ABSTRACT
The aim of this study was to investigate the use of ESM-1 (endothelial cell-specific molecule-1) as a new biomarker for the pathogenesis of rheumatoid arthritis (RA). The study cohort was divided into four groups according to the DAS28 disease activity score: 16 patients were classified as being in remission (DAS28 < 2.6), 16 patients exhibited low disease activity (DAS28: 2.6–3.2), 20 patients exhibited moderate disease activity (DAS28: 3.2–5.1) and 16 patients exhibited high disease activity (DAS28 > 5.1); 20 healthy subjects were included as a control group. Serum samples were gathered from the patients with documented seropositivity for rheumatoid factor (RF) and anti-cyclic citrullinated peptide antibodies in order to assess RF IgM and ESM-1. ESM-1 levels were significantly higher in patients with RA than in healthy subjects (p = 0.035). The data presented here strongly indicate ESM-1 as an attractive target for the treatment of inflammation-related diseases, such as RA.
Introduction
Endothelial cell-specific molecule-1 (ESM-1) is an important immunomodulatory protein secreted by endothelial linings of the lungs and kidneys.[Citation1,Citation2] Expression of this protein is regulated by multiple cytokines, with tumour necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) strongly enhancing expression, while interferon-γ (IFN-γ) is suppressive; no effect is seen in response to IL-4. Consistent with a basal secretion of ESM-1 by vascular endothelial cells, circulating ESM-1 is found in the sera of healthy subjects and is increased in patients with acute and severe sepsis.[Citation3]
Under normal circumstances, the endothelium is responsible for maintaining vascular homeostasis.[Citation4] Chronic inflammation may disrupt this balance, leading to endothelial dysfunction, and contributing to the pathogenesis of other diseases, such as rheumatoid arthritis (RA).[Citation5,Citation6] Indeed, many markers of endothelial cell activation and dysfunction are strongly correlated with the inflammatory process seen in RA.[Citation7]
One such marker, ESM-1, an endothelial cell-specific proteoglycan, is commonly used as a biomarker for endothelial cell activation or dysfunction.[Citation8,Citation9] While ESM-1 has recently been identified as a key player in inflammatory conditions,[Citation10] it is not clear whether levels of ESM-1 in the circulation are associated with endothelial function or disease activity in patients with RA. Here, we investigated the use of ESM-1 as a new biomarker for RA pathogenesis.
Subjects and methods
A total of 68 enrolled patients receiving treatment at the rheumatology outpatient clinics of the School of Medicine Hospital, Sakarya University (Sakarya, Turkey), between April 2012 and September 2013 were enrolled in this study, along with 20 healthy controls. All patients fulfilled the minimum ACR/EULAR 2010 criteria (American College of Rheumatology/European League against Rheumatism) for RA classification at the time of enrolment, along with seropositivity for both rheumatoid factor (RF) Immunoglobulin (IgG) and anti-cyclic citrullinated peptide (anti-CCP) antibodies.[Citation11] Exclusion criteria included a history of cancer, any cardiovascular disorders, haematological abnormality, any acute or other infection, a granulomatous chronic disease, a metabolic disease and anti-TNF use (anti-TNF therapy).
All relevant study-related protocols were approved by the Ethics Committee of Sakarya University. This work was performed as part of a laboratory-based study, with no direct involvement with the affected patients. All clinical data, including specimen source and relevant patient information, were carefully recorded from laboratory and clinical request forms.
The study cohort was divided into four groups according to DAS28 score: 16 patients were classified as being in remission (DAS28 < 2.6) (RG), 16 patients exhibited low disease activity (DAS28: 2.6–3.2) (LDAG), 20 patients exhibited moderate disease activity (DAS28: 3.2–5.1) (MDAG) and 16 patients exhibited high disease activity (DAS28 > 5.1) (HDAG); 20 healthy subjects were included as a control group.
Clinical characteristics, including age, gender, body mass index and smoking habits, were recorded for all patients, along with disease-specific data, such as time elapsed from first clinical symptoms (TEFCS), time elapsed after diagnosis (TEAD), duration of delay in diagnosis (DD), the existence of clinical remission, use of relevant disease-modifying antirheumatic drugs (DMARDs) and family history of disease. In order to evaluate the patients' quality of life, a health assessment questionnaire (HAQ) disability index consisting of 20 questions was used.[Citation12] Disease remission was defined using the ACR/EULAR 2011 remission criteria, involving C-reactive protein (CRP) expression, the number of swollen and fragile joints and the patient's global evaluation.[Citation13]
Serum samples were collected from the patients with documented seropositivity for RF and anti-CCP antibodies in order to assess RF IgM and ESM-1. RF IgG was measured by nephelometry using an IMMAGE Immunochemistry System (Beckman Coulter, Pasadena, CA, USA), with a level of 20 U/mL considered positive. Anti-CCP antibodies were measured using an enzyme-linked immunosorbent assay (ELISA; Abbott Laboratories, Green Oaks, IL, USA) and read on an ARCHITECT i1000SR immunoassay analyzer (Abbott Diagnostics, Lake Forest, IL, USA), with levels >5 arbitrary units defined as positive. RF IgM was assessed using a fluorescence-based immunoassay method in accordance with the manufacturer's instructions (i-CHROMA, BodiTech Med Inc., Chuncheon, Korea). ESM-1 (Aviscera Bioscience, Santa Clara, CA, USA) was assessed by ELISA using a cut-off of 0.39 ng/mL. The erythrocyte sedimentation rate (ESR) was measured immediately after blood collection using a Greiner SRS 20/II instrument (Vacuette Greiner, Kremsmünster, Austria).
