ABSTRACT
Ganoderma lucidum is a traditional medicinal macrofungus in China, which has two kinds of key bioactive compounds -- polysaccharides and triterpenoids. To improve the polysaccharide and triterpenoid production from G. lucidum, the preparation and regeneration conditions of protoplasts were optimized. This was done by systematic trials with various parameters, and protoplast mutation was subsequently performed. A mycelium that was cultivated for seven days and treated with 0.33 mL of 1% snailase and 0.66 mL of 0.5% cellulase solution for 2.5 h at 30 °C in the presence of osmotic pressure stabilizer mannitol (0.5 mol/L), had the best conditions, in which the resultant protoplasts were 6.40 × 105/mL and the regeneration rate was 6.25%. The resultant protoplasts were subjected to subsequent mutation by lithium chloride or by the combination of lithium chloride and Triton X-100. The highest yields of intracellular polysaccharide and triterpenoid in two mutant strains were 37.50 and 40.81 mg/g, which were increased with 568.45% and 373.43%, respectively, as compared to the original strain. Furthermore, the yields of intracellular polysaccharides and triterpenoids in the second generation and the third generation of the mutants were comparable to that of the first generation, which showed genetic stability of the mutants for the production of polysaccharides and triterpenoids.
Abbreviations
| GLP | = | Ganoderma lucidum polysaccharide |
| s.d. | = | Standard deviation |
| UV | = | Ultraviolet |
| EMS | = | Ethyl methyl sulfone |
| NTG | = | Nitrosoguanidine |
Introduction
Ganoderma lucidum is a well-known medicinal macrofungus in China, which creates multiple bioactive compounds.[Citation1] Polysaccharides and triterpenoids are recognized as two kinds of key ingredients, which are responsible for the pharmacological activities of this fungus.[Citation2,Citation3] Modern pharmaceutical researches have reported a list of medicinal effects of G. lucidum polysaccharides, such as immunomodulating and antitumour activities. [Citation4,Citation5] Shi et al. [Citation4] reported that four G. lucidum polysaccharides (GLP-I, GLP-II, GLP-III and GLP-IV) from G. lucidum showed a significant stimulation of the macrophage proliferation. Also, higher nitric oxide production was observed after treatment with 40 μg/mL Ganoderma lucidum polysaccharide (GLP).[Citation4] In addition, triterpenoids have been demonstrated to possess cytotoxic, antiangiogenic and antitumour effects, as well as anticomplement, antiandrogenic and α-glucosidase inhibitory activities.[Citation6–11] Liu et al. [Citation8] reported that the triterpenoid fraction of G. lucidum might be beneficial for curing benign prostatic hyperplasia. The experiments of Fatmawatia et al. [Citation11] indicated that triterpenoid isolated from the fruiting body of G. lucidum was found to inhibit α-glucosidase in vitro.
However, the content of polysaccharides and triterpenoids in natural species is rather low. Thus, species improvement for higher yield by mutation techniques, including spore and protoplast mutation, has drawn the attention of the academic and industry communities. The spore mutation has several drawbacks, such as strong stimulation requirement, low mutation rate and difficult germination. On the other hand, the protoplast mutation is a rapid and convenient method, which possesses several advantages, such as high stimulation sensitivity, high mutation rate and easy screening procedure. Therefore, the protoplast mutation has a wide application in the breeding of diverse set of strains. For instance, Mukherjee et al. [Citation12] described an efficient way for regeneration and mutagenesis of protoplasts in the mushroom Volvariella volvacea. Though the biological activity, extraction and purification of polysaccharides and triterpenoids from G. lucidum have been well documented in recent years,[Citation4–11,Citation13,Citation14] there are few researches on the mutagenesis of G. lucidum.[Citation15] The aim of the present paper is to describe a new approach for substantial improvement of polysaccharides and triterpenoids production from G. lucidum.
Materials and methods
Materials and reagents
G. lucidum was obtained from Jiangxi Peigongtang Biological Technology Co. Ltd. (Nanchang, China). Snailase and cellulase were purchased from Ruibio and Amano Enzyme Manufacturing (China) Ltd. All other chemicals were obtained from various commercial sources and were of analytical or higher grade.
