ABSTRACT
Different inducers for laccase production in Pleurotus ostreatus (ACCC 52857) were screened: carbon and nitrogen source, phenolic compounds and metal ions. Among the tested substances, yeast extract and copper showed the strongest effect on laccase activity. Laccase activity increased during the early phase of cultivation in the presence of yeast extract, peaking on the 6th day and decreasing thereafter. Copper-induced laccase activity increased both in a dose-dependent and a time-dependent manner. The highest laccase activity was obtained with 2 mmol/L Cu2+, while the mycelial growth was inhibited approximately 27%. Thus, the time-dependent effect of copper on laccase activity was examined. The results showed that the best laccase production was induced when copper was added during the mid-logarithmic phase of cultivation (the 5th day). A positive synergistic effect of yeast extract and copper on the laccase production was observed. Laccase activity dramatically increased upon the addition of copper to medium containing 1% yeast extract on the 5th day of cultivation. The highest activity (8533.33 ± 1228.94 U/mL) was observed on the 13th day of cultivation, increased more than 80 folds compared to the original level.
Introduction
White-rot fungi uniquely secrete extracellular enzymes essential for lignin degradation, including manganese peroxidase (MnP; EC 1.11.1.13), lignin peroxidase (LiP; EC 1.11.1.14) and laccase (Lac; EC 1.10.3.2), and exhibit a strong capacity for degradation of lignin and synthetic chemicals similar in structure to lignin. Laccases are multi-copper enzymes that catalyse the oxidation of a broad variety of phenolic compounds and aromatic amines, reducing oxygen to water.[Citation1] Due to the wide range of substrates, laccases show potential for use in various applications, such as pulp bleaching in paper, food, textile and dye industries, and detoxification of environmental pollutants, wastewater treatment, biofuel and biosensor.[Citation2–5] Fungal laccases likely have additional functions in nature, including lignocellulose degradation, soil organic matter cycling, fungal plant--pathogen/host interaction and defence, and stress responses on diverse environmental challenges.[Citation6]
The biological characteristics of laccases and practical uses for these enzymes in industry and biotechnology have attracted much research attention. However, the production of laccase in white-rot fungi is relatively low under natural conditions and cannot satisfy the demands of practical use in industry and biotechnology. Thus, much attention has been focused on increasing laccase production in white-rot fungi. The synthesis and secretion of laccase is influenced by many factors, including nutrient levels, culture conditions, development stages and the addition of inducers to the culture medium.[Citation7–13] The effects of these factors vary among the fungal species and could regulate different isoenzymes in the same strain.[Citation14–16] Laccase activity in Pleurotus sajor-caju is enhanced when cultured with glucose and pure casein,[Citation17] whereas laccase gene transcription in Phanerochaete chrysosporium is triggered when the glucose and nitrogen concentrations in the medium reach a certain low level.[Citation18,Citation19] Aromatic compounds structurally related to lignin, such as xylidine, ferulic acid and veratric acid, are routinely added to fungal cultures to increase laccase production.[Citation12,Citation15,Citation20,Citation21] The regulation of laccase expression by metals is widespread in fungi.[Citation10] Copper induces the laccase activity in almost all fungi, and in Trametes versicolor,[Citation7] P. sajor-caju,[Citation21] Ceriporiopsis subvermispora,[Citation22] Pleurotus ostreatus [Citation23] and Trametes pubescens,[Citation24] the copper-mediated regulation of laccase occurs at the transcription level. Other metals, such as Fe, Mn and Zn, also increase the laccase activity. Notably, the same metal can exert opposing effects in different species. Compared with the control, laccase activity significantly increased in the presence of Fe2+ in Pleurotus eryngii,[Citation25] while Fe2+ markedly inhibited laccase production in Ganoderma lucidum.[Citation26]
Pleurotus ostreatus is a white-rot basidiomycete living as a saprophyte on dead or decaying wood secreting different enzymes involved in lignin degradation. The genome of P. ostreatus encodes 12 putative laccase-encoding genes, and 6 of these genes have been isolated and characterized.[Citation10] Lacc2 and Lacc10 are highly expressed under submerged conditions.[Citation11,Citation27]
Previous work in our lab showed that laccase activity and the degree of induction differed among different P. ostreatus strains isolated from different districts in China (unpublished data). A similar result was obtained in Flammulina velutipes.[Citation28] In this study, we screened the laccase inducers in P. ostreatus strain ACCC 52857 and examined the effects of the inducers and their time-dependent addition.