SPSS statistical software was used for all statistical analyses (IBM SPSS statistics version 20.0). Quantitative variables (clinical and laboratory) are presented as the mean, standard deviation (SD) and range. Relationships between categorical values were analysed by one-way analysis of variance (ANOVA) and Pearson correlation test. P values ≤ 0.05 were considered statistically significant.
Results and discussion
The age, sex and smoking status were similar between RA patients and healthy controls. Average TEFCS, TEAD and DD times were 48.56 ± 39.02, 44.18 ± 34.09 and 6.12 ± 8.4 months, respectively. Patients with RA had significantly higher RF IgG and anti-CCP levels, as compared to healthy controls (p < 0.001 and p < 0.05, respectively). A full list of assay results and clinical characteristics of patients is shown in .
Table 1. Demographic and clinical characteristics of the study groups.
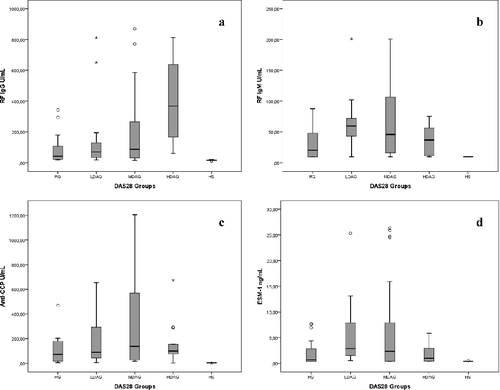
TEFCS and TEAD times, along with DMARDs' usage rates, were all significantly different between groups (p < 0.001); no such difference was observed for DD (). Anti-CCP levels were substantially higher in the MDAG group relative to RG; however, this difference was not statistically significant (p > 0.05). Similarly, while all groups were significantly different from each other (p < 0.01), the difference in RF IgM levels between the MDAG and RG groups was just above the threshold for statistical significance (p = 0.054).
Table 2. Clinic and laboratory parameters in the study groups.
No statistically significant differences in ESM-1 levels were observed between disease activity groups; however, these significant differences were observed when compared against healthy controls (p < 0.05). DMARD use was significantly higher among HDAG patients, as compared to other disease groups, although surprisingly, these patients also exhibited significantly lower ESM-1 expression. Associations between RF IgG (a), RF IgM (b), anti-CCP (c) and ESM-1 (d) in relation to disease activity are shown in
Accelerated atherosclerosis is a significant issue among patients with RA, with chronic endothelial dysfunction driving premature onset of atherosclerosis. The significant differences in ESM-1 expression observed in our study, along with increased ESR and CRP values in patients with RA relative to healthy controls are in agreement with reported evidence that patients with RA have an elevated risk of cardiovascular disease, possibly due to similarities in the inflammatory process in RA and atherosclerosis.[Citation14,Citation15]
Recent insights into the molecular mechanisms underlying RA pathogenesis have placed new emphasis on the role of endothelial cell dysfunction. Imaging methods, such as carotid intima-media thickness (ccIMT) and flow-mediated vasodilatation (FMD), can be used to determine atherosclerosis and endothelial dysfunction, and to assess cardiovascular risk in patients with autoimmune arthritis.[Citation16] A clinical study, evaluating the influence of chronic inflammation on endothelial function demonstrated that young to middle-aged patients with RA, with low disease activity and without clinically overt atherosclerotic disease or traditional cardiovascular risk factors, has an altered pattern of flow-mediated vasodilatation in the brachial artery. Endothelial dysfunction in these patients was related to serum CRP levels, consistent with a role for chronic inflammation.[Citation17]
More routine testing for RA can be performed using circulating biomarkers, such as soluble VCAM-1, von Willebrand factor, pentraxin-3, asymmetric dimethyl-L-arginine, soluble E-selectin, monocyte chemotactic protein-1 and osteoprotegerin.[Citation18] Many of these circulating factors used as biomarkers of disease progression are produced in response to inflammatory mediators commonly associated with RA and can themselves contribute to RA pathogenesis.
