 or 70 °C for 60 min at 10-min increments. Enzyme activity at starting time was assumed as 100% stability. Enzyme assays were performed in triplicates. The Km was done with freshly prepared enzyme mixed with various concentrations of ABTS (from 0.005 to 2 mmol/L) added to McIIvaine buffer (pH 2.2) at 50 °C for 20 min. There were three replicates of each treatment.
Degradation of synthetic dyes
The ability of purified laccase to decolourize three synthetic dyes was studied. The reaction mixture for degradation experiments contained a substrate pollutant solution and enzyme preparation in a total of 200 μL of 0.2 mol/L citrate--phosphate buffer (pH 2.2) at 37 °C for each time period of 0, 20, 40, 60, 90 and 120 min, and then subsequently incubated in the dark. ABTS, as the mediator, was added if necessary. Decolourization rate was defined as follows: decolourization (%) = 100 × (At0 − Atf)/At0, where At0 is the absorbance of the assay mixture before incubation and Atf is the absorbance after incubation. The decolourization of MG, CV and orange G was determined by measuring the absorbance at 620, 590 and 470 nm, respectively.
Effects of metal ions and inhibitors
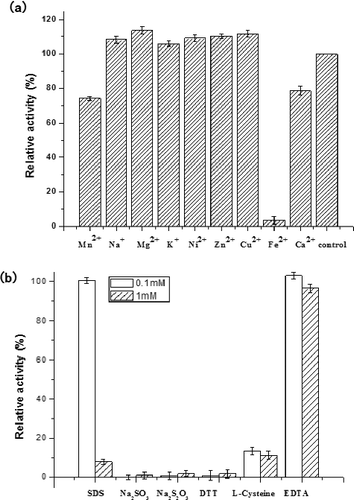
The effect of metal ions was assayed in McIlvaine buffer (pH 2.2) containing 0.5 mmol/L of ABTS with or without 10 mmol/L metal ions (e.g. Mn2+, Na+, Mg2+, K+, Ni2+, Zn2+, Cu2+, Fe2+ and Ca2+). Inhibitor studies were conducted in McIlvaine buffer (pH 2.2) containing different inhibitors, such as 0.1 and 1 mmol/L of dithiothreitol (DTT), L-cysteine, sodium thiosulphated, sodium sulphite and SDS or 1 mmol/L and 10 mmol/L EDTA.
Data analysis
All enzymological assays and dye decolourization tests were performed at least in triplicate. Data presented in figures are mean values from three independent experiments with standard deviation (±SD). Statistical analysis was performed using the SPSS 13.0 statistical software.
Results and discussion
Expression of LBLCC3I gene in P. pastoris
The LBLCC3I gene was synthesized and sequenced to check its correctness by cloning into the pMD18-T vector. The presence of the expression plasmid for secretion of the recombinant product was verified by restriction enzyme Bgl II digestion (data not shown).
The plasmid and the control vector were digested by Bgl II and transformed into P. pastoris GS115. Transformants produced coloured zones on BMMY solid plates containing ABTS. A large number of fungal laccases have been expressed earlier in P. pastoris by other researchers. But the stability of each different laccase is diverse in culture medium. In order to achieve high levels of foreign protein production, expression conditions need to be determined. In addition, the pH value of the medium has a pivotal role in the yield of protein. In the fermentation process involving P. pastoris, the pH of the medium often drops to 3 or below. Therefore, the expression medium has to be buffered for better results. In this research, LBLCC3I could not be detected in control group BMM and MM, but in BMGY, which contains yeast extract and peptone. Transformants cultured in BMGY in shake flask at 30 °C for three days produced laccase at an amount of about 498 µg/mL. Yeast extract and peptone were very important for the stability of LBLCC3I during fermentation. It was supposed that LBLCC3I was specifically susceptible to extracellular proteases.
Characterization of LBLCC3I
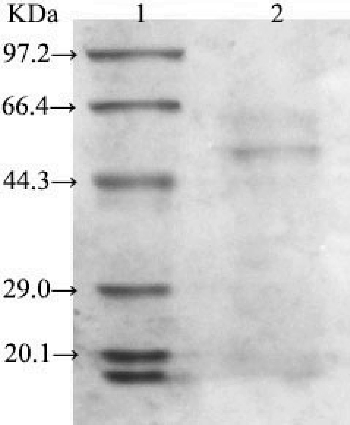
After three consecutive days of fermentation, the enzyme LBLCC3I was purified by affinity chromatography with an Ni+ column. The purified laccase appeared as a single protein band on the SDS-PAGE gel (). As determined by SDS-PAGE, the molecular mass of the purified laccase was 56 kDa. With ABTS as a substrate, the Km value of the heterologous laccase was 0.187 mmol/L at pH 2.2 and 50 °C.