Materials and methods
Strain and culture conditions
Pleurotus ostreatus (ACCC 52857) was collected from Anhui Province in China and was deposited in the Agricultural Culture Collection of China. It was identified based on the ITS sequence analysis and morphological characteristics using Chinese fungi [Citation29] as a reference. In preliminary experiments, it showed a slightly higher growth rate, especially at low temperatures, compared to other strains (unpublished data).
The mycelia were cultivated in potato dextrose agar (PDA) medium containing 200 g/L potato extract, 20 g/L D-glucose, 3 g/L KH2PO4, 1.5 g/L MgSO4 and 20 g/L agar, at 25 °C. After 7 days of growth, the mycelia were stored at 4 °C, and the stocks were transferred to fresh PDA every three months.
Liquid PDB medium (PDA medium without agar) was used in shaken cultures. The pre-cultures were prepared by homogenization of an agar plug sized about 4 cm2 containing P. ostreatus mycelia in 100 mL of PDB medium in a 250 mL flask for 7 days at 25 °C with agitation at 160 r/min. The pre-cultures were homogenized again and subsequently, 5 mL of homogenized pre-cultures were transferred to 100 mL fresh liquid medium in 250 mL flasks, followed by incubation at 25 °C, with shaking (160 r/min).
Medium for screening
The glucose in the PDB medium was replaced with other carbon resources, including fructose, mannose, galactose, sucrose, lactose, maltose, starch and cellulose, at a final concentration of 2%.
The nitrogen sources, including (NH4)2SO4, NH4NO3, urea, yeast extract, peptone or malt extract, were added to the PDB medium at a final concentration of 1%. The effect of different concentrations of yeast extract on laccase activity was tested, and the final concentration of yeast extract was 0.5%, 1% and 2%, respectively.
Aromatic compounds [phenol, pyrocatechol, pyrogallol, syringic acid, vanillic acid, ferulic acid, caffeic acid, gallic acid, veratryl alcohol, vanillin, guaiacol and 2,2-azino-bis-3(ethylbenzthiazoline-6-sulfonic acid) (ABTS)] and metal ions (KCl, NaCl, MgSO4, ZnSO4, MnSO4, CaCl2, FeSO4, Fe2(SO4)3, CuSO4, Pb(NO3)2 and CdCl2) were dissolved and sterilized through filtration, and freshly added to the medium before cultivation. The final concentrations of Pb(NO3)2 and CdCl2 were 400 and 100 μmol/L, respectively, and the other compounds were added at a final concentration of 1 mmol/L. The effect of copper concentration on laccase activity was tested, and the final concentration of Cu2+ in the medium was 0.5, 1, 1.5, 2 and 2.5 mmol/L, respectively.
Determination of the mycelial dry weight
The mycelia were separated from the liquid medium by centrifugation at 3000 r/min (Eppendorf 5810R) for 5 min and washed three times with ddH2O. The mycelia were dried at 60 °C to constant weight (n = 5).
Enzyme assay
Laccase activity was determined at room temperature using ABTS as a substrate. The reaction mixture contained 2.3 mL of sodium acetate buffer (pH = 4.5), 0.2 mL of 1 mmol/L ABTS and 0.5 mL of diluted enzyme. After incubation for 2 min at room temperature, laccase activity was determined as the increase in the absorbance at 420 nm [ϵ = 36,000 L/(mol·cm)]. One unit of laccase activity was defined as the amount of enzyme required to oxidize 1 μmol of ABTS per minute.
Effect of time on copper-induced laccase activity
The mycelia were incubated in 100 mL PDB medium at 25 °C, with shaking (160 r/min). Cu2+ was added to the medium on the 3rd, 5th, 8th and 11th day of cultivation at a final concentration of 2 mmol/L. Laccase activities were measured from the following day to the end of cultivation, and the biomass was also measured after incubation.
Synergistic effects of yeast extract and copper on laccase activity
The mycelia were incubated in 100 mL PDB medium containing 1% yeast extract, and Cu2+ was added to the medium to a final concentration of 2 mmol/L on the 5th day of cultivation. Laccase activities were measured from the 3rd day to the end of the cultivation period.