The data presented here support a role for endothelial dysfunction due to chronic inflammation in patients with RA. We found an association between RF IgM levels and ESM-1 in MDAG (r = 0.300; p < 0.01). Such an observation is consistent with several earlier studies showing the presence of anti-CCP antibodies and RF IgM, associated with impaired endothelial function independently of cardiovascular risk factors, in patients with RA.[Citation19] Existing preclinical and clinical data strongly support the use of ESM-1 as a validated therapeutic target in RA.
With respect to therapeutic strategies for RA treatment, anti-TNF-α therapies have proven particularly effective at reducing inflammation, as well as improving endothelial function.[Citation20] Sarrazin et al.[Citation10] suggest that inflammatory cytokines such as TNF-α, and pro-angiogenic growth factors such as vascular endothelial growth factor, the fibroblast growth factor-2 and hepatocyte growth factor/scatter factor, strongly induced the expression, synthesis and secretion of ESM-1 by human endothelial cells. Likewise, several in vitro studies have shown that ESM-1 expression is highly upregulated in response to TNF-α, and in a time-dependent manner.[Citation2] While exposure to pro-inflammatory cytokines, such as TNF-α leads to endothelial dysfunction,[Citation21,Citation22] the data presented here suggest a more complicated association between disease severity and ESM-1 expression, as peak ESM-1 expression was observed in MDAG patients, with the HDAG group exhibiting levels not statistically different from other groups, including controls. Our results seem to indicate a two-way interaction of disease activity in RA and pro-inflammatory cytokines, such as TNF-α.
Alternatively, the decrease in ESM-1 levels observed in HDAG patients may be the result of greater immunosuppression due to increased DMARD use in the HDAG group. In this regard, we further examined the relationship between ESM-1 levels in the MDAG and HDAG groups. Our data demonstrated a significant association between the number of DMARDs taken and ESM-1 levels in HDAG individuals, which is likely to be caused by a response to synthetic DMARD therapy. It has been shown that short-term treatment with anti-TNF was able to increase circulating endothelial progenitor cells concurrently with a proportional decrease in disease activity, suggesting that therapeutic intervention aimed at suppressing the inflammatory process might positively affect endothelial function.[Citation23]
Our findings suggest that inflammatory processes are not only a driving force in disease pathogenesis in RA patients, but they may also play a role in the activation and damage of the endothelium lining. The data presented here are suggestive of endothelial function being restored after DMARD use, though more research will be necessary before definitive conclusions can be reached.
Alternatively, ESM-1 may exert a separate role in the pathogenesis of atherosclerosis in patients with RA. Circulating ESM-1 may represent a new marker that correlates with cardiovascular risk, as well as the severity of disease in patients with psoriasis vulgaris.[Citation24] In patients with Behcet's disease, serum ESM-1 levels correlated moderately with disease activity but significantly with CRP and ESR.[Citation25] In concordance with these observations, we have first shown that patients with RA had significantly higher serum ESM-1 levels than those of healthy subjects. Second, we observed increased cardiovascular morbidity and mortality in patients with RA, which may be explained by endothelial-dependent mechanisms.
As with all research, this study has several limitations. First, the relationship between ESM-1, atherosclerosis and cardiovascular risk may be an artefact of the study design, with exclusion criteria masking important disease-related outcomes. That is, although this study may have demonstrated a significant association between RA and ESM-1 levels, these data fail to demonstrate a direct link between ESM-1, atherosclerotic mechanisms and cardiovascular events. To answer these questions, further prospective studies should be designed to determine the relationship between atherosclerosis and endothelial dysfunction markers such as ESM-1 in patients with RA; incorporation of imaging techniques, along with early determination of ccIMT and FMD, may provide useful insights into the mechanisms underlying these associations. The second limitation of our study was that pro-inflammatory mediators and cytokines, which are abundantly produced in RA and exert numerous effects in endothelial cells, were not assayed.
Conclusions
Taken together, the results from this study provide insight into the mechanisms by which endothelial function can diverge from normal homeostatic processes to those resulting in cardiovascular disorders, as in the case of RA. The data presented here strongly indicate ESM-1 as an attractive target for the treatment of inflammation-related diseases, such as RA.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Béchard D, Gentina T, Delehedde M, et al. Endocan is a novel chondroitin sulfate/dermatan sulfate proteoglycan that promotes hepatocyte growth factor/scatter factor mitogenic activity. J Biol Chem. 2001;276:48341–48349.
- Lassalle P, Molet S, Janin A, et al. ESM-1 is a novel human endothelial cell-specific molecule expressed in lung and regulated by cytokines. J Biol Chem. 1996;271:20458–20464.
