ABSTRACT
The establishment of a method for the removal of abundant proteins (APs) and enrichment of low-abundance proteins (LAPs) might contribute to further studies on the physiological functions of LAPs in animals and humans without the influence of APs. The aim of our study was to investigate the effect of ultrasonic treatment on the removal of APs and enrichment of LAPs in defatted soybean meal using isopropanol. The optimal isopropanol concentration for the removal APs was initially shown to be 50% without ultrasonic treatment. The highest protein concentration was achieved at 50% isopropanol concentration with 1 h of total extraction time (ultrasonic treatment and water-bath vibrating) and when the sample was treated with ultrasound at 400 W for 10 min. Under these conditions, the protein concentration increased by 26.42% when compared to the control (no ultrasonic treatment). Furthermore, no β-conglycinin and glycinin was detected by sodium dodecyl sulphate–polyacrylamide gel electrophoresis and mass spectrometry.
Introduction
Soybean proteins usually extracted from defatted soybean flakes constitute a protein mixture with complex components.[Citation1] The extraction or detection of low-abundance proteins (LAPs) from this mixture can be very challenging not only because of their lower content, but also because most of them escape the standard protein extraction techniques [Citation2] and are shielded by highly abundant proteins (APs), β-conglycinin and glycinin.[Citation3] β-Conglycinin and glycinin, which are the major APs and represent 60%–80% of the soybean storage proteins, are becoming a focus of research.[Citation4–6] Yet, due to the lack of a satisfactory method for the removal of APs and enrichment of LAPs, comprehensive studies on the physiological function of LAPs in soybean meal in animals or humans have not been conducted so far.
Recently, with the quick development of research techniques in proteomics, the separation and identification of soybean LAPs has become a focus of attention. Some studies found that isopropanol resulted in the depletion of abundant seed-storage proteins and was more suitable for preferential enrichment of LAPs in soybean, when compared to trichloroacetic acid (TCA) protein extraction.[Citation7,Citation8] On the other hand, acoustic cavitations induced by ultrasonic waves facilitate the disintegration of particles [Citation9] and the release of cellular content into the extraction medium. Some studies have proved that high-power ultrasound treatment is a useful method for increasing the extraction of intracellular compounds from plant materials,[Citation9–11] and affects the protein properties.[Citation12–14]
However, to the best of our knowledge, reports on the ultrasonic-assisted extraction of LAPs from defatted soybean meal by isopropanol are still absent. Hence, the objective of our work was to determine the optimal conditions of combined ultrasonic treatment and isopropanol for the removal of APs and enrichment of LAPs in defatted soybean meal. The present work may contribute to further studies on the physiological function of LAPs in animals and humans without the influence of APs.
Materials and methods
Materials and chemicals
Defatted soybean meal (crude protein, 55%) was purchased from Jilin Fengzheng Soybean Food Limited Company (China). Isopropanol was of analytical grade. Ammonium bicarbonate, acetonitrile and trifluoroacetic acid (TFA) were all of chromatographic grade. Trypsin (Promega, USA) was of sequencing grade, treated with TPCK (L-1-tosylamide-2-phenylethyl chloromethyl ketone). Ultrapure water was self-made (Thermo, USA). An ultrasonic KQ-500DE cleaner (China) was used for ultrasonic treatment of the samples. A 170-kD protein molecular mass marker (Thermo, USA) was used in electrophoretic analyses.
Experimental set-up
Four extraction experiments were set to determine the effects of ultrasonic treatment on the enrichment of LAPs and removal of APs using isopropanol. In the first experiment, the optimal isopropanol concentration for the removal of APs was determined without any ultrasonic treatment. Two experiments were conducted to investigate the proper ultrasonic power for 10 min and the proper ultrasonic time at 400 W, with a total extraction time of 1 h (ultrasonic treatment and water-bath vibrating) [Citation8] and at an optimal isopropanol concentration determined in the first experiment. Based upon the three experiments, the fourth experiment was set for further confirmation of the ultrasonic treatment conditions for the removal of APs and enrichment of LAPs using isopropanol.