Data analysis
All samples were prepared in triplicate and the data presented in figures are mean values; the error bars represent the standard deviation.
Results and discussion
Mycelial growth and laccase production in liquid PDB
The mycelia of P. ostreatus (ACCC 52857) grew well in liquid PDB medium, as shown in . The average growth rate was 2.42 g/L per day from the 3rd to the 7th day of cultivation, followed by a subsequent reduction in the growth rate, averaging 1.13 g/L per day from the 8th to the 12th day of cultivation. After 14 days of cultivation, the biomass was 19.20 ± 1.68 g/L.
Figure 1. Time course of laccase activity and biomass accumulation of P. ostreatus (ACCC 52857) cultivated in PDB medium.
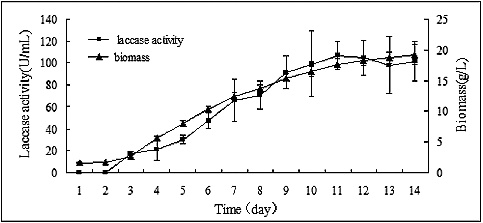
The laccase activity gradually increased with mycelial growth (). The highest laccase activity (106.62 ± 13.18 U/mL) was obtained by the 11th day of cultivation and became relatively stable thereafter. Considering the mycelial growth and laccase activity in the medium, the laccase inducers in P. ostreatus (ACCC 52857) were screened on the 8th day.
Screening the laccase inducers in Pleurotus ostreatus (ACCC 52857)
In this study, when the mycelia of P. ostreatus (ACCC 52857) were incubated in the medium for screening different laccase inducers, laccase activity was measured on the 8th day of cultivation. The laccase activity in PDB medium was set as control.
The results shown in demonstrated that the tested carbon resources could be used to maintain mycelial growth, while there was no evidence showing that any of these compounds promoted laccase production compared with the control ((A)).
Figure 2. Effects of potential inducers of laccase activity in P. ostreatus (ACCC 52857). (A) Different carbon sources, (B) nitrogen resources, (C) different metal ion solutions and (D) different aromatic compounds.
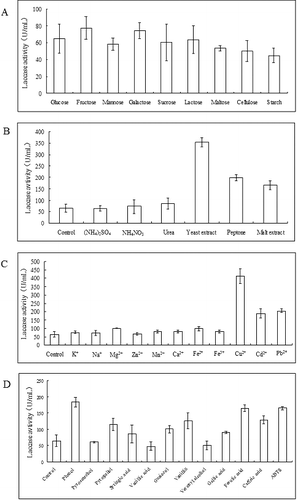
Laccase activity did not significantly differ compared with the control upon the addition of inorganic nitrogen sources and urea to the medium. The laccase activities were significantly increased in the presence of the other organic nitrogen sources, yeast extract, peptone and malt extract. Laccase activity was, respectively, 353.33 ± 20.5 U/mL, 198.53 ± 13.58 U/mL and 165.67 ± 19.74 U/mL on the 8th day of cultivation, showing increases of 5.5-, 3- and 2.6-fold, respectively, compared with the control ((B)).
No obvious effect on laccase production was observed after the addition of essential metal ions, such as K+, Na+, Mg2+, Zn2+, Mn2+, Ca2+, Fe2+ and Fe3+, to the medium, whereas other two toxic heavy metals, Cd2+ and Pb2+, generated a nearly threefold increase in laccase activity ((C)). The laccase activity was, respectively, 189.98 ± 28.07 U/mL and 205.54 ± 13.05 U/mL on the 8th day of cultivation in medium supplemented with 100 μmol/L Cd2+ or 400 μmol/L Pb2+, separately. Although the regulation mechanism remains unknown, the increase in laccase activity might reflect the functions or physiological roles of this enzyme in P. ostreatus. Copper, the cofactor of laccase, was the strongest inducer among the substances tested. Laccase activity in P. ostreatus (ACCC 52857) was markedly increased in the presence of 1 mmol/L copper in the medium. The activity peaked to 412.50 ± 46.24 U/mL, showing a greater sixfold increase compared with the control ((C)).
