ABSTRACT
The extracellular adenosine triphosphate (ATP) regulates different cellular functions through activating purinergic receptors as a signalling molecule or neurotransmitter. P2X7 is highly expressed in murine small luteal cells. In this study, murine luteal cells were cultured in vitro and treated with P2X7 agonists – ATP and 2′(3′)-O-(4-benzoyl-benzoyl)-adenosine 50-triphosphate (BzATP) and with P2X7 antagonist – brilliant blue G (BBG). We found that ATP and BzATP increased the production of progesterone and had no influence on the production of estradiol. BBG reversed the effect of BzATP and ATP. Further studies demonstrated that ATP and BzATP promoted the expression of CYP11A. These results revealed that P2X7 receptor activation is involved in the steroid synthesis in corpus luteum.
KEYWORDS:
Introduction
The main function of corpus luteum is the secretion of progesterone, which controls the oestrous cycle and is also necessary for the blastocyst implantation. When pregnancy does not occur, the progesterone is decreased and structural luteolysis follows. The progesterone production is strictly controlled by nervous and humoral regulation. Noradrenergic stimulation regulates the progesterone production in luteal cells.[Citation1] Endocrine and paracrine molecules, such as luteinizing hormone, human chorionic gonadotropin (hCG), insulin-like growth factor system and prostaglandin F2α (PGF2α), also regulate the secretion of progesterone in luteal cells.[Citation2–6]
As a signalling molecule or neurotransmitter, extracellular adenosine triphosphate (ATP) regulates different cellular functions through activating purinergic receptors.[Citation7] Some studies have shown that purinergic signalling is activated and plays an important role in ovaries.[Citation8–11] ATP activated the caspase signalling pathway and induced apoptosis in human granulosa-luteal cells.[Citation12,Citation13] In our previous study, we found that P2X7 was highly expressed in murine small luteal cells.[Citation14] Activation of P2X7 inhibited luteal cell proliferation, [Citation14] suggesting that the P2X7 purinergic signalling plays important role in corpus luteum. Studies have also shown that purinergic receptors, such as P2Y2 and P2Y4, were involved in the synthesis of steroids in granulose cells.[Citation15] However, the role of P2X7 purinergic signalling on steroid synthesis of luteal cells is still unclear. In this study, we explored the influence of P2X7 purinergic signalling on progesterone and estradiol in corpus luteum.
Materials and methods
Animals and treatment
Immature Kunming mice (26-day-old) were purchased from Animal Facility of Nanchang University and housed in a temperature and light controlled facility with free access to water and food. The experimental protocols were approved by the ethical committee of Nanchang University. For each experiment, six mice were injected intraperitoneally with 36 IU of pregnant mare's serum gonadotropin (NingBo Biological Technology) to induce follicular maturation. Seventy two hours later, they were administered with 36 IU hCG (NingBo Biological Technology) to induce ovulation and luteinization. The ovaries were collected seven days after the hCG treatment.
Chemicals
P450 side-chain cleavage enzyme gene (CYP11A, BA4136) was purchased from Boster (Wuhan China). β-Actin (A5316) was purchased from Sigma. Brilliant SYBR Green Quantitative Polymerase Chain Reaction (qPCR) Master Mix (FP-202) was purchased from Tiangen (Beijing, China). Collagenase (type I), DNase I, 2′(3′)-O-(4-benzoyl-benzoyl)-adenosine 50-triphosphate (BzATP), ATP and brilliant blue G (BBG), a P2X7 specific antagonist, were purchased from Sigma.
Luteal cell culture
The procedure of cells’ isolation from luteinized ovaries was performed as described in previous studies.[Citation14,Citation16] First, the luteinized ovaries were taken out and the fat and capsule tissue, were removed. After the mechanical dissection, the ovaries were digested in medium containing 1 mg/mL collagenase (Sigma, St. Louis, Missouri), 0.025% trypsin (Sigma, St. Louis, Missouri) and 0.02 mg/mL Dnase I (Sigma, St. Louis, Missouri) for 10 min at 37 °C. The digested suspension was filtered through 75 μm strainer to remove the debris and then was centrifuged (Xiangyi centrifuge instrument) at 500 g for 5 min. Following two washes with medium, the cells were inoculated in Dulbecco's modification of Eagle's medium/F12 culture medium (HyClone), supplemented with 5% (v/v) foetal bovine serum, 100 IU/mL penicillin (Solarbio) and 100 µg/mL streptomycin sulphate (Solarbio) and cultured overnight for adhesion. After this period, the cells were cultured only in fresh medium (control) or in fresh medium with 100 μmol/L BzATP, 100 μmol/L ATP, 1 μmol/L BBG,100 μmol/L BzATP + 1 μmol/L BBG or 100 μmol/L ATP + 1 μmol/L BBG.