Soybean protein extraction
In the four extraction experiments, 5 mL solutions (containing ultrapure water and isopropanol, V:V) of known concentrations of isopropanol (35%, 40%, 45%, 50% and 55%) were added into clean tubes containing 500 mg defatted soybean meal powder. Then, the solution was ultrasound treated at 400 W for certain time (0 min, 5 min, 10 min, 15 min and 20 min) or for 10 min at certain ultrasound power (0 W, 200 W, 300 W, 400 W and 500 W). After 1 h of extraction (ultrasonic treatment and water-bath vibrating), each extract supernatant was obtained by centrifugation at 12,000 r min−1 for 15 min at 4 °C. Finally, the protein concentration in each supernatant was measured by a Coomassie Brilliant Blue kit (Jiancheng Institute of Biological Engineering, Nanjing; UV-7502C spectrophotometer, Xinmao, Shanghai).
Soybean protein identification
The proteins in the isopropanol extracts were further identified by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and mass spectrometry (MS). According to Savithiry et al. [Citation8] and Hari et al. [Citation15], 20 μL isopropanol extracts were resolved by SDS-PAGE on a 12% resolving gel (or 15% to determine the proper resolving gel concentration for the detection of low-molecular-weight proteins) at 100 V using a Mini-PROTEAN Tetra System (Bio-Rad, USA). The proteins were visualized by staining the gel with Coomassie Brilliant Blue R-250 (CBB) for about 1 h and fading overnight.
Protein bands were excised manually from the CBB-stained gel and washed twice with ultrapure water for 15 min. After this, three washes were performed with 25 mmol L−1 ammonium bicarbonate in 50% acetonitrile to destain the protein bands. The excised protein bands were washed three times with ultrapure water and dehydrated with 200 μL acetonitrile until they turned fully opaque and shrunk significantly in size. The bands excised from the gel were digested with trypsin buffer (Promega, Madison, USA) after casting away the redundant acetonitrile. The digestion was performed overnight at 37 °C after incubation at 4° C for 30 min. The supernatant was removed into a clean tube and extracted three times in 50% acetonitrile containing 0.1% TFA. After this, the mixture was vacuum freeze-dried (LyoQUEST-55 vacuum freeze dryer, Telstar, Spain).
A 5800 MALDI TOF/TOF analyzer (Applied Biosystem, Framingham, MA, USA) was used for analyzing all samples. The mass spectra (m/z 800–4000) were acquired in a positive ion reflector mode. Twenty most intense ions were selected for subsequent MS/MS sequencing analysis in a 2 kV mode. Protein identification was performed by searching MS/MS spectra and peptide mass was obtained from the NCBI protein database using the search engine Matrix Science (http://www.matrixscience.com). The following search parameters were applied: Viridiplantae (green plants) chosen as a taxonomical unit, peptide tolerance of 1.2 Da, MS/MS tolerance of 0.6 Da, one incomplete cleavage was allowed and oxidation of methionine as variable modification. According to the MASCOT probability analysis (p < 0.05), only significant hits were accepted for protein identification.
Results and discussion
Isopropanol is known to facilitate the enrichment of LAPs and the removal of APs in soybean [Citation7] and 30%, 40% and 50% isopropanol extracts exhibited more LAPs than 60%, 70% and 80% isopropanol extracts analyzed by one-dimensional PAGE.[Citation8] The results in our study () showed that the higher isopropanol concentration, the lower protein concentration in the isopropanol extracts. The protein concentration in 35% isopropanol extracts was significantly higher than that in 40%, 45%, 50% and 55% isopropanol extracts (p < 0.01). SDS-PAGE analysis () revealed that the 35% and 40% isopropanol extracts contained more protein bands. In the extracts with 50% isopropanol and above, the number of protein bands decreased or the intensity of their staining weakened, which indicated a reduction in the protein content. The results shown in and suggested that the proteins in the isopropanol extracts decreased significantly (p < 0.05) or great significantly (p < 0.01) with the increase of the isopropanol concentration.