The effects of several aromatic compounds on laccase activity in P. ostreatus (ACCC 52857) are shown in (D). Laccase activity increased more than 2.5-fold in the presence of phenol, ferulic acid and ABTS in the medium on the 8th day of cultivation. Although it is well accepted that aromatic compounds could induce the laccase gene transcription and increase laccase activity, the effects of aromatic compounds on laccase production in P. ostreatus (ACCC 52857) were less pronounced than those of copper and yeast extract. Thus, yeast extract and copper were used in the following experiments.
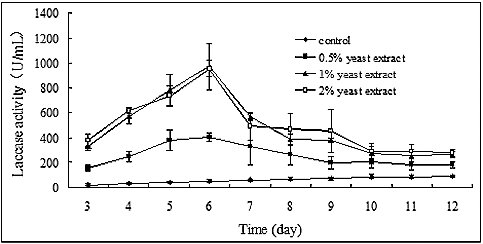
Effect of yeast extract on laccase production
Yeast extract is a complex mixture which is not only a good organic nitrogen resource for the promotion of mycelial growth, but also an adequate inducer of laccase activity in P. ostreatus (ACCC 52857), although the precise components responsible for the induction of laccase production have not been identified yet. As shown in , laccase activity rapidly increased upon the addition of yeast extract in the medium. On the 3rd day of cultivation, laccase activities were 151.67 ± 23.79 U/mL, 331.67 ± 40.00 U/mL and 379.87 ± 47.14 U/mL in the presence of 0.5%, 1% and 2% yeast extract, showing an increase of 7.5-, 16.5- and 18.9-fold, respectively, compared with the control (20.14 ± 8.11 U/mL). Laccase activity increased with incubation time, peaking on the 6th day of cultivation (970 ± 182.07 U/mL) and gradually decreasing thereafter. Laccase activity was much higher with 1% than with 0.5% yeast extract in the medium, while there was no considerable difference in laccase activity between 1% and 2% yeast extract.
It is noteworthy that laccase activity decreased after 6 days of cultivation. It could be hypothesized that laccase molecules that have been synthesized during earlier stages of growth might later become destroyed or inactivated by some unknown mechanism, leading to instability. Moreover, since yeast extract is a good nitrogen source to maintain cell growth, the concentration of yeast extract will decrease with the mycelial growth. Thus, it is likely that the concentration of inducers also decreases, which may possibly explain the downward trend in the induction effect after 6 days of cultivation.
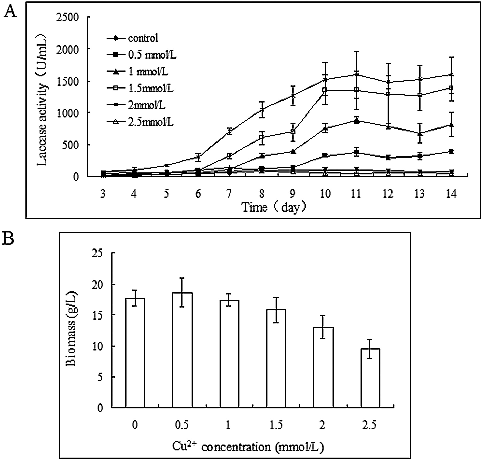
Effect of copper concentration on laccase production and mycelial growth
The effect of copper on laccase activity was dose dependent ((A)), i.e. laccase activity increased with increasing copper concentration in the medium. Laccase activity slowly increased during the early mid-logarithmic phase of mycelial growth (from the 3rd to the 6th day), followed by a rapid increase in the next few days (from the 7th to the 10th day). The highest laccase activity (1595.83 ± 358.57 U/mL) was obtained after the addition of 2 mmol/L Cu2+ to the medium after 11 days of cultivation. However, after the addition of 2.5 mmol/L Cu2+ to the medium, the laccase activity was lower compared to that in the control group, which may suggest that the high copper concentration inhibited the mycelial growth. Thus, the biomass was measured after incubation and the results showed that, whereas copper increased laccase activity, it inhibited the mycelial growth ((B)). When the Cu2+ concentration was less than 1 mmol/L in the medium, the mycelial growth did not considerably differ from that in the control group. After 14 days of cultivation, the biomass was 13.03 ± 1.85 g/L with 2 mmol/L Cu2+, 73% of that observed in the control group (17.75 ± 1.32 g/L). The mycelial growth was inhibited nearly 50% compared to the control in the presence of 2.5 mmol/L Cu2+: the biomass was only 9.49 ± 1.50 g/L after 14 days of incubation.