Hormone measurements
Luteal cells were cultured in serum free medium and treated with ATP, BzATP, BBG, ATP + BBG or BzATP + BBG. After 48 h, we harvested the media supernatant and stored it at –80 °C for further hormone measurement. The estradiol (pg/106 cells) and progesterone (ng/106 cells) values were measured with a commercial radioimmunoassay kit at a commercial laboratory (Beijing Sino-uk institute of Biological Technology). For BBG, ATP + BBG and BzATP + BBG groups, only the progesterone quantity was measured. The intra- and inter-assay coefficients of variation were <10%. The cross-reactivity with other peptides and steroid hormones in these kits did not exceed 4%. The sensitivity of the progesterone and estradiol are 0.25 ng/mL and 2 pg/mL, respectively. The total cell number was counted and used to correct the estradiol and progesterone production, because the values of the estradiol and progesterone production will depend on the number of cells in the wells.
RNA extraction and real-time PCR
Steroidogenic acute regulatory protein (StAR), CYP11A and 3β-hydroxysteroid dehydrogenase (3β-HSD) are involved in steroid hormone synthesis. We examined the mRNA expression of StAR, CYP11A and 3β-HSD after treatment with ATP and BzATP, and the effect of BBG on CYP11A mRNA expression, induced by ATP and BzATP through qPCR.[Citation17–21] Luteal cells were lysed with Trizol Reagent. Total RNA was extracted according to the manufacturer's instructions (Invitrogen). Total RNA (2 µg) was reverse transcribed in a mixture with final volume of 25 µL, containing random primers, Moloney murine leukaemia virus reverse transcriptase (M-MLV RT), reaction buffer, dNTP and RNasin following M-MLV First Strand Kit (Invitrogen, C28025032).The sequences of the specific primers are listed in . Real-time PCR was performed in a 20 μL reaction volume containing 10μL of 2 × Brilliant SYBR Green qPCR Master Mix, 1 μL cDNA, 0.5 μmol/L primers and 300 nmol/L of ROX reference dye, by using the ABI thermal cycler 7500 (Applied Biosystems, Carlsbad, California). The thermal cycling conditions were 94 ℃ for 15 min, followed by 40 cycles at 94 °C for 15 s, 60 °C for 30 s and 72 °C for 30 s. Melting curve analyses were conducted after the real-time PCR. The mRNA expression level was normalized against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA level and analysed using the comparative Ct method.
Table 1. Oligonucleotides used for real-time PCR.
Western blotting
Luteal cells were lysed in radioimmune precipitation assay lysis buffer (50 mmol/L Tris (pH 7.4), 150 mmol/L NaCl, 1% NP-40, 0.5% sodium deoxycholate) with Mini protease-inhibitor cocktail tablets (Roche, Mannheim, Germany). We tested the total protein concentration through Bradford assay (Bio-Rad Laboratories, Hercules, CA). Samples with equal amounts of protein were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (Bei jing liu yi biotechnology Co., Ltd) and transferred to nitrocellulose membranes. The membrane was blocked with 5% non-fat dry milk and was incubated overnight with primary CYP11A antibody (1:500) at 4 °C. The membrane was then washed with Tris-buffered saline with Tween-20 (TBST, pH 8.0) and incubated with horseradish peroxidase (HRP)-labelled anti-rabbit secondary antibody (1:2000) for 1 h at room temperature. Specific signals were detected on autoradiography film by using the enhanced chemiluminescence kit (Thermo pierce). Protein expression level was normalized against β-actin.
Statistical analysis
The data were evaluated for statistical differences by using SPSS computer software. Data are expressed as mean ± standard error of the mean (SEM) One-way analysis of variance followed by a least-significant-difference test was used for statistical comparisons among multiple groups. The significance was assured when the P value was less than 0.05.