Figure 1. Effect of different isopropanol concentrations on protein concentration in the isopropanol extracts. Note: Different capital letters indicate significant differences at p < 0.01, and different lowercase letters indicate significant differences at p < 0.05.
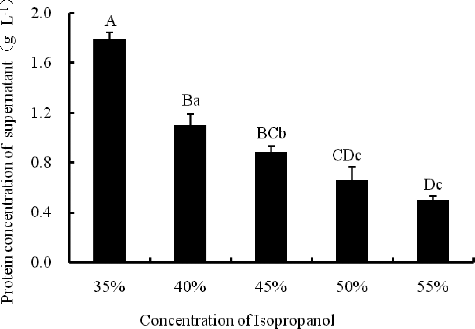
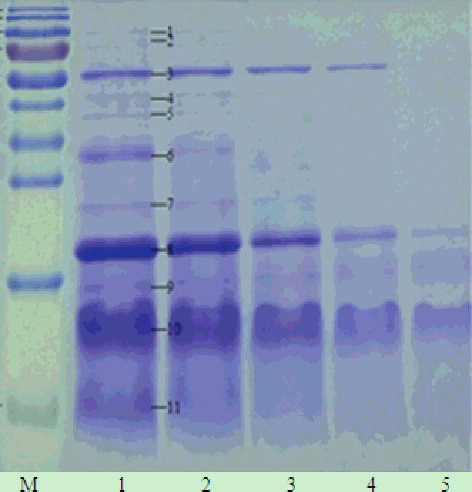
Figure 2. One-dimensional electrophoretogram of isopropanol extracts at different isopropanol concentrations. Lane M, 170 kD protein marker (from top to bottom: 170, 130, 100, 70, 55, 40, 35, 25, 15 and 10 kD); Lanes 1–5, extracts with 35%, 40%, 45%, 50% and 55% isopropanol, respectively. Note: 15% resolving gel; no ultrasonic treatment.
The proteins in the extracts with 35% isopropanol were identified by MS to determine the effect of isopropanol concentration on the removal of APs. Soybean trypsin inhibitor (STI), β-amylase and soybean agglutinin (SBA) were found to be the three most abundant proteins (). It has been reported that STI accounts for 6% of the total protein content of soybeans.[Citation16] STI has been studied for the isolation and release of Bowman–Birk inhibitor (BBI) [Citation17,Citation18] and for its effects on animals.[Citation19,Citation20] Beta-amylase, the main amylase of soybean, has been found to be the major enzyme for starch breakdown in leaves.[Citation21] SBA, accounting for about 5%–7% of the soybean proteins, is a tetrameric glycoprotein with a subunit molecular mass of 29,495 Da.[Citation22] SBA has been studied to determine its effect on growth performance,[Citation23] nitrogen metabolism,[Citation24,Citation25] gastrointestinal development in animals,[Citation25,Citation26] the effect of glycosylation on its properties [Citation27] and the effect of galactose on its molten globule state.[Citation28]
Table 1. Proteins in isopropanol extracts at 35% isopropanol identified by MS.
Furthermore, many other LAPs, such as phospholipase D alpha 1-like, glyceraldehyde-3-phosphate dehydrogenase-like and oleosin 16 kDa-like, which could not be obtained by traditional extraction techniques, were also easily detected at lower isopropanol concentrations. In addition, no glycinin was found in the extracts, and the α-subunit of β-conglycinin was only detected in the extracts with low isopropanol concentration (35%, 40% and 45%) ( and ). Therefore, we chose 50% as the reasonable isopropanol concentration to further investigate the effect of ultrasonic treatment on the enrichment of LAPs in defatted soybean meal.
Ultrasonic treatment produces localized cavitations which facilitate disintegration of particles.[Citation9] High-power ultrasound has been reported as a useful method to increase the extraction of intracellular compounds from plant materials.[Citation10] In our study, increasing ultrasonic power (0∼400 W) for 10 min increased the protein concentration in the isopropanol extracts. Exceeding 400 W, the protein concentration slightly decreased, but nevertheless it remained higher (p < 0.01) than that in the control (). Consistent with the change trend of protein concentration, the number of the protein bands increased and the intensity of their staining was enhanced as the ultrasonic power increased up to 400 W ().