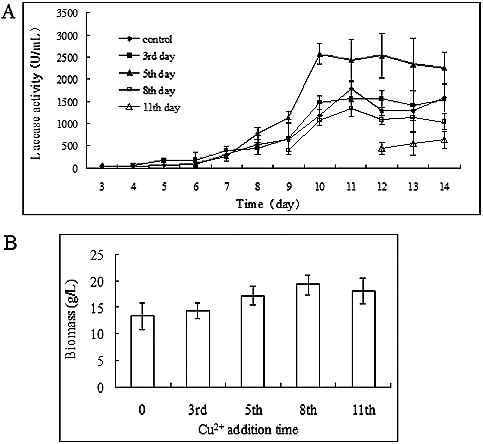
Effect of time on copper-induced laccase production
Because both the strongest induction effect of copper and increased mycelial growth are needed for the production of laccase, the time-dependent effect of copper induction might be critical to induce laccase activity in P. ostreatus (ACCC 52857). Considering the toxic effect of 2 mmol/L Cu2+ on the mycelial growth of P. ostreatus (ACCC 52857), we investigated the laccase activity upon the addition of copper at different time points of the cultivation process. As shown in , the changes in laccase activities were similar ((A)) and there was no difference in the biomass when Cu2+ was added before incubation or on the 3rd day of cultivation ((B)), as the mycelial growth was inhibited at nearly the same level. Although increased biomass accumulation was also observed when copper was added on the 8th and 11th day of cultivation, the addition of copper to the culture medium on the 5th day showed the best induction of laccase production. This suggests that mycelia age is an additional factor in laccase production. When 2 mmol/L copper was added to the medium on the 5th day of cultivation, the laccase activity reached 2575 ± 247.31 U/mL on the 10th day ((A)). It could be speculated that when the cells are too old, they cannot withstand the toxic effect of high copper concentrations. Thus, the addition of copper to the medium during a later stage of fungal development showed lower laccase activity, although increased biomass was observed.
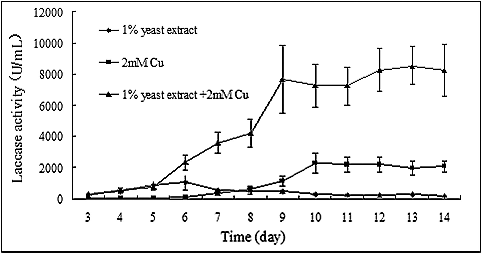
Synergistic effects of yeast extract and copper on laccase production
Yeast extract and copper were the most powerful inducers of laccase production in P. ostreatus (ACCC 52857) among the substances we tested. However, varying changes in laccase activities were observed, suggesting that these inducers have different functions. The synergistic effects of yeast extract and copper on laccase production were further investigated. The change in laccase activity is shown in . There was a positive synergistic effect of yeast extract and copper on laccase production. The laccase activity dramatically increased upon the addition of copper to the medium from the 6th to the 9th day of cultivation. The highest activity, reaching 8533.33 ± 1228.94 U/mL, was obtained on the 13th day of cultivation, with a more than 80-fold increase compared to the original level ().
Final remarks
Due to their ability to catalyse the oxidation of a wide variety of substrates, laccases have attracted special interest in the research of their characteristics and application in industrial uses. In order to meet the demand for low-cost laccase, different strategies have been developed to enhance laccase production.[Citation30–32] Many studies have focused on the use of industrial and agricultural waste or by-products to increase laccase production. These materials might contain laccase inducers that promote laccase production. Because agricultural waste and by-products are complex mixtures, the precise identity and the concentration of the inducer in the medium remains unknown, which makes the regulation mechanism remain elusive.
Our results that the changes in laccase activity induced by yeast extract and copper showed different patterns, likely indicate that these inducers function in different manners. A positive synergistic effect was observed when these inducers were used together. Moreover, Palmieri et al. [Citation33] showed that copper also inhibits extracellular proteolytic activity, thus impairing laccase degradation. In this study, laccase activity dramatically increased after the addition of copper to the medium, and this activity remained stable after 2 weeks in shaken cultures. It is reasonable to speculate that, although the regulation mechanism remains unknown, copper and its metabolism play multiple roles in cell growth, laccase gene transcription and laccase activity stability in P. ostreatus (ACCC 52857).