Preparation of protoplasts in G. lucidum
G. lucidum was transferred to potato dextrose agar (20% potatoes, 2% glucose, 1.8% agar powder) slant for growth, until the slant was filled with mycelia. Five millilitres of double distilled water were added into the test tube with mycelia. Spore suspension was obtained after gently shaking and 2.5 mL were inoculated into 100 mL of potato dextrose broth (20% potatoes, 2% glucose). After this, incubation followed on a rotary shaking incubator (Changzhou Boyuan Instrument Co., Ltd.) at 28 °C and 150 r/min.
The mycelia at different growth intervals were washed twice with osmotic stabilizer solution, and 200 mg of wet mycelia were used for enzymolysis by adding 1 mL of enzyme solution (snailase and cellulose). The resultant mixture was filtered through cotton wool to remove undigested fragments and then was centrifuged (Anting Scientific Instrument Co., Ltd.) for 2 min at 10,000 r/min. The precipitation was washed with osmotic stabilizer solution twice and then was suspended again. The suspension was observed under a light microscope (Phenix Optics Scientific Instrument Co., Ltd.) to ensure the absence of mycelial fragments. The number of protoplasts (×105/mL) was determined by using blood counting chamber.
Regeneration of protoplasts in G. lucidum
The protoplasts suspension was diluted to 1 × 105/mL by an osmotic stabilizer. Suspension (0.1 mL) was inoculated onto regeneration medium plate containing 1% (w/v) maltose, 0.4% (w/v) glucose, 0.4% (w/v) yeast extract and 1.8% (w/v) agar and then was incubated for 1–2 days. The colony number was determined. Protoplasts regeneration rate was calculated by using the following formula: protoplasts regeneration rate (%) = (number of colonies × dilution fold) / (0.1 × protoplasts concentration) × 100.
Effect of mycelia age on the preparation and regeneration of protoplasts in G. lucidum
In order to study the effect of mycelia age on the preparation and regeneration of protoplasts in G. lucidum, mycelia cultivated for 5, 6, 7 and 8 days were used for enzymolysis at 28 °C for 3 h by adding 0.5 mL of 1% (m/v) snailase solution and 0.5 mL of 1% (m/v) cellulase solution in the presence of 0.5 mol/L KCl (Tianjin Yongda Chemical Reagent Co., Ltd.), as osmotic stabilizer solution.
Effect of different kinds of osmotic stabilizers on the preparation and regeneration of protoplasts in G. lucidum
In order to study the effect of different kinds of osmotic stabilizer solutions on the preparation and regeneration of protoplasts in G. lucidum, mycelia cultivated for seven days were used for enzymolysis at 28 °C for 3 h by adding 0.5 mL of 1% (m/v) snailase solution and 0.5 mL of 1% (m/v) cellulase solution in the presence of 0.5 mol/L KCl, 0.5 mol/L MgSO4·7H2O, 0.5 mol/L mannitol and 0.5 mol/L sucrose (Tianjin Yongda Chemical Reagent Co., Ltd.), as osmotic stabilizer solution.
Effect of different enzyme compositions on the preparation and regeneration of protoplasts in G. lucidum
Snailase and cellulase were prepared in osmotic stabilizer solution. The mixtures were filter-sterilized and stored at 4 °C. We studied the effect of different enzyme compositions, shown in , on the preparation and regeneration of protoplasts in G. lucidum. The mycelia were cultivated for seven days and were used for enzymolysis at 28 °C for 3 h with varying enzyme compositions in the presence of 0.5 mol/L mannitol, as osmotic stabilizer solution.
Table 1. Different enzyme compositions used for mycelia enzymolysis.
Effect of different enzymolysis time on the preparation and regeneration of protoplasts in G. lucidum
In order to study the effect of enzymolysis time on the preparation and regeneration of protoplasts in G. lucidum, the mycelia cultivated for seven days were used for enzymolysis at 28 °C for 1.5, 2, 2.5 and 3 h by adding 0.66 mL of 0.5% (m/v) snailase solution and 0.33 mL of 1% (m/v) cellulase solution in the presence of 0.5 mol/L mannitol, as osmotic stabilizer solution.