Figure 3. Effect of ultrasonic power on protein concentration with 10 min ultrasonication at 50% isopropanol. Note: Different capital letters indicate significant differences at p < 0.01, and different lowercase letters indicate significant differences at p < 0.05.
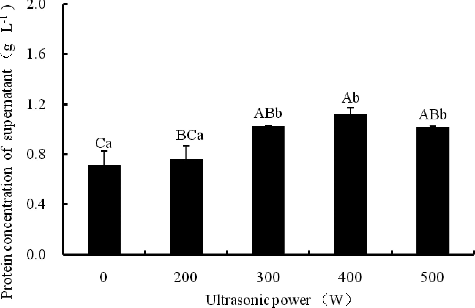
Figure 4. One-dimensional electrophoretogram of isopropanol extracts at different ultrasonic power and 50% isopropanol. Lane M, 170 kD protein maker; Lanes 1–5, isopropanol extracts at 0, 200, 300, 400 and 500 W ultrasonic power, respectively. Note: 12% resolving gel; 10 min ultrasonication time.
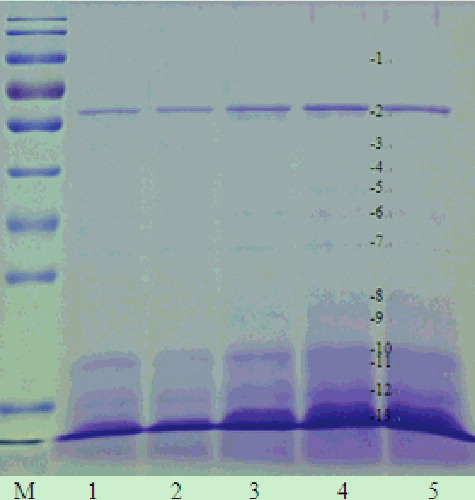
The proteins in the extracts obtained under the condition of 50% isopropanol and ultrasonic treatment (400 W, 10 min) were identified and the results were shown in . Compared to the protein extracts with 35%, 40% and 45% isopropanol without any ultrasonic treatment ( and ), no α-subunit of β-conglycinin was detected in the 50% isopropanol extracts at any ultrasonic power for 10 min. Furthermore, the detection of many LAPs, such as oleosin 1-like isoform 1, TOM1-like protein 2-like isoform X2, putative lactoylglutathione lyase-like isoform X3, iron-superoxide dismutase and some uncharacterized and unknown proteins, indicated that the ultrasonic power facilitated the solubilization of some LAPs from defatted soybean meal ( and ). Hence, we considered that 400 W was the more suitable ultrasonic power for the enrichment of LAPs in defatted soybean meal.
Table 2. Proteins identified by MS in isopropanol extracts treated at different ultrasonic power for 10 min.
As shown in , the ultrasonic treatment increased the protein concentration in isopropanol extracts at any ultrasonication time set in our experiments. The observed trend was similar to that of the effect of different ultrasonic power ( and ): the protein concentration in the isopropanol extracts increased and reached a maximum following ultrasonic treatment for 10 min at 400 W (p < 0.01) and then decreased again at longer ultrasonic treatments. Overall, in all samples treated by ultrasound, the protein concentration was significantly higher (p < 0.05 or p < 0.01) (except for 200 W, 10 min) than that of those in the control (no ultrasonic treatment). Likewise, the beta-amylase, soybean trypsin inhibitor, putative lactoylglutathione lyase-like and oleosin 1-like proteins were detected in the extracts (400 W, 10 min) as protein bands, and yet neither glycinin nor β-conglycinin α-subunit was detected by SDS-PAGE and MS ( and ).
Figure 5. Effect of ultrasonication time on protein concentration in the extracts at 400 W and 50% isopropanol. Note: Different capital letters indicate significant differences at p < 0.01 and different lowercase letters indicate significant differences at p < 0.05.