Moreover, the biological function of laccase remains unknown. Interestingly, in our study, the highest laccase activity was observed after the addition of copper at levels that inhibit mycelial growth. The obtained results that lead and cadmium, which are toxic to living cells with no obvious function, promoted the laccase activities are in agreement with similar results reported by other authors.[Citation34,Citation35] The phenolic compounds, ferulic acid and vanillin, presenting the most toxic effect on the mycelial growth of Pleurotus pulmonarius, were very efficient laccase inducers.[Citation15] In another fungal species, Sclerotinia sclerotiorum, laccase activity was upregulated via stress pathways. Certain chemical stress conditions that reduce biomass production may increase laccase activity.[Citation36] These observations suggest that the function of laccase might be involved in detoxification or stress responses, but the mode of action remains unknown. In addition, laccase activity continuously increased with mycelial growth in the control group, even though the activity was low, suggesting that laccase enzymes perform certain functions during normal fungal growth.
Conclusions
In this study, the laccase inducers in P. ostreatus strain (ACCC 52857) were screened. Yeast extract and copper showed the strongest effect on laccase activity among the tested substances. Even though they induced laccase activities in a different manner, there existed a positive synergistic effect of yeast extract and copper on laccase production in P. ostreatus strain ACCC 52857. Considering the toxic effect of copper, mycelia age is a critical factor in laccase production. The highest activity was observed when copper was added on the 5th day of cultivation. Further research is needed to focus on the intrinsic biological properties and laccase expression regulation mechanism which may provide the basic information to enhance laccase production.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Giardina P, Faraco V, Pezzella C, et al. Laccases: a never-ending story. Cell Mol Life Sci. 2010;67:369–385.
- Kunamneni A, Plou FJ, Ballesteros A, et al. Laccases and their applications: a patent review. Recent Pat Biotechnol. 2008;2:10–24.
- Bragazzi NL, Pechkova E, Scudieri D, et al. Recombinant laccase: II. Medical biosensor. Crit Rev Eukaryot Gene Expr. 2012;22:197–203.
- Kudanga T, Le Roes-Hill M. Laccase applications in biofuels production: current status and future prospects. Appl Microbiol Biotechnol. 2014;98:6525–6542.
- Zeeb B, Fischer L, Weiss J. Stabilization of food dispersions by enzymes. Food Funct. 2014;5:198–213.
- Kües U, Rühl M. Multiple multi-copper oxidase gene families in basidiomycetes - what for? Curr Genom. 2011;12:72–94.
- Collins PJ, Dobson A. Regulation of laccase gene transcription in Trametes versicolor. Appl Environ Microb. 1997;63:3444–3450.
- Terrón MC, González T, Carbajo JM, et al. Structural close-related aromatic compounds have different effects on laccase activity and on lcc gene expression in the ligninolytic fungus Trametes sp. I-62. Fungal Genet Biol. 2004;41:954–962.
- Lettera V, Piscitelli A, Leo G, et al. Identification of a new member of Pleurotus ostreatus laccase family from mature fruiting body. Fungal Biol. 2010;114:724–730.
- Piscitelli A, Giardina P, Lettera V, et al. Induction and transcriptional regulation of laccases in fungi. Curr Genom. 2011;12:104–112.
- Castanera R, Pérez G, Omarini A, et al. Transcriptional and enzymatic profiling of Pleurotus ostreatus laccase genes in submerged and solid-state fermentation cultures. Appl Environ Microbiol. 2012;78:4037–4045.
- Yang Y, Wei F, Zhuo R, et al. Enhancing the laccase production and laccase gene expression in the white-rot fungus Trametes velutina 5930 with great potential for biotechnological applications by different metal ions and aromatic compounds. PLOS One. 2013;8:e79307.
- Bertrand B, Martínez-Morales F, Tinoco R, et al. Induction of laccases in Trametes versicolor by aqueous wood extracts. World J Microbiol Biotechnol. 2014;30:135–142.
- Mansur M, Suárez T, González AE. Differential gene expression in the laccase gene family from basidiomycete I-62 (CECT 20197). Appl Environ Microbiol. 1998;64:771–774.
- de Souza CG, Tychanowicz GK, de Souza DF, et al. Production of laccase isoforms by Pleurotus pulmonarius in response to presence of phenolic and aromatic compounds. J Basic Microbiol. 2004;44:129–136.