Results and discussion
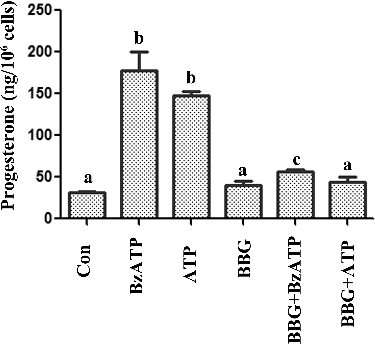
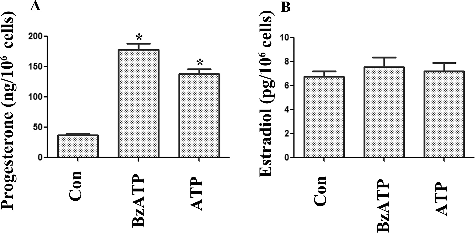
P2X7 receptors are extracellular ATP-gated ion channels, which are expressed in different cell types. P2X7 activation is involved in numerous physiological and pathological processes, such as the induction of the inflammatory cascade, tumour progression and pain mechanisms.[Citation22–24] P2X7 signalling is also related to cell proliferation and apoptosis.[Citation25,Citation26] Studies have shown that P2X7 is expressed in porcine ovarian theca cells and murine/human ovarian surface epithelium and is involved in ATP-induced apoptotic cell death.[Citation14,Citation27,Citation28] In a previous study, we also reported that P2X7 was expressed in murine small luteal cells.[Citation14] The activation of P2X7 receptors decreased the proliferation of luteal cells.[Citation14] These data implied that P2X7 signalling is involved in corpus luteum formation. However, the main function of corpus luteum is the secretion of progesterone, which is useful for the control of the oestrous cycle and is necessary for the implantation of the blastocyst. The influence of P2X7 signalling on steroid synthesis is not clear. In this study, we explored the influence of P2X7 activation on steroid synthesis in luteal cells. We cultured murine luteal cells and harvested the media supernatant to measure the progesterone and estradiol after treatment with ATP and BzATP. As shown in (A) and (B), ATP and BzATP treatment increased the progesterone secretion and had no effect on estradiol. To confirm that the progesterone increase was dependent on P2X7 signalling, we employed BBG, as a P2X7 specific antagonist.[Citation29] The results showed that BBG partially reversed the influence of ATP and BzATP on the progesterone production (). These results suggested that P2X7 signalling was involved in regulating the progesterone synthesis in luteal cells.
Figure 1. Influence of P2X7 purinergic signalling on (A) progesterone and (B) estradiol secretion in luteal cells.
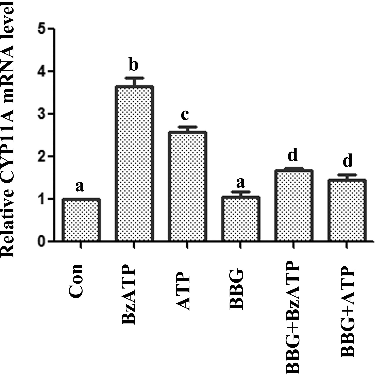
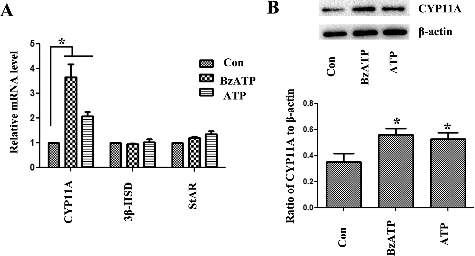
To analyse the possible mechanism, we found that ATP and BzATP promoted the protein level and expression of CYP11A. There was no obvious change in 3β-HSD and StAR expression levels (). CYP11A cleaves the cholesterol to pregnenolone. It is an important step in progesterone biosynthesis.[Citation18] BBG also reversed the change of CYP11A induced by ATP and BzATP (), suggesting that ATP and BzATP possibly influenced the progesterone production through regulating CYP11A.
Figure 3. mRNA level of (A) CYP11A, 3β-HSD, StAR and (B) protein level of CYP11A in luteal cells assessed by real-time PCR and Western blotting.