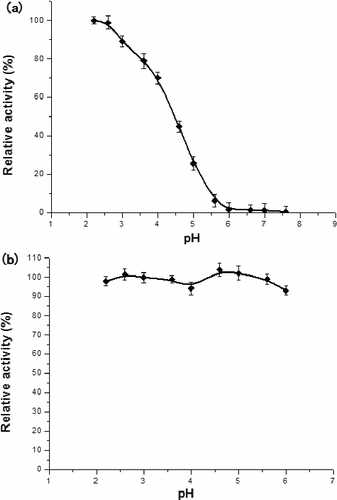
The optimum pH value of the catalytic reaction by LBLCC3I was 2.2 ((a)), indicating that the recombinant enzyme was more acid-resistant than other laccases from Lentinula edodes,[Citation8] Panus rudis[Citation9] and Albatrellus dispansus.[Citation10] Furthermore, the enzyme was stable at a wide range of pH values, with most residual activity being up to 95% after incubation at 4 °C for 24 h ((b)). This characteristic makes LBLCC3I a good candidate for the potential applications.
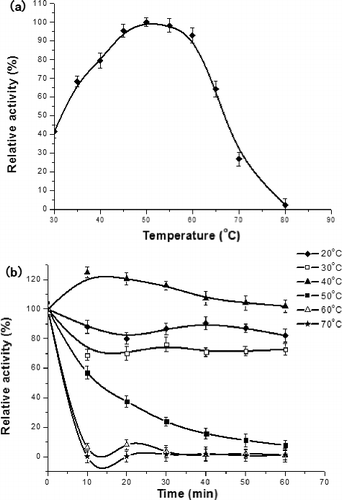
The optimum reaction temperature and thermal stability of LBLCC3I were determined with ABTS as the substrate at pH 2.2 ((a)). The recombinant laccase had a maximum activity at 50 °C. Apparently, it was not stable at temperatures higher than 60 °C during prolonged incubation ((b)). The residual activities were nearly reduced to zero after incubation at 60 and 70 °C for 10 min.
Degradation of synthetic dyes by LBLCC3I
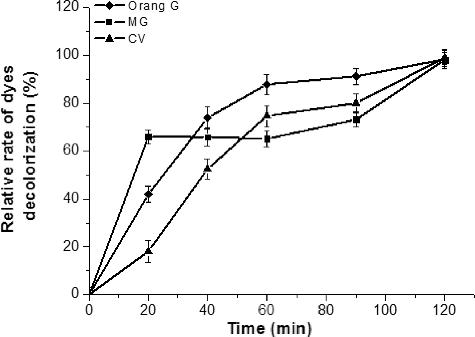
In our research, we found that MG, CV and orange G could not be degraded by LBLCC3I unless the redox mediator ABTS was present. However, some laccases could efficiently decolourize dyes without ABTS.[Citation4,Citation11] Notwithstanding, the redox mediator could remarkably improve the decolourization efficiency of other laccases.[Citation12,Citation13] The degradation rate depends on the structure of the dye and the structure and redox-potential of the enzyme.[Citation13] The decolourization rate was proportional to the incubation time. In the presence of ABTS (0.05 mmol/L), the decolourization rates of MG, CV and orange G were nearly 100% after 120 min (). As a mediator, ABTS could facilitate the reactions. ABTS can transfer electrons between the laccase and the dyes, and helps the enzyme oxidize the non-substrate dyes.
Effects of metal ions and inhibitors on laccase activity
The effect of inorganic salts on the activity of LBLCC3I is shown in (a). Inhibition studies on several inorganic salts revealed that LBLCC3I was inhibited by typical inhibitors of laccase, such as Ca2+, Fe2+ and Mn2+. Fe2+ is known to inhibit most laccases, such as laccases from Ganoderma lucidum,[Citation12] G. lucidum 7071-9,[Citation14] Monilinia fructigena,[Citation15] Pleurotus ostreatus [Citation11] and Paraconiothyrium variabile.[Citation16] In this study, we found that Fe2+ could decrease the activity of LBLCC3I at a low concentration; on the other hand, Fe2+ could serve as a terminator for the laccase catalysed reaction. Effluents from textile dyeing facilities contain metals used in dye production technologies or in dye molecules themselves. That is why the effect of metal ions on LBLCC3I is important for its application. The metal ions used in this study are the most common metals present in industrial effluents. Most of the tested had little effect on the activity of LBLCC3I, except Fe2+. Therefore, in order to make better use of LBLCC3I, it is necessary to study the possible ways to improve the stability of LBLCC3I in the presence of Fe2+. Some typical laccase inhibitors showed a significant effect on the activity of LBLCC3I, except 0.1 mmol/L SDS, 0.1 mmol/L EDTA and 1 mmol/L EDTA ((b)). LBLCC3I was strongly inhibited by sodium thiosulphate, sodium sulphite, DTT, L-cysteine and 1 mmol/L SDS. This was assumed to be the result of interference of the copper atoms in the enzyme active site or the disulphide bonds essential for enzyme activity. These findings are in accordance with the general properties of laccase from a diverse range of fungal sources.[Citation17,Citation18]
Conclusions
In this research, the laccase gene from L. bicolor was modified and expressed in P. pastoris. The properties of the laccase and its ability to degrade some synthetic dyes were investigated. The laccase showed high decolourization rates for MG, CV and orange G with ABTS. These results suggest that the recombinant laccase may be used to treat industrial effluents containing synthetic dyes.