- Xiao YZ, Hong YZ, Li JF, et al. Cloning of novel laccase isozyme genes from Trametes sp. AH28-2 and analyses of their differential expression. Appl Microbiol Biotechnol. 2006;71:493–501.
- Bettin F, Montanari Q, Calloni R, et al. Production of laccases in submerged process by Pleurotus sajor-caju PS-2001 in relation to carbon and organic nitrogen sources, antifoams and Tween 80. J Ind Microbiol Biotechnol. 2009;36:1–9.
- Jeffries TW, Choi S, Kirk TK. Nutritional regulation of lignin degradation by Phanerochaete chrysosporium. Appl Environ Microbiol. 1981;42:290–296.
- Wang P, Hu X, Cook S, et al. Effect of culture conditions on the production of ligninolytic enzymes by white rot fungi Phanerochaete chrysosporium (ATCC 20696) and separation of its lignin peroxidase. World J Microbiol Biotechnol. 2008;24:2205–2212.
- Munoz C, Guillen F, Martinez AT, et al. Induction and characterization of laccase in the ligninolytic fungus Pleurotus eryngii. Curr Microbiol. 1997;34:1–5.
- Soden DM, Dobson AD. Differential regulation of laccase gene expression in Pleurotus sajor-caju. Microbiology. 2001;147:1755–1763.
- Alvarez JM, Canessa P, Mancilla RA, et al. Expression of genes encoding laccase and manganese-dependent peroxidase in the fungus Ceriporiopsis subvermispora is mediated by an ACE1-like copper-fist transcription factor. Fungal Genet Biol. 2009;46:104–111.
- Faraco V, Giardina P, Sannia G. Metal-responsive elements in Pleurotus ostreatus laccase gene promoters. Microbiology. 2003;149:2155–2162.
- Galhaup C, Goller S, Peterbauer CK, et al. Characterization of the major laccase isoenzyme from Trametes pubescens and regulation of its synthesis by metal ions. Microbiology. 2002;148:2159–2169.
- Stajić M, Vukojević J. Interaction of trace elements and ligninolytic enzymes in Pleurotus eryngii. Biol Trace Elem Res. 2011;143:1202–1208.
- Murugesan K, Kim YM, Jeon JR, et al. Effect of metal ions on reactive dye decolorization by laccase from Ganoderma lucidum. J Hazard Mater. 2009;168:523–529.
- Pezzella C, Lettera V, Piscitelli A, et al. Transcriptional analysis of Pleurotus ostreatus laccase genes. Appl Microbiol Biotechnol. 2013;97:705–717.
- Janusz G, Czuryło A, Frąc M, et al. Laccase production and metabolic diversity among Flammulina velutipes strains. World J Microbiol Biotechnol. 2015;31:121–133.
- Yu L, Guer G. Chinese fungi. China: Science Press; 2014.
- Gu C, Zheng F, Long L, et al. Engineering the expression and characterization of two novel laccase isoenzymes from Coprinus comatus in Pichia pastoris by fusing an additional ten amino acids tag at N-terminus. PLOS One. 2014;9:e93912.
- Ramírez-Cavazos LI, Junghanns C, Nair R, et al. Enhanced production of thermostable laccases from a native strain of Pycnoporus sanguineus using central composite design. J Zhejiang Univ Sci B. 2014;15:343–352.
- Wang F, Hu JH, Guo C, et al. Enhanced laccase production by Trametes versicolor using corn steep liquor as both nitrogen source and inducer. Bioresour Technol. 2014;166:602–605.
- Palmieri G, Bianco C, Cennamo G, et al. Purification characterization and functional role of a novel extracellular protease from Pleurotus ostreatus. Appl Environ Microbiol. 2001;67:2754–2759.
- Baldrian P, Gabriel J. Lignocellulose degradation by Pleurotus ostreatus in the presence of cadmium. FEMS Microbiol Lett. 2003;220:235–240.
- Baldrian P, Valásková V, Merhautová V, et al. Degradation of lignocellulose by Pleurotus ostreatus in the presence of copper, manganse, lead and zinc. Res Microbiol. 2005;156:670–676.
- Coman C, Moţ AC, Gal E, et al. Laccase is upregulated via stress pathways in the phytopathogenic fungus Sclerotinia sclerotiorum. Fungal Biol. 2013;117:528–539.