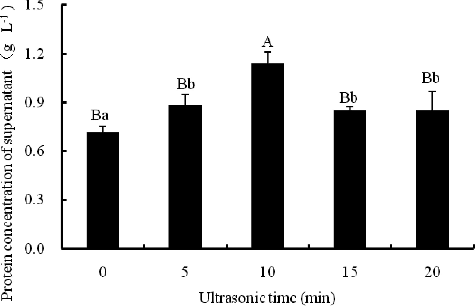
Figure 6. One-dimensional electrophoretogram of isopropanol extracts at different ultrasonication time and 50% isopropanol. Lane M, 170 kD protein marker; Lanes 1–5, isopropanol extracts at 0, 5, 10, 15 and 20 min ultrasonication time, respectively. Note: 12% resolving gel; 400 W ultrasonic power.
Table 3. Proteins identified by MS in isopropanol extracts treated with 400 W for different ultrasonication time.
It has been reported that ultrasound treatment did not modify the peptide profile, but decreased the size of the soy flakes by nearly 10-fold and increased the soybean protein isolate yield by 13% and 34% after ultrasonication for 120 s at low and high power, respectively.[Citation10,Citation12] Both with (400 W, 10 min) and without ultrasonic treatment, the protein concentration in the isopropanol extracts decreased as the isopropanol concentration increased. The protein concentration with ultrasonic treatment was much higher than that in the samples without any ultrasonic treatment at the same isopropanol concentration (p < 0.01). For example, the protein concentration increased by 26.42% when compared to the control (no ultrasonic treatment) at 50% isopropanol (). In SDS-PAGE, the number of the protein bands was similar at the same isopropanol concentration with or without ultrasonic treatment, and yet the staining intensity of the protein bands was different, which indicated the difference in the protein concentration with or without ultrasonic treatment. That is to say, the ultrasound treatment facilitated the dissolving of the proteins in defatted soybean meal at the same isopropanol concentration ().
Figure 7. Protein concentration of extracts at different isopropanol concentrations with or without ultrasonic treatment (400 W, 10 min). Note: Different capital letters indicate significant differences at p < 0.01 and different lowercase letters indicate significant differences at p < 0.05.
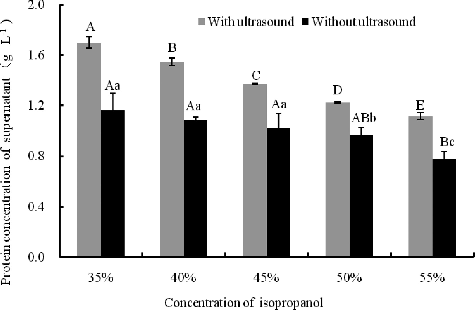
Figure 8. One-dimensional electrophoretogram of isopropanol extracts with or without ultrasonic treatment at different isopropanol concentrations. Lane M, 170 kD protein marker; Lanes 1, 3, 5, 7, 9, without ultrasonic treatment, and Lanes 2, 4, 6, 8, 10, with ultrasonic treatment of isopropanol extracts of 35%, 40%, 45%, 50% and 55% isopropanol, respectively. Note: 12% resolving gel; ultrasonic treatment (400 W, 10 min).
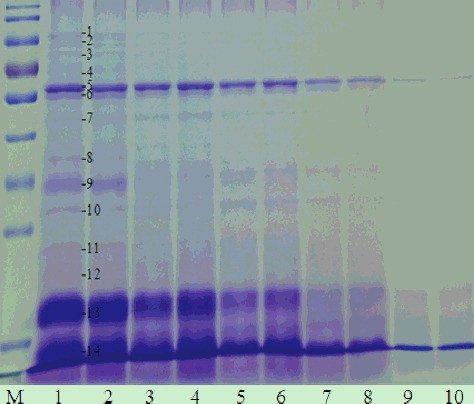
Whey proteins, which make up from 9% to 15.3% of the soybean proteins, are composed of lipoxygenase (LOX, 102 kDa), β-amylase (61.7 kDa), lectin (33 kDa) and Kunitz trypsin inhibitors (KTI, 20 kDa).[Citation29] The results shown in and indicated that the amount of SBA, TI (trypsin inhibitor), β-amylase and lipoxygensase were the highest among the proteins of isopropanol extracts. Some protein bands (band 1, 2, 3, 4, 8 and 12) in the SDS-PAGE electrophoretogram even disappeared at high isopropanol concentrations. Further, we also found that protein band 11, which was identified as the alpha subunit of beta-conglycinin, completely disappeared when the isopropanol concentration reached 50%. This was also in accordance with our result shown in and .