Disclosure statement
The funders had no role in the study design, data collection and analysis, decision to publish or preparation of this manuscript.
Additional information
Funding
References
- Liu W, Chao Y, Liu S, Bao H, et al. Molecular cloning and characterization of a laccase gene from the basidiomycete Fome lignosus and expression in Pichia pastoris. Appl Microbiol Biotechnol. 2003;63:174–181.
- Otterbein L, Record E, Longhi S, et al. Molecular cloning of the cDNA encoding laccase from Pycnoporus cinnabarinus I-937 and expression in Pichia pastoris. Eur J Biochem. 2000;267:1619–1625.
- Soden DM, O'Callaghan J, Dobson ADW. Molecular cloning of a laccase isozyme gene from Pleurotus sajor-caju and expression in the heterologous Pichia pastoris host. Microbiology. 2002;148:4003–4014.
- Lu L, Zhao M, Liang S, et al.. Production and synthetic dyes decolourization capacity of a recombinant laccase from Pichia pastoris. J Appl Microbiol. 2009;107:1149–1156.
- Kanhere HA, Trochsler MI, Kanhere MH, et al. Pancreaticoduodenectomy: Outcomes in a low-volume, specialised hepato pancreato biliary unit. World J Surg. 2014;38:1484–1490.
- Zheng M, Chi Y, Yi H, Shao S. Decolorization of Alizarin red and other synthetic dyes by a recombinant laccase form Pichia pastoris. Biotechnol Lett. 2014;36:39–45.
- Lin YQ, Zhang Z, Tian YS, et al. Purification and characterization of a novel laccase from Coprinus cinereus and decolorization of different chemically dyes. Mol Biol Rep. 2013;40(2):1487–1494.
- Nagai M, Sakamoto Y, Nakade K, et al. Purification of a novel extracellular laccase from solid-state culture of the edible mushroom Lentinula edodes. Mycoscience. 2009;50:308–312.
- Zhang M, Wu F, Wei Z, et al. Characterization and decolorization ability of a laccase from Panus rudis. Enzyme Microb Technol. 2006;39:92–97.
- Wang H, Ng TB. A novel laccase with fair thermostability from the edible wild mushroom (Albatrella dispansus). Biochem Biophys Res Commun. 2004;319:381–385.
- Kumar VV, Sathyaselvabala V, Premkumar MP, et al. Biochemical characterization of three phase partitioned laccase and its application in decolorization and degradation of synthetic dyes. J Mol Catal B Enzyme. 2014;74:63–72.
- Murugesan K, Kim Y, Jeon J, et al. Effect of metal ions on reactive dye decolorization by laccase from Ganoderma lucidum. J Hazard Mater. 2009;168:523–529.
- Nyanhongo GS, Gomes J, Gubitz GM, et al. Decolorization of textile dyes by laccases from a newly isolated strain of Trametes modesta. Water Res. 2002;36:1449–1456.
- Sun J, Peng R, Xiong A, et al. Secretory expression and characterization of a soluble laccase from the Ganoderma lucidum strain 7071-9 in Pichia pastoris. Mol Biol Rep. 2012;39:3807–3814.
- Bao W, Peng R, Zhang Z, et al. Expression, characterization and 2,4,6-trichlorophenol degradation of laccase from Monilinia fructigena. Mol Biol Rep. 2011;39:3871–3877.
- Forootanfar H, Faramarzi MA, Shahverdi AR, et al. Purification and biochemical characterization of extracellular laccase from the ascomycete Paraconiothyrium variabile. Bioresour Technol. 2011;102:1808–1814.
- Halaburgi VM, Sharma S, Sinha M, et al. Purification and characterization of a thermostable laccase from the ascomycetes Cladosporium cladosporioided and its applications. Process Biochem. 2011;46:1146–4452.
- Telke AA, Ghodake GS, Kalyani DC, et al. Biochemical characteristics of a textile dye degrading extracellular laccase from a Bacillus sp. ADR Bioresour Technol. 2011;102:1752–1756.