- Bechard D, Meignin V, Scherpereel A, et al. Characterization of the secreted form of endothelial-cell-specific molecule 1 by specific monoclonal antibodies. J Vasc Res. 2000;37:417–425.
- Tousoulis D, Charakida M, Stefanadis C. Endothelial function and inflammation in coronary artery disease. Heart. 2006;92:441–444.
- Kharbanda RK, Walton B, Allen M, et al. Prevention of inflammation-induced endothelial dysfunction: a novel vasculo-protective action of aspirin. Circulation. 2002;105:2600–2604.
- Steyers CM III, Miller FJ Jr. Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci. 2014;15:11324–11349.
- Spinelli FR, Di Franco M, Metere A, et al. Decrease of asymmetric dimethyl arginine after anti-TNF therapy in patients with rheumatoid arthritis. Drug Dev Res. 2014;75:S67–S69.
- Mosevoll KA, Lindås R, Wendelbo O, et al. Systemic levels of the endothelium-derived soluble adhesion molecules endocan and E-selectin in patients with suspected deep vein thrombosis. SpringerPlus [Internet]. 2014;3:571. Available from: http://www.springerplus.com/content/3/1/571
- Adekola H, Romero R, Chaemsaithong P, et al. Endocan, a putative endothelial cell marker, is elevated in preeclampsia, decreased in acute pyelonephritis, and unchanged in other obstetrical syndromes. J Matern Fetal Neonatal Med. 2014;28:1–12.
- Sarrazin S, Adam E, Lyon M, et al. Endocan or endothelial cell specific molecule-1 (ESM-1): a potential novel endothelial cell marker and a new target for cancer therapy. Biochim Biophys Acta. 2006;1765:25–37.
- Aletaha D, Neogi T, Silman AJ, et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–1588.
- Cohen JD, Dougados M, Goupille P, et al. Health assessment questionnaire score is the best predictor of 5-year quality of life in early rheumatoid arthritis. J Rheumatol. 2006;33:1936–1941.
- Klarenbeek NB, Koevoets R, van der Heijde DM, et al. Association with joint damage and physical functioning of nine composite indices and the 2011 ACR/EULAR remission criteria in rheumatoid arthritis. Ann Rheum Dis. 2011;70:1815–1821.
- Sattar N, McCarey DW, Capell H, et al. Explaining how “high-grade” systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation. 2003;108:2957–2963.
- Gonzalez-Gay MA, Gonzalez-Juanatey C, Martin J. Inflammation and endothelial dysfunction in rheumatoid arthritis. Clin Exp Rheumatol. 2006;24:115–117.
- Soltész P, Dér H, Kerekes G, et al. A comparative study of arterial stiffness, flow-mediated vasodilation of the brachial artery, and the thickness of the carotid artery intima-media in patients with systemic autoimmune diseases. Clin Rheumatol. 2009;28:655–662.
- Vaudo G, Marchesi S, Gerli R, et al. Endothelial dysfunction in young patients with rheumatoid arthritis and low disease activity. Ann Rheum Dis. 2004;63:31–35.
- Klimek E, Skalska A, Kwaśny-Krochin B, et al. Differential associations of inflammatory and endothelial biomarkers with disease activity in rheumatoid arthritis of short duration. Mediat Inflamm [Internet]. 2014;2014:681635. Available from: http://dx.doi.org/10.1155/2014/681635
- Hjeltnes G, Hollan I, Førre Ø, et al. Anti-CCP and RF IgM: predictors of impaired endothelial function in rheumatoid arthritis patients. Scand J Rheumatol. 2011;40:422–427.
- Korkosz M, Gąsowski J, Surdacki A, et al. Disparate effects of anti-TNF-α therapies on measures of disease activity and mediators of endothelial damage in ankylosing spondylitis. Pharmacol Rep. 2013;65:891–897.
- Sen A, Most P, Peppel K. Induction of microRNA-138 by pro-inflammatory cytokines causes endothelial cell dysfunction. FEBS Lett. 2014;588:906–914.
- Moelants EA, Mortier A, Van Damme J, et al. Regulation of TNF-α with a focus on rheumatoid arthritis. Immunol Cell Biol. 2013;91:393–401.
- Spinelli FR, Metere A, Barbati C, et al. Effect of therapeutic inhibition of TNF on circulating endothelial progenitor cells in patients with rheumatoid arthritis. Mediat Inflamm [Internet]. 2013;2013:537539. Available from: http://dx.doi.org/10.1155/2013/537539
- Balta I, Balta S, Demirkol S, et al. Elevated serum levels of endocan in patients with psoriasis vulgaris: correlations with cardiovascular risk and activity of disease. Br J Dermatol. 2013;169:1066–1070.
- Balta I, Balta S, Koryurek OM, et al. Serum endocan levels as a marker of disease activity in patients with Behçet disease. J Am Acad Dermatol. 2014;70:291–296.