Table 4. Proteins identified by MS in isopropanol extracts with or without ultrasound treatment.
Taken together, our results showed that high concentration of isopropanol (50%, V: V) was more suitable for removing the APs from defatted soybean meal than lower concentrations of isopropanol (35%, 40% and 45%) and that ultrasonic treatment plays an important role in the enrichment of LAPs. Previous studies that have verified TCA/acetone [Citation30] or isopropanol/acetone as the suitable method for LAPs extraction from soybean [Citation8] have focused only on detection of LAPs by proteomics techniques and not on determination of the amount of total LAPs. In our research, not only the LAPs (no APs) in extracts were preliminarily identified by SDS-PAGE and MS, but also the optimal conditions of ultrasonic treatment were confirmed by determination of the protein concentration. This may provide new opportunities for the detection of LAP components in soybean and studies of their physiological function.
Conclusions
In this study, ultrasound was demonstrated to have a positive effect on the removal of APs and enrichment of LAPs in extracts obtained from defatted soybean meal using isopropanol extraction. The protein concentration was increased by 26.42% when compared with the control (without ultrasonic treatment). The optimal conditions for the removal of APs and enrichment of LAPs were 50% isopropanol, 1 h total extraction time and a 10-min ultrasound treatment at 400 W. Further experiments would be carried to prepare a sufficient amount of LAPs extracts to identify the components and study their physiological functions in animals and humans without the influence of APs.
Acknowledgments
The authors would like to thank the Testing Center of Yangzhou University for providing the 5800 MALDI TOF/TOF analyzer. The authors are also grateful to Yuyang Wang (Testing Center of Yangzhou University) for the operation guidance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Grieshop CM, Kadzere CT, Clapper GM, et al. Chemical and nutritional characteristics of United States soybeans and soybean meals. J Agric Food Chem. 2003;51:684–691.
- Marmagne A, Rouet MA, Ferro M, et al. Identification of new intrinsic proteins in Arabidopsis plasma membrane proteome. Mol Cell Proteomics. 2004;3:675–691.
- Herman EM, Helm RM, Jung R, et al. Genetic modification removes an immunodominant allergen from soybean. Plant Physiol. 2003;132:36–43.
- Krishnan HB, Kim WS, Jang SC, et al. All three subunits of soybean β-conglycinin are potential food allergens. J Agric Food Chem. 2009;57:938–943.
- Eun HK, Sang IL, Sangsuk O. Effect of enzymatic hydrolysis of 7S globulin, a soybean protein, on its allergenicity and identification of its allergenic hydrolyzed fragments using SDS-PAGE. Food Sci Biotechnol. 2006;15:128–132.
- Peng S, De FL, Zhe JL. Effects of glycinin on IgE-mediated increase of mast cell numbers and histamine release in the small intestine. J Nutr Biochem. 2008;19:627–633.
- Hari BK. A simple and rapid method to isolate low molecular weight proteinase inhibitors from soybean. Korean J Crop Sci. 2004;49:342–348.
- Savithiry SN, Hari BK, Sukla L. An efficient extraction method to enhance analysis of low abundant proteins from soybean seed. Anal Biochem. 2009;394:259–268.
- Khanal SK, Montalbo M, Srinivasan G, et al. Ultrasound enhanced glucose release from corn in ethanol plants. Biotechnol Bioeng. 2007;98:978–985.
- Bishnu K, Buddhi PL, Stephanie JJ. Enhancing protein and sugar release from defatted soy flakes using ultrasound technology. J Food Eng. 2010;96:270–278.
- Bu G, Liu H, Chen F, et al. Effects of different factors on the forward extraction of soy protein in reverse micelle systems. Afr J Biotechnol. 2012;11:7247–7257.