Effect of different enzymolysis temperatures on the preparation and regeneration of protoplasts in G. lucidum
In order to study the effect of enzymolysis temperature on the preparation and regeneration of protoplasts in G. lucidum, the mycelia cultivated for seven days were used for enzymolysis at 25, 30, 35 and 40 °C for 2.5 h by adding 0.66 mL of 0.5% (m/v) snailase solution and 0.33 mL of 1% (m/v) cellulase solution in the presence of 0.5 mol/L mannitol, as osmotic stabilizer.
G. lucidum protoplast mutation by using lithium chloride or combination of lithium chloride and Triton X-100
The prepared protoplasts were inoculated to 100 mL potato dextrose broth containing LiCl (Tianjin Yongda Chemical Reagent Co., Ltd.) with different concentrations (0.1%, 0.2%, 0.3%, 0.4% and 0.5%) or LiCl with the same concentrations plus 1 mL Triton X-100 (Beijing Solarbio Science and Technology Co., Ltd.), and then cultured at 30 °C (150 r/min) for 3–5 days. The culture was diluted and plated on regenerating solid medium (1% maltose, 0.4% glucose, 0.4% yeast extract, 1.8% agar powder) for colony counting. The lethality was calculated according to the following equation, by using the sample not treated with mutagens as a control: lethality rate (%) = (number of colonies in the control − number of colonies in the samples treated with LiCl and Triton X-100)/number of colonies in the control.
Screening of mutants
A single colony from the regenerating solid medium with rapid growth and large diameter was transferred to potato dextrose broth for further screening. After culturing at 30 °C (150 r/min) for 3–5 days, the fermented broths were filtered to obtain mycelia, which were then dried and crushed into powder. One gram of powder was mixed with 50 mL methanol and was allowed to stand overnight. The extraction was filtered through filter paper and the filtrates were dried for determination of the cellular polysaccharides and triterpenoids of G. lucidum.
Determination of cellular polysaccharide in G. lucidum
The determination of G. lucidum cellular polysaccharide (mg/g) was performed according to the method described by Toba et al.[Citation16]
Determination of cellular triterpenoid in G. lucidum
The intracellular triterpenoid (mg/g) of G. lucidum was assayed by using the colorimetric method. The samples were mixed with 0.40 mL vanillin acetum (5%) and 1.00 mL perchloric acid. The mixtures were heated in water bath at 60 °C for 15 min. Perchloric acid (5.00 mL) was added once again and the absorbance of the mixtures was measured at 548 nm after 15 min.
Heredity stability of the mutants for the production of polysaccharide and triterpenoid
The second and third generations of the mutants were obtained by transferring from the first one. They were subjected to the assay of cellular polysaccharide and triterpenoid to assess the heredity stability of the mutants.
Statistical analysis
The experimental data were presented as mean ± standard deviation (s.d.). Statistical analysis was performed by using SPSS 11.5 software.
Results and discussion
Effects of different factors on the preparation and regeneration of protoplasts in G. lucidum
The optimum cultivation time was observed to be seven days, as indicated in . At this time, the number of protoplasts and regeneration rate were found to be 8.80 × 105/mL and 0.53%, respectively. Shorter or longer cultivation time was not conducive for the preparation and regeneration of protoplasts. Generally speaking, the thick cell walls of G. lucidum cultivated for longer time resulted in difficult preparation of protoplasts. However, shorter culturing time may give rise to incomplete organelles in the plasma and hence may have a negative effect on the regeneration of protoplasts in G. lucidum. Reyes et al. [Citation17] reported that V. volvacea, cultivated for five days, was suitable for regeneration of the protoplasts. However, Fulvia fulva mycelia from 24–48 h liquid cultures had the best culture time for protoplasts release,[Citation18] which illustrated that the cultivation time was notably varied among different species.
Table 2. Effect of different cultivation time on protoplasts preparation and regeneration.