Mitogen-activated protein kinases (MAPKs) include a large family of kinases that respond to diverse physiological stimuli. Several MAPK signalling pathways were involved in progesterone synthesis in corpus luteum. Extracellular regulated protein kinases1/2 (ERK1/2) activation is required for progesterone production in human granulosa-lutein cells.[Citation30] Studies of water buffalo showed that c-Jun N-terminal kinase (JNK) and p38 MAPK played an important role in the PGF2α-mediated apoptotic cell death in the corpus luteum.[Citation31] Studies of bonnet monkey showed that all three MAPKs appeared to be constitutively active in the mid-luteal-phase corpus luteum. During the late-luteal phase, ERK1/2 and p38MAPK decreased. However, JNK-1/2 activities increased significantly. hCG also activated ERK1/2 and p38MAPK activity, but inhibited JNK-1/2. Intraluteal administration of p38 kinase inhibitor (SB203580) inhibited the luteal function.[Citation32] These studies revealed that there is a disagreement about the p38MAPK function in luteal cells. Our previous studies showed that ATP and BzATP inhibited the p38MAPK signalling pathway in luteal cells.[Citation14] The study of granulosa cell tumours showed that inhibition of p38MAPK signalling contributed to the increase of progesterone production induced by staurosporine.[Citation33] Therefore, p38MAPK signalling is possibly involved in regulating the progesterone production induced by ATP and BzATP. Further studies should be done to explore the mechanism in details.
Conclusions
The results from this study demonstrated that P2X7 signalling was involved in progesterone synthesis in murine corpus luteum through regulating CYP11A. The finding will help to explain the physiological and pathological effects of ATP on corpus luteum, as an endocrine molecule or neurotransmitter. Further researches need to be done to explore the exact mechanism.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Kotwica J, Bogacki M, Rekawiecki R. Neural regulation of the bovine corpus luteum. Domest Anim Endocrinol. 2002;23:299–308.
- Auletta FJ, Flint AP. Mechanisms controlling corpus luteum function in sheep, cows, nonhuman primates, and women especially in relation to the time of luteolysis. Endocr Rev. 1988;9:88–105.
- Niswender GD, Juengel JL, McGuire WJ, et al. Luteal function: the estrous cycle and early pregnancy. Biol Reprod. 1994;50:239–247.
- Miyamoto A, Shirasuna K, Sasahara K. Local regulation of corpus luteum development and regression in the cow: impact of angiogenic and vasoactive factors. Domest Anim Endocrinol. 2009;37:159–169.
- Kumagai A, Yoshioka S, Sakumoto R, et al. Auto-amplification system for prostaglandin F2alpha in bovine corpus luteum. Mol Reprod Dev. 2014;81:646–654.
- Uniyal S, Panda RP, Chouhan VS, et al. Expression and localization of insulin-like growth factor system in corpus luteum during different stages of estrous cycle in water buffaloes (Bubalus bubalis) and the effect of insulin-like growth factor I on production of vascular endothelial growth factor and progesterone in luteal cells cultured in vitro. Theriogenology. 2015;83:58–77.
- Burnstock G, Verkhratsky A. Long-term (trophic) purinergic signalling: purinoceptors control cell proliferation, differentiation and death. Cell Death Dis. 2010;1:e9 (1–10).
- Tai CJ, Kang SK, Tzeng CR, et al. Adenosine triphosphate activates mitogen-activated protein kinase in human granulosa-luteal cells. Endocrinology. 2001;142:1554–1560.
- Tai CJ, Kang SK, Leung PC. Adenosine triphosphate-evoked cytosolic calcium oscillations in human granulosa-luteal cells: role of protein kinase C. J Clin Endocrinol Metab. 2001;86:773–777.
- Tai CJ, Kang SK, Cheng KW, et al. Expression and regulation of P2U-purinergic receptor in human granulosa-luteal cells. J Clin Endocrinol Metab. 2000;85:1591–1597.
- Martinez-Ramirez AS, Vazquez-Cuevas FG. Purinergic signaling in the ovary. Mol Reprod Dev. 2015 ;82:839–848.
- Tai CJ, Chang SJ, Chien LY, et al. Adenosine triphosphate induces activation of caspase-3 in apoptosis of human granulosa-luteal cells. Endocr J. 2005;52:327–335.
- Park DW, Cho T, Kim MR, et al. ATP-induced apoptosis of human granulosa luteal cells cultured in vitro. Fertil Steril. 2003;80:993–1002.
