ABSTRACT
Giardia is the most frequently reported intestinal parasite worldwide. The aim of this study was to investigate the ghrelin, melatonin, glucose and cholesterol concentration in male patients infected with Giardia lamblia. We enrolled 66 patients with Giardiasis and the control groups consisted of healthy subjects (n = 30). The results demonstrated that there was a significant decrease (P < 0.05) in ghrelin levels, while the melatonin, glucose and cholesterol levels were significantly increased (P < 0.05) in giardiasis patients as compared to the healthy group. The obtained results suggest that ghrelin and melatonin could serve as biomarkers in patients infected with G. lamblia.
Introduction
Giardia lamblia is the most frequently reported intestinal parasite worldwide. It is a microscopic parasite widespread in streams and rivers around the world. Giardia can cause acute or chronic diarrhoea, contributing to nutritional deficiency or may remain asymptomatic.[Citation1,Citation2] Giardiasis is prevalent particularly in areas with poor sanitation and unsafe water supply. That is why common names of the disease are ‘beaver fever’ or ‘backpacker's diarrhoea’. Symptoms include abdominal cramps, bloating, nausea and bouts of watery diarrhoea. Giardia infections usually last a few weeks but intestinal problems may persist for a long time after the causative agent has been eliminated from the body of the patient. The disease may cause severe damage to the surface of the mucosal barrier in the small intestine and, consequently, affect nutrient absorption. There are several drugs for treatment of giardiasis that are generally effective, but some patients may prove unresponsive to therapy. That is why prevention is considered the most effective measure against giardiasis.[Citation3,Citation4]
The mechanism of pathogenesis lies in destruction of the mucosal barrier in the small intestine due to Giardia-induced enterocyte apoptosis, resulting in inflammation and malabsorption of sugars and fats.[Citation5] It can also lead to the formation of mucus plugs that provide a protective environment for infectious organisms. Additionally, the nutrients undigested owing to colonic hypermotility facilitate the proliferation of undesirable microflora.[Citation6,Citation7]
Intestinal parasites are known to cause anaemia, poor physical growth, poor intellectual development and impaired cognitive function. Because parasite infection of the gastrointestinal tract induces detrimental effects on host tissues and host physiology, different processes which tend to attenuate the effect of the loss of appetite, the intestinal malabsorption or the increased tissue losses have been assessed.[Citation8,Citation9] Ghrelin is a hormone that is produced and released mainly by the stomach, with small amounts also released by the small intestine, pancreas, brain, kidney, myocardium, hypothalamus and pituitary gland. Ghrelin is transported in the circulation bound to very high-density lipoprotein (VHDL) and high-density lipoprotein (HDL). Changes in ghrelin levels have been reported in cases of parasitic infections.[Citation10,Citation11] Acylated ghrelin affects glucose metabolism by modulating insulin secretion, amino-acid uptake and bone formation, appetite, increases food intake, energy balance, gastrointestinal motility, cardiac performance and anxiety.[Citation12,Citation13]
Malabsorption of disaccharides is a main complication of giardiasis. However, many patients infected with G. lamblia do not experience symptoms or damage to the small intestine. Another hormone of interest is melatonin. It is the main hormone secreted by the pineal gland. Secondary sources are the retina, gut, skin, platelets, bone marrow and probably other structures, whose systemic contribution is insignificant.[Citation14,Citation15] It can stimulate innate immune cells, primarily leukocytes, which represent an important anti-bacterial mechanism; however, very little is known about its influence on protozoan infections.[Citation16,Citation17] Melatonin has been discussed [Citation17] to have immunomodulatory effects in cases of toxoplasmosis,[Citation18,Citation19] malaria [Citation20,Citation21] and Chagas’ disease.[Citation22]
The aim of this work was to study the serum ghrelin, melatonin, glucose and cholesterol levels in patients with giardiasis.
Subjects and methods
Subjects and collection of samples
From January to August 2012, 96 samples were collected from 66 male patients and 30 healthy control subjects (age range 18–40 years) who attended the clinics in AL-Sadder Teaching Hospital and AL-Hakeem Hospital in AL-Najaf Province.