Osmotic pressure stabilizers help protoplasts maintain their normal shape. Therefore, the choice of efficient pressure stabilizer plays an important role in the preparation and regeneration of protoplasts. The effects of different osmotic pressure stabilizers on the protoplast regeneration and preparation were investigated in the present study. The results are shown in . KCl, sucrose and mannitol had almost similar influences on the preparation of protoplasts, whereas mannitol was the best choice for the regeneration of protoplasts with a rate of 1.64%. In a similar study, 0.6 mol/L mannitol was fit for a V. volvacea protoplasts yield with a relatively high regeneration rate.[Citation17]
Table 3. Effect of different osmotic pressure stabilizers on protoplasts preparation and regeneration.
Filamentous fungi have a complex cell wall composition and thus appropriate enzymes are essential for protoplast preparation. Generally, enzyme composites are more suitable than single enzymes.[Citation17] As shown in , the fourth enzyme composite was the most suitable for protoplast preparation, but had low regeneration rate. Nevertheless, the second enzyme composite not only gave rise to a high number of protoplasts, but was also favourable for protoplasts regeneration. Reyes et al. [Citation17] used a combination of 2% Novozyme 234 and 0.2% chitinase for treating hyphal strands of V. volvacea, which produced a high yield of protoplasts.
Table 4. Effect of different enzyme composites on protoplasts preparation and regeneration.
The effect of different enzymolysis time on the preparation and regeneration of protoplasts in G. lucidum were also investigated (). The number of protoplasts initially increased with the increase of the enzymolysis time and then decreased. The regeneration rate had a similar curve with the increase of the enzymolysis time. The optimal enzymolysis time for regeneration of protoplasts was 2 h.
Table 5. Effect of different enzymolysis time on protoplasts preparation and regeneration.
The optimal temperature for preparation and regeneration of protoplasts was found to be 30 °C (). The lower and higher temperatures had a negative influence on the enzyme activity and physiological activity, and thus lowered the number of protoplasts and regeneration rate.
Table 6. Effects of different enzymolysis temperatures on protoplasts preparation and regeneration.
G. lucidum protoplast mutation and heredity stability of the mutants
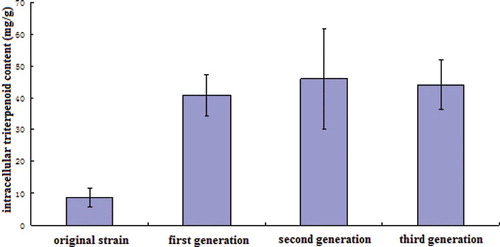
In order to improve the polysaccharide and triterpenoid production of G. lucidum, the resultant protoplasts were subjected to mutagenesis by using lithium chloride or lithium chloride plus Triton X-100. The effects of lithium chloride with diverse concentrations or lithium chloride plus Triton X-100 on mutagenesis were studied. indicated that the lethality increased with the increase of the concentration of LiCl. The addition of Triton X-100 remarkably increased the lethality caused by LiCl concentrations ranging from 0.1% to 0.3%. After mutagenesis, the mutants were used for screening. The highest yields of intracellular polysaccharides and triterpenoids in two mutant strains screened after mutagenesis were 37.50 and 40.81 mg/g, respectively, which were increased with 568.45% and 373.43%, as compared to the original strain ( and ). There was a significant difference between two mutant strains and the original strain (P < 0.01). However, polysaccharide and triterpenoid contents in fruiting bodies of 12 strains from Ganoderma genus (G. lucidum 05, G. lucidum 06, G. applanatum, G. tsugae, G. capense, G. lucidum Huizhou, G. lucidum Xinzhou, G. lucidum Jingda, G. lucidum Zhongzhi, G. lucidum Xianzhi, G. lucidum Xianyuan and Japanese G. lucidum) varied from 7.5 to 11.6 mg/g and from 4.1 to 11.9 mg/g, respectively.[Citation19] The polysaccharide and triterpenoid contents of the mutants were remarkably higher than that of these strains. LiCl is often used as a chemical mutagen to improve the performance of strains. Kang et al. [Citation20] reported the mutagenesis of mycelial fragments of Flammulina velutipes by using 0.8% LiCl and combined mutagen (ultraviolet (UV) + LiCl + ethyl methyl sulfone (EMS)) to obtain high-temperature-tolerant strains. Alcaligenes faecalis WT10 was subjected to mutagenesis by using a combined LiCl–UV irradiation and low energy N+-beam implantation technique to improve its nitrilase activity for industrial applications.[Citation21] Peng et al. [Citation22] used LiCl to enhance the degradation of phenol by Rhodococcus ruber SD3. Wang et al. [Citation23] successfully performed a protoplast mutation of the endophytic fungus Tubercularia sp. TF5 with UV and nitrosoguanidine (NTG) to produce 860 mutagenesis strains. Li et al. [Citation24] developed a protoplast mutagenesis method to enhance the arachidonic acid production by Mortierella isabellina. To the best of our knowledge, this is the first report of protoplast mutagenesis of G. lucidum by using the combination of LiCl and Triton X-100. Triton X-100 is a non-ionic surfactant, which aids in the improvement of the cell membrane permeability. When used in mutagenesis, Triton X-100 helps the mutagen to enter the cell and facilitates the protoplast mutation.