- Wang J, Liu S, Nie Y, et al. Activation of P2×7 receptors decreases the proliferation of murine luteal cells. Reprod Fertil Dev. 2015 ;27:1262–1271.
- Kamada S, Blackmore PF, Oehninger S, et al. Existence of P2-purinoceptors on human and porcine granulosa cells. J Clin Endocrinol Metab. 1994;78:650–656.
- Wang J, Liu S, Peng L, et al. Notch signaling pathway regulates progesterone secretion in murine luteal cells. Reprod Sci. 2015 ;22:1243–1251.
- Irusta G, Parborell F, Peluffo M, et al. Steroidogenic acute regulatory protein in ovarian follicles of gonadotropin-stimulated rats is regulated by a gonadotropin-releasing hormone agonist. Biol Reprod. 2003;68:1577–1583.
- Chien Y, Cheng WC, Wu MR, et al. Misregulated progesterone secretion and impaired pregnancy in Cyp11a1 transgenic mice. Biol Reprod. 2013;89:91 (1–10).
- Luu-The V. Assessment of steroidogenesis and steroidogenic enzyme functions. J Steroid Biochem Mol Biol. 2013;137:176–182.
- Bain PA, Yoo M, Clarke T, et al. Multiple forms of mouse 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4 isomerase and differential expression in gonads, adrenal glands, liver, and kidneys of both sexes. Proc Natl Acad Sci USA. 1991;88:8870–8874.
- Carreau S, de Vienne C, Galeraud-Denis I. Aromatase and estrogens in man reproduction: a review and latest advances. Adv Med Sci. 2008;53:139–144.
- Roger S, Pelegrin P. P2×7 receptor antagonism in the treatment of cancers. Expert Opin Investig Drugs. 2011;20:875–880.
- Baudelet D, Lipka E, Millet R, et al. Involvement of the P2×7 purinergic receptor in inflammation: an update of antagonists series since 2009 and their promising therapeutic potential. Curr Med Chem. 2015;22:713–729.
- Ursu D, Ebert P, Langron E, et al. Gain and loss of function of P2×7 receptors: mechanisms, pharmacology and relevance to diabetic neuropathic pain. Mol Pain. 2014;10:37 (1–11).
- Zou J, Vetreno RP, Crews FT. ATP-P2×7 receptor signaling controls basal and TNFalpha-stimulated glial cell proliferation. Glia. 2012;60:661–673.
- Li J, Chen J, Chen G. P2×7 receptor and apoptosis. Crit Care Med. 2014;42:e804–e805.
- Vazquez-Cuevas FG, Cruz-Rico A, Garay E, et al. Differential expression of the P2×7 receptor in ovarian surface epithelium during the oestrous cycle in the mouse. Reprod Fertil Dev. 2013;25:971–984.
- Vazquez-Cuevas FG, Juarez B, Garay E, et al. ATP-induced apoptotic cell death in porcine ovarian theca cells through P2×7 receptor activation. Mol Reprod Dev. 2006;73:745–755.
- Ji X, Naito Y, Hirokawa G, et al. P2X(7) receptor antagonism attenuates the hypertension and renal injury in Dahl salt-sensitive rats. Hypertens Res. 2012;35:173–179.
- Dewi DA, Abayasekara DR, Wheeler-Jones CP. Requirement for ERK1/2 activation in the regulation of progesterone production in human granulosa-lutein cells is stimulus specific. Endocrinology. 2002;143:877–888.
- Yadav VK, Sudhagar RR, Medhamurthy R. Apoptosis during spontaneous and prostaglandin F(2alpha)-induced luteal regression in the buffalo cow (Bubalus bubalis): involvement of mitogen-activated protein kinases. Biol Reprod. 2002;67:752–759.
- Yadav VK, Medhamurthy R. Dynamic changes in mitogen-activated protein kinase (MAPK) activities in the corpus luteum of the bonnet monkey (Macaca radiata) during development, induced luteolysis, and simulated early pregnancy: a role for p38 MAPK in the regulation of luteal function. Endocrinology. 2006;147:2018–2027.
- Zhang H, Vollmer M, De Geyter M, et al. Apoptosis and differentiation induced by staurosporine in granulosa tumor cells is coupled with activation of JNK and suppression of p38 MAPK. Int J Oncol. 2005;26:1575–1580.