The collection of samples was approved by the Institutional Ethics Committee of the Faculty of Science at the University of Kufa and all participants signed informed consent forms. Faecal samples were collected in clean containers. Blood samples were also drawn from the same patients by vein-puncture into specimen tubes and were kept for 30 min at room temperature to clot. Then serum was obtained by centrifugation at 1000 r/min (Beckman Coulter, Allegra® X-15R benchtop centrifuge, UK) for 10 min.
Sample processing
The blood samples were centrifuged at 3000 r/min for 5 min to separate the serum. The supernatants were collected in new sterile tubes and each sample of serum was divided into two parts: each of them was stored at −20 ºC until use for determination of the ghrelin and melatonin concentration. The haematological parameters were determined on ethylenediaminetetraacetic acid blood using Ruby (Abbott, Chicago, IL, USA) in the haematology laboratory at AL-Sadder Medical City in Najaf Province.
Freshly voided stool specimens were processed and examined microscopically using a ×40 objective lens for intestinal parasites as described by Paniker.[Citation23] Ten ×40 objective fields of each stool smear were examined before a slide was considered negative.
Measurement of serum ghrelin concentration
The serum ghrelin concentration was measured using an enzyme-linked immunosorbent assay (ELISA) specific kit (Cusabio Biotech Co., Ltd., Cambridge, MA, USA), according to the manufacturer's instructions. A Biokit ELx800 Reader and ELx50 Washer (Barcelona, Spain) were used. Briefly, the procedure was as follows: 50 μL of standard or sample, 25 μL of primary antiserum and 25 μL of biotinylated peptide were added in the wells of 96-well immunoplates. Following incubation at room temperature for 2 h, the immunoplates were washed four times with 350 μL/well of assay TBS buffer (Tris-buffered saline, 1X). Then, 100 μL/well of horseradish peroxidase-conjugated streptavidin solution was added and the mixture was incubated at room temperature for 1 h. The immunoplates were washed four times with 350 μL/well of assay buffer (1X). Then, 100 μL/well of TMB (3,3′,5,5′-tetramethylbenzidine) substrate solution was added. After incubation for 1 h at room temperature, the reaction was terminated with 100 μL/well of stop solution, and the absorbance (optical density, O.D.) was read at 450 nm. The results were calculated by using a standard curve generated by the ELISA device.
Measurement of serum melatonin concentration
A specific ELISA kit (Cusabio Biotech Co., Ltd., Cambridge, MA, USA) was used following the manufacturer's instructions. Fifty microlitres of each standard, control and sample were pipetted into the respective wells of 96-well immunoplates. Then, 50 μL of melatonin–biotin was pipetted into each well, 50 μL of melatonin antiserum was added, and the plate was covered with adhesive foil and incubated for 14–20 h at 2–8 °C. After that, the plate was washed three times with 250 μL of wash buffer (TBS buffer, 1X). The excess solution was removed by tapping the inverted plate on a paper towel. Then, 150 μL of freshly prepared enzyme conjugate was pipetted into each well and the plate was covered with new adhesive foil to be incubated 120 min at room temperature (18–25 °C) on an orbital shaker. After that, 200 μL of freshly prepared PNPP (p-nitrophenyl phosphate) substrate solution was pipetted into each well. Following incubation for 20–40 min at room temperature (18–25 °C) on an orbital shaker (500 r/min), the substrate reaction was stopped by adding 50 μL of stop solution into each well. The contents were mixed briefly by gently shaking the plate and the optical density was measured at 405 nm. The results were calculated by using a standard curve generated by the ELISA device.
Measurement of serum glucose concentration
The glucose concentration was determined using an automatic analyzer (Olympus Optical Co., Tokyo. Japan) in the Biochemistry Laboratory at AL-Sadder Teaching Hospital in AL-Najaf, AL-Shraf, Iraq.
Measurement of serum cholesterol concentration
A CHOD–POD enzymatic colorimetric test kit was used (Cypress Diagnostics, Langdrop, Belgium), according to the manufacturer's protocol. Measurements were performed at 505 nm using a biochemistry analyzer (CYANStart Cy004, Cypress Diagnostics, Langdrop, Belgium).