- Bishnu K, Buddhi PL, David G. Functional properties of soy protein isolates produced from ultrasonicated defatted soy flakes. J Am Oil Chem Soc. 2009;86:1021–1028.
- Jonathan O'S, Brian M, Cal F, et al. The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocoll. 2016;53:141–154.
- Hu H, Li-Chan ECY, Wan L, et al. The effect of high intensity ultrasonic pre-treatment on the properties of soybean protein isolate gel induced by calcium sulfate. Food Hydrocoll. 2013;32:303–311.
- Hari BK, Nathan WO, Savithiry SN. A rapid and simple procedure for the depletion of abundant storage proteins from legume seeds to advance proteome analysis: a case study using glycinemax. Proteomics. 2009;9:3174–3188.
- Rackis JJ, Wolf WJ, Baker EC. Protease inhibitors in plant foods: content and inactivation. In: Friedman M, editor. Nutritional and toxicological significance of enzyme inhibitors in foods. Vol. 199, Advances in experimental medicine and biology. New York: Plenum Press; 1986. p. 299–349.
- Conor F, Paul M, Julien M. Isolation of Bowman-Birk-inhibitor from soybean extracts using novel peptide probes and high gradient magnetic separation. Food Chem. 2012;134:1831–1838.
- Manoj HP, Savithiry SN, Thomas TYW, et al. Imbibition of soybean seeds in warm water results in the release of copious amounts of Bowman–Birk Protease Inhibitor, a putative anticarcinogenic agent. J Agric Food Chem. 2012;60:3135–3143.
- Ester S, Miguel AS, Ana R. Effect of diets containing a purified soybean trypsin inhibitor on growth performance, digestive proteases and intestinal histology in juvenile sea bream (Sparus aurata L.). Aquac Res. 2010;41:187–198.
- Gu CM, Li SJ, Zhao LL. et al. The effects of soybean trypsin inhibitor on free radicals levels in pancreatic mitochondria of mice. J Food Nutr Res. 2014;2:357–362.
- Daniel CF, Michaela S, Tabea M. β-AMYLASE4, a noncatalytic protein required for starch breakdown, acts upstream of three active β-amylases in Arabidopsis chloroplasts. Plant Cell. 2008;20:1040–1058.
- Shalini B, Aparna S, Munishwar NG. Removal and recovery of antinutritional factors from soybean flour. Food Chem. 2005;89:497–501.
- Steven DH, Anant SB, Paul BB. Soybean lectins and trypsin inhibitors, but not oligosaccharides or the interactions of factors, impact weight gain of rainbow trout (Oncorhynchus mykiss). Aquaculture. 2010;306:310–314.
- Li ZT, Li DF, Qiao SY, et al. Anti-nutritional effects of a moderate dose of soybean agglutinin in the rat. Arch Anim Nutr. 2003;57:267–277.
- Li ZT, Li D, Qiao S. Effects of soybean agglutinin on nitrogen metabolism and on characteristics of intestinal tissues and pancreas in rats. Arch Anim Nutr. 2003;57:369–380.
- Zang J, Li D, Piao X. Effects of soybean agglutinin on body composition and organ weight in rats. Arch Anim Nutr. 2006;60:245–253.
- Swagata H, Avadhesha S, Chaitali M. Impact of glycosylation on stability, structure and unfolding of soybean agglutinin (SBA): an insight from thermal perturbation molecular dynamics simulations. Glycoconj J. 2015;32:371–384.
- Parvez A, Farha N, Ali SA, et al. Effect of galactose on acid induced molten globule state of soybean agglutinin: biophysical approach. J Mol Struct. 2015;1099:149–153.
- Iwabuchi S, Yanauchi F. Electrophoretic analysis of whey proteins presents in soybean globulin fractions. J Agr Food Chem. 1987;35:205–209.
- Savithiry SN, Chenping X, Wesley MG, et al. Assessment of the natural variation of low abundant metabolic proteins in soybean seeds using proteomics. J Plant Biochem Biotechnol. 2012;21:30–37.