Figure 1. Relationship between lithium chloride with different concentrations and lethality rate (A) or lithium chloride with different concentrations plus Triton X-100 and lethality rate (B).
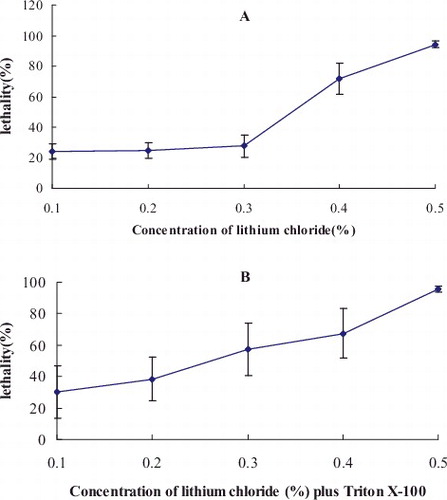
Figure 2. Comparison of intracellular polysaccharide content between the original strain and mutants.
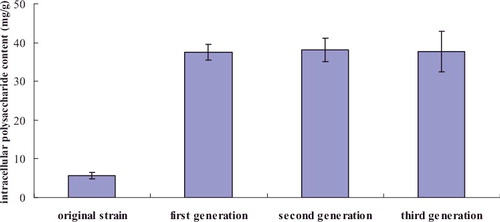
To assess the heredity stability of the mutants, the yields of polysaccharides and triterpenoids of the second and third generations were determined. The intracellular polysaccharide content in the second generation and in the third generation of mutants was 38.11 and 37.69 mg/g, respectively, whereas the intracellular triterpenoid content in the second generation and in the third generation of mutant was 46.02 and 44.13 mg/g, respectively ( and ). The data clearly indicated the stability of the mutants for the production of polysaccharides and triterpenoids.
Conclusions
The best conditions for the preparation and regeneration of protoplasts in G. lucidum were the following: mycelium cultivation for seven days and treatment with 0.33 mL of 1% snailase and 0.66 mL of 0.5% cellulase solution for 2.5 h at 30 °C in the presence of osmotic pressure stabilizer mannitol (0.5 mol/L). The mutagenesis of G. lucidum protoplasts by using lithium chloride and Triton X-100 resulted in a remarkable improvement of the polysaccharides and triterpenoids production from two mutant strains, which increased with 568.45% and 373.43%, respectively, as compared to the original strain. The present paper reported a novel and effective method for substantial improvement of the polysaccharides and triterpenoids yield from G. lucidum.
Dısclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgements
The authors thankfully acknowledge the financial support from Jiangxi provincial science and technology correspondent project and Jiangxi Normal University.
Additional information
Funding
References
- Chen S, Xu J, Liu C, et al. Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nat Commun. 2012;3:913.
- Jie L, Kuniyoshi S, Fumiko K, et al. Anti-androgenic activities of the triterpenoids fraction of Ganoderma lucidum. Food Chem. 2007;100:1691–1696.
- Zhou XW, Li QZ, Yin YZ, et al. Identification of medicinal Ganoderma species based on PCR with specific primers and PCR-RFLP. Planta Med. 2008;74:197–200.
- Shi M, Zhang Z, Yang Y. Antioxidant and immunoregulatory activity of Ganoderma lucidum polysaccharide (GLP). Carbohydr Polym. 2013;95:200–206.