Statistical analysis
Data were analysed using the software packages GraphPad Prism for Windows (5.04, GraphPad Software Inc., San Diego, CA USA). Data are presented as the mean values with standard error of the mean (±SEM). Comparisons between the patients and the healthy group were made by using t-test. A P-value of less than 0.05 was considered to indicate significant difference.
Results and discussion
Serum ghrelin levels in giardiasis patients and healthy subjects
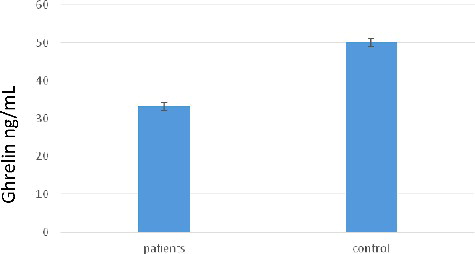
The results presented in showed that the serum ghrelin concentration in giardiasis patients (33.245 ± 0.847 ng/mL) was significantly lower (P < 0.05) compared to the mean value observed in the healthy group (50.102 ± 1.121 ng/mL).
Ghrelin concentrations reduced in patients with intestinal parasitic infections are thought to compensate for an increase in the glucose concentration; hence, the relationship between insulin and ghrelin.[Citation11] The decreased ghrelin concentration in the patients observed in this study is in agreement with the suggestion that this could be the main reason for the loss of appetite in patients with parasitic infections.[Citation24] Another possible cause–effect relationship that might explain, at least in part, the decreased endogenous ghrelin levels in infected patients might be to decrease the lipid peroxidation that increased as a result of the parasitic infection.[Citation25–27]
Serum melatonin levels in giardiasis patients and healthy subjects
It is known that the phagocytic capacity of amoebae involves leukophagocytosis because amoebae are constantly in contact with leukocytes in vivo and must be able to destroy them to survive.[Citation28] By analogy, G. lamblia, which is a protozoan, could be hypothesized to have a similar mechanism involved in its pathogenesis.
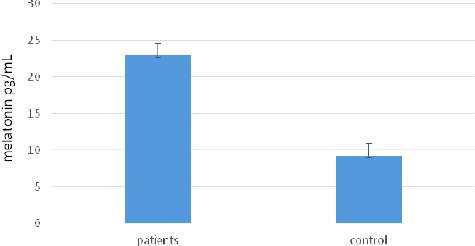
That is why the next step in our experiments was to determine the serum melatonin concentration in the study cohort. The obtained results () showed that the serum melatonin concentration was higher in the giardiasis patients (22.876 ± 1.643 pg/mL) than in the healthy group (9.213 ± 0.221 pg/mL). The increase was over twofold and the statistical analysis revealed this difference to be significant (P < 0.05). It could be speculated that the increased levels of melatonin might be due to leukocytosis caused by G. lamblia. Interactions between G. lamblia and white blood cells may lead to increased levels of melatonin, which is a known immunomodulator.[Citation29]
Serum glucose levels in giardiasis patients and healthy subjects
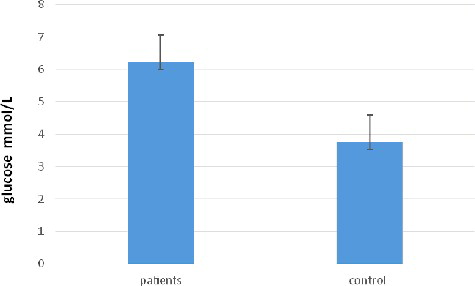
Some authors have associated the decrease in ghrelin concentrations in patients with parasitic infections with increased glucose levels.[Citation30] shows the serum glucose concentration observed in the giardiasis patients (6.231 ± 0.841 mmol/L) as compared with that in the group of healthy subjects (3.751 ± 0.241 mmol/L). Therefore, these results could support the suggestion that the decrease in the serum ghrelin level in patients with G. lamblia may be due to the increased glucose concentration in these patients as compared to that in the control group. This observation is in agreement with the hypothesis that ghrelin may play a role in regulating the long-term energy balance in intestinal parasitic infections.[Citation11,Citation31]
Serum cholesterol levels in giardiasis patients and healthy subjects
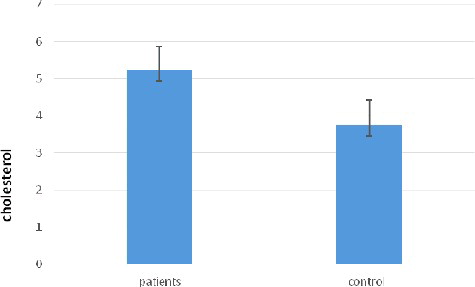
A comparison between the serum cholesterol levels in the giardiasis patients and the healthy group is shown in . The results revealed that there was a significant increase (P < 0.05) in the serum cholesterol levels in giardiasis patients (5.217 ± 0.661 mmol/L) as compared to the healthy group (3.751 ± 0.292 mmol/L). Ghrelin has been shown to interact with human plasma lipoproteins and to be linked with VHDL and HDL in the circulation.[Citation16] In turn, HDL might play major roles in the transport and metabolism of lipid hydroperoxides in vivo.[Citation32] Further research is needed to throw more light on the complex relationships between serum ghrelin and HDL levels and the enhanced lipid peroxidation in patients with parasitic infections.[Citation33]
Final remarks
Overall, our results showed that the serum ghrelin concentration was significantly decreased in patients infected with G. lamblia as compared to the healthy group (), whereas the serum melatonin (), serum glucose () and serum cholesterol () levels were significantly increased in giardiasis patients as compared to the healthy group. However, it should be noted that our research included a relatively small number of male patients only. Within the limitations of our study, based on our results and other reports, we hypothesize that the increase in the levels of serum melatonin in patients with G. lamblia might be due to phagocytosis activity. To further explore this hypothesis, studies involving a larger number of patients and a more comprehensive battery of indicators are needed.
Conclusions
The results from this study showed that there was a significant increase in the serum concentration of glucose, cholesterol and melatonin in males infected with G. lamblia, whereas the level of serum ghrelin in giardiasis patients was decreased in comparison to that in the control group. These results suggest a positive relationship between phagocytosis activity and the level of ghrelin and melatonin in patients with giardiasis.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ali SA, Hill DR. Giardia intestinalis. Curr Opin Infect Dis. 2003;16:453–460.
- Carvalho-Costa FA, Gonçalves AQ, Lassance SL, et al. Giardia lamblia and other intestinal parasitic infections and their relationships with nutritional status in children in Brazilian Amazon. Rev Inst Med Trop S Paulo. 2007;49:147–153.
- Oberhuber G, Kastner N, Stolte M, et al. Histologic analysis of 567 cases. Scand J Gastroenterol. 1997;32:51–54.
- Kliegman MD, Robert M, Behrman MD, et al. Nelson textbook of pediatrics. 18th ed. Philadelphia (PA): Saunders; 2007. p. 1462–1464.
- Nazer H. Giardiasis [Internet]. New York: Medscape, WebMD LLC; c1994–2016 [ cited 2016 Jan 15]. Available from: http://emedicine.medscape.com/article/176718-overview
- Wright SG. Protozoan infections of the gastrointestinal tract. Infect Dis Clin North Am. 2012;26:323–339.
- World Health Organization. The evidence is in: deworming helps meet the Millennium Development Goals [Internet]. Geneva: World Health Organization; c2005 [cited 2015 Oct 23]. Available from: http://www.who.int/iris/handle/10665/68876
- Carlini VP, Monzon ME, Varas MM, et al. Ghrelin increases anxiety-like behavior and memory retention in rats. Biochem Biophys Res Commun. 2002;299:739–743.
- Hoste H. Adaptive physiological processes in the host during gastrointestinal parasitism. Int J Parasitol. 2001;31:231–244.
- Parvin Z, Iraj MD, Minoo S, et al. Effects of Toxoplasma gondii infection on anxiety, depression and ghrelin level in male rats. J Parasit Dis. 2014;38:1–6.
- Erensoy A, Aydin S, Kelestimur N, et al. The change of ghrelin levels in intestinal parasitic infections. JMB. 2010;29:34–38.
- Nagaya N, Kangawa K. Ghrelin improves left ventricular dysfunction and cardiac cachexia in heart failure. Curr Opin Pharmacol. 2003;3:146–151.