- Paterson RRM. Ganoderma - a therapeutic fungal biofactory. Phytochemistry. 2006;67:1985–2001.
- Nguyen VT, Tung NT, Cuong TD, et al. Cytotoxic and anti-angiogenic effects of lanostane triterpenoids from Ganoderma lucidum. Phytochem Lett. 2015;12:69–74.
- Min BS, Gao JJ, Hattori M, et al. Anticomplement activity of triterpenoids from the spores of Ganoderma lucidum. Planta Med. 2001;67:811–814.
- Liu J, Shimizu K, Konishi, F, et al. Anti-androgenic activities of the triterpenoids fraction of Ganoderma lucidum. Food Chem. 2007;100:1691–1696.
- Smina TP, Mathew J, Janardhanan KK, et al. Antioxidant activity and toxicity profile of total triterpenes isolated from Ganoderma lucidum (Fr.) P. Karst occurring in South India. Environ. Toxicol Phar. 2011;32:438–446.
- Ruan W, Popovich DG. Ganoderma lucidum triterpenoid extract induces apoptosis in human colon carcinoma cells (Caco-2). Biomed Prev Nutr. 2012;2:203–209.
- Fatmawatia S, Shimizu K, Kondo R. Ganoderol B: a potent - α-glucosidase inhibitor isolated from the fruiting body of Ganoderma lucidum. Phytomedicine. 2011;18:1053–1055.
- Mukherjee M, Sengupta S. Mutagenesis of protoplasts and regeneration of mycelium in the mushroom Volvariella volvacea. Appl Environ. Microb. 1986;52:1412–1414.
- Ma C, Feng M, Zhai X, et al. Optimization for the extraction of polysaccharides from Ganoderma lucidum and their antioxidant and antiproliferative activities. J Taiwan Inst Chem E. 2013;44:886–894.
- Jiang H, Sun P, He J, et al. Rapid purification of polysaccharides using novel radial flow ion-exchange by response surface methodology from Ganoderma lucidum. Food Bioprod Process. 2012;90:1–8.
- Li G, Yang F, Li R, et al. A study on the breeding of new Ganoderma lucidum by UV induced mutagenesis. Acta Microbiologica Sinica. 2001;41:229–233.
- Toba T, Uemura H, Itoh T. A new method for the quantitative determination of microbial extracellular polysaccharide production using a disposable ultrafilter membrane unit. Lett Appl Microbiol. 1992;14:30–32.
- Reyes RG, Eguchi F, Iijima T, et al. Regeneration of protoplasts from hyphal strands of Volvariella volvacea. J. Wood Sci. 1998;44:401–407.
- Harling R, Kenyon L, Lewis BG, et al. Conditions for efficient isolation and regeneration of protoplasts from Fulvia fulva. J Phytopathol. 1988;122:143–146.
- Zhong FL, Wu XQ, Li MY, et al. Analysis and evaluation of polysaccharides and triterpenes contents of fruit body of Ganoderma lucidum strains. Edible Fungi China. 2009;28:38–40.
- Kang LZ, Han F, Lin JF, et al. Breeding of new high-temperature-tolerant strains of Flammulina velutipes. Sci Hortic. 2013;151:97–102.
- Xue YP, Xu SZ, Liu ZQ, et al. Enantioselective biocatalytic hydrolysis of (R,S)-mandelonitrile for production of (R)-(-)- mandelic acid by a newly isolated mutant strain. J Ind Microbiol Biotechnol. 2011;38:337–345.
- Peng R, Yang G, Wang Q, et al. Isolation and mutagenesis of a novel phenol-degrading strain. Adv Mater Res. 2013;64:588–594.
- Wang M, Liu S, Li Y, et al. Protoplast mutation and genome shuffling induce the endophytic fungus Tubercularia sp. TF5 to produce new compounds. Curr Microbiol. 2010;61:254–260.
- Li L, Yu C, Han Y. Enhancement of arachidonic acid production by Mortierella isabellina through protoplast regeneration mutagenesis. J Northeast Agr Uni. (English edition). 2011;18:65–72.