- Leite-Moreira A, Soares J. Physiological, pathological and potential therapeutic roles of ghrelin. Drug Discov Today. 2007;12:276–288.
- Stefulj J, Hortner M, Ghosh M, et al. Gene expression of the key enzymes of melatonin synthesis in extrapineal tissues of the rat. J Pineal Res. 2001;30:243–247.
- Liu C, Fukuhara C, Wessel JH, et al. Localization of Aa-nat mRNA in the rat retina by fluorescence in situ hybridization and laser capture microdissection. Cell Tissue Res. 2004;315:197–201.
- França EL, Feliciano ND, Silva KA, et al. Modulatory role of melatonin on superoxide release by spleen macrophages isolated from alloxan-induced diabetic rats. Bratisl Med J. 2009;110:517–522.
- França-Botelho AC, França JL, Oliveira FMS, et al. Melatonin reduces the severity of experimental amoebiasis. Parasit Vectors [Internet]. 2011 [cited 2016 Jan 25];4:62. Available from: http://www.parasitesandvectors.com/content/4/1/62
- Baltaci AK, Mogulkoc R, Turkoz Y, et al. The effect of pinealectomy and zinc deficiency on nitric oxide levels in rats with induced Toxoplasma gondii infection. Swiss Med Wkly. 2004;134:359–363.
- Avunduk AM, Avunduk MC, Baltaci AK, et al. Effect of melatonin and zinc on the immune response in experimental Toxoplasma retinochoroiditis. Ophthalmologica. 2007;221:421–425.
- Hotta CT, Markus RP, Garcia CR. Melatonin and N-acetyl-serotonin cross the red blood cell membrane and evoke calcium mobilization in malarial parasites. Braz J Med Biol Res. 2003;36:1583–1587.
- Budu A, Peres R, Bueno VB, et al. N1-acetyl-N2-formyl-5-methoxykynuramine modulates the cell cycle of malaria parasites. J Pineal Res. 2007;42:261–266.
- Santello FH, Frare EO, dos Santos CD, et al. Melatonin treatment reduces the severity of experimental Trypanosoma cruzi infection. J Pineal Res. 2007;42:359–363.
- Paniker CK. Textbook of medical parasitology. 2nd ed. New Delhi: Jaypee Brothers; 1989.
- De Vriese C, Hacquebard M, Gregoire F, et al. Ghrelin interacts with human plasma lipoproteins. Endocrinol. 2007;148:2355–2362.
- Primo-Parmo SL, Sorenson RC, Teiber J, et al. The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene family. Genomics. 1996;33:498–507.
- Kılıc E, Yazar S, Saraymen R. Lipid peroxidation level in patients with blastocystosis. Inonu Universitesi Tıp Fakultesi Dergisi. 2003;10:1–3.
- Kılıc E, Yazar S, Saraymen R, et al. Serum lipid peroxidation level in patients with Taeniasis saginata. Turkiye Parazitoloji Dergisi. 2004;2:91–93.
- Moraes LCA, França EL, Pessoa RS, et al. The effect of IFN-γ and TGF-β in the functional activity of mononuclear cells in the presence of Entamoeba histolytica. Parasit Vectors [Internet]. 2015 [cited 2016 Jan 25];8:413. Available from: http://dx.doi.org/10.1186/s13071-015-1028-6
- Carrillo-Vico A, Lardone PJ, Álvarez-Sánchez N, et al. Melatonin: buffering the immune system. Int J Mol Sci. 2013;14:8638–8683.
- Ashraf A, Mick G, Meleth S, et al. Insulin treatment reduces pre-prandial plasma ghrelin concentrations in children with type 1 diabetes. Med Sci Monit. 2007;13: CR533–537.
- Toshinai K, Mondal MS, Nakazato M, et al. Upregulation of ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun. 2001;281:1220–1225.
- Shao B, Heinecke J. HDL, lipid peroxidation, and atherosclerosis. J Lipid Res. 2009;50:599–601.
- Aydin S, Aydin S, Croteau GA, et al. Ghrelin, nitrite and paraoxonase/arylesterase concentrations in cement plant workers. JMB. 2010;29